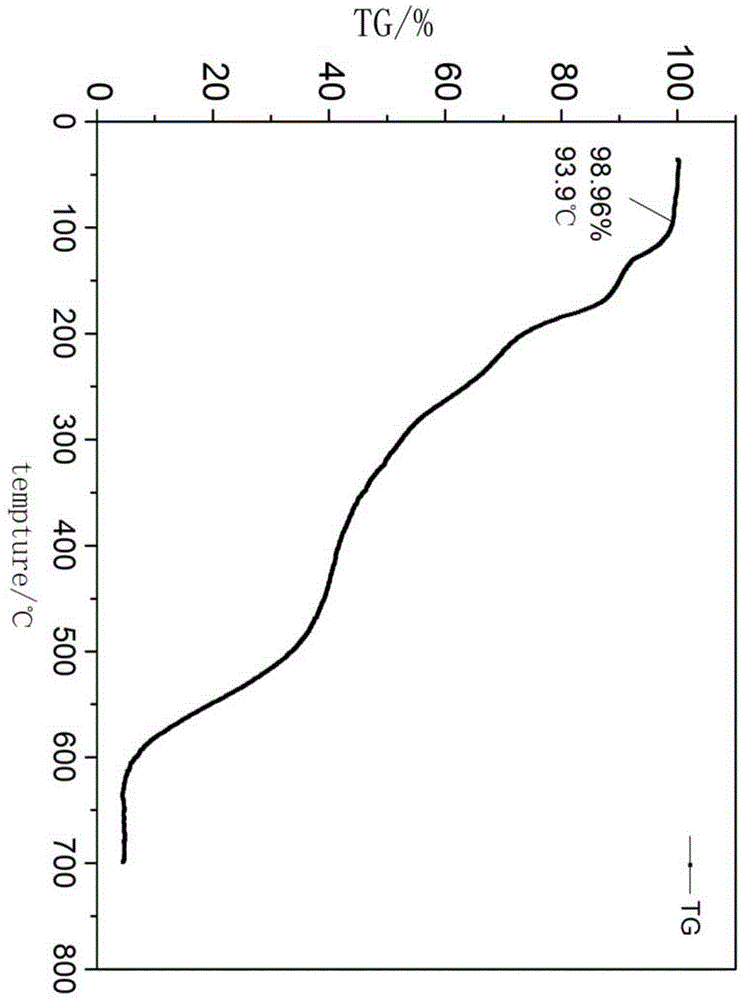
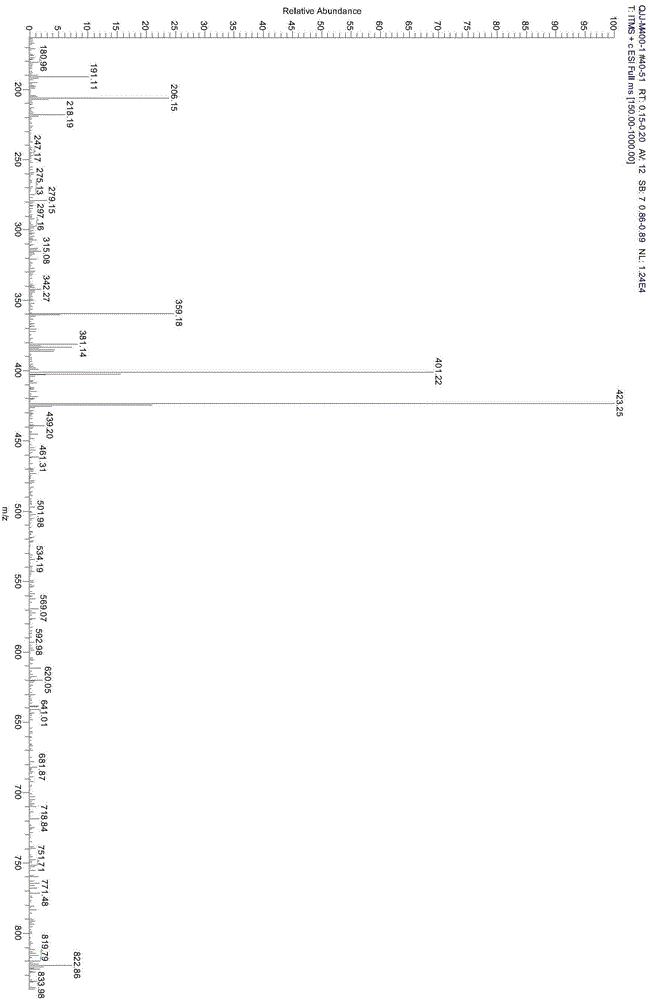
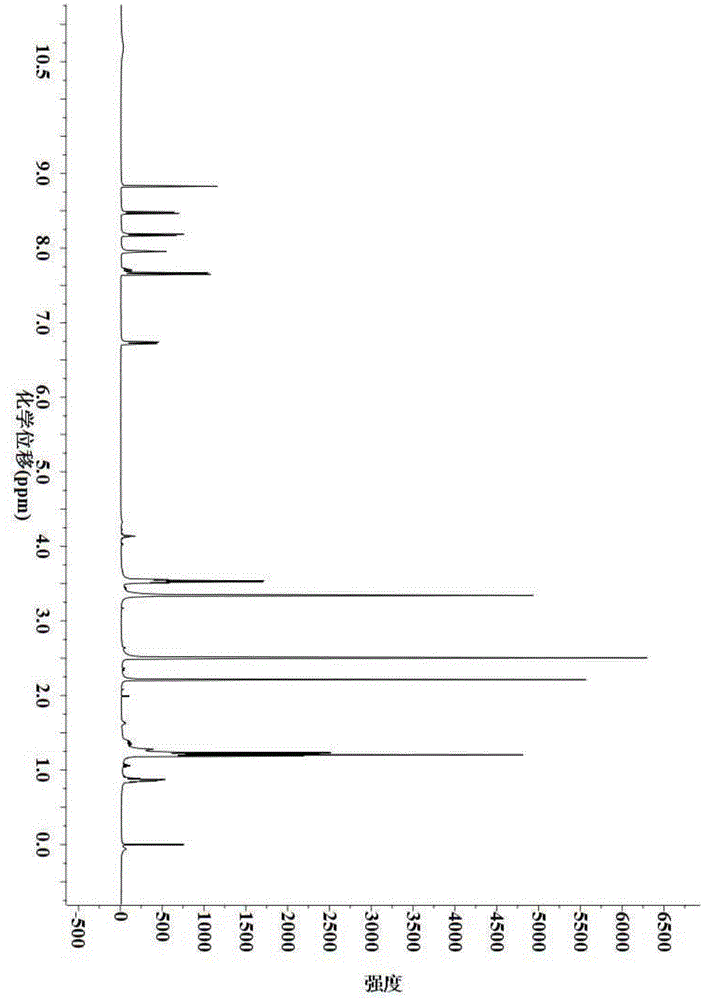
Preparation method for weak-base arylamine diazonium salt and method for preparing tertiary amine weak-base arylamine azo dye by weak-base arylamine diazonium salt
A kind of aromatic amine azo, weak basic technology, applied in the direction of azo dyes, monoazo dyes, organic dyes, etc., can solve the problem of large amount of acid, shorten the reaction time, broad application prospects, beneficial to the environment protective effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0032] A clean preparation method of N,N-diethyl-4-(2,4-dinitrophenylazo)-3-acetamidoaniline dye, the method includes the following steps:
[0033] Diazotization reaction: Dissolve 1.84g of 2,4-dinitroaniline in 60mL of diethylene glycol dimethyl ether, and add 4.32g of 1,5-naphthalene to the 2,4-dinitroaniline solution Disulfonic acid, add 1.72g of tert-butyl nitrite under stirring at 20°C, react at 20°C for 1h, and filter with suction to obtain diazonium salt;
[0034] Coupling reaction: Dissolve 2.06g of N,N-diethyl-3-acetamidoaniline and 2.88g of 1,5-naphthalenedisulfonic acid in 100mL of water and add the weight obtained in the diazotization step at 0°C. Nitrogen salt, adjust the pH to 4-6 with anhydrous sodium carbonate, react for 10 min at 0℃, adjust the pH to neutral, filter with suction, wash, and dry to obtain N,N-diethyl-4-(2,4-dinitro) Phenylazo)-3-acetamidoaniline dye, the yield was 83.50%.
Embodiment 2
[0036] A clean preparation method of N,N-diethyl-4-(2,6-dichloro-4-nitrophenylazo)-3-acetamidoaniline dye, the method includes the following steps:
[0037] Diazotization reaction: Dissolve 2.07g of 2,6-dichloro-4-nitroaniline in 40mL of ethylene glycol dimethyl ether, and add 4.32 to the 2,6-dichloro-4-nitroaniline solution g of 1,5-naphthalenedisulfonic acid, add 1.72g of tert-butyl nitrite with stirring at 25°C, react at 25°C for 0.5h, and filter with suction to obtain the diazonium salt;
[0038] Coupling reaction: Dissolve 2.06g of N,N-diethyl-3-acetamidoaniline and 2.88g of 1,5-naphthalenedisulfonic acid in 100mL of water and add the weight obtained in the diazotization step at 0°C. Nitrogen salt, adjust the pH to 4-6 with anhydrous sodium carbonate, react for 10 min at 0℃, adjust the pH to neutral, filter with suction, wash, and dry to obtain N,N-diethyl-4-(2,6-dichloro -4-nitrophenylazo)-3-acetamidoaniline dye, the yield was 78.77%.
Embodiment 3
[0040] A clean preparation method of N,N-diethyl-4-(2-cyano-4-nitrophenylazo)-3-acetamidoaniline dye, the method includes the following steps:
[0041] Diazotization reaction: Dissolve 1.63 g of 2-cyano-4-nitroaniline in 30 mL of diethylene glycol dimethyl ether, and add 3.17 g of 1 to the 2-cyano-4-nitroaniline solution ,5-Naphthalenedisulfonic acid, add 1.26g of tert-butyl nitrite with stirring at 0℃, react at 0℃ for 20min, and filter with suction to obtain diazonium salt
[0042] Coupling reaction: Dissolve 2.06g of N,N-diethyl-3-acetamidoaniline and 2.88g of 1,5-naphthalenedisulfonic acid in 100mL of water and add the weight obtained in the diazotization step at 0°C. Nitrogen salt, adjust the pH to 4-6 with anhydrous sodium carbonate, react for 10 min at 0°C, adjust the pH to neutral, filter with suction, wash, and dry to obtain N,N-diethyl-4-(2-cyano-4 -Nitrophenylazo)-3-acetamidoaniline dye with a yield of 84.47%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com