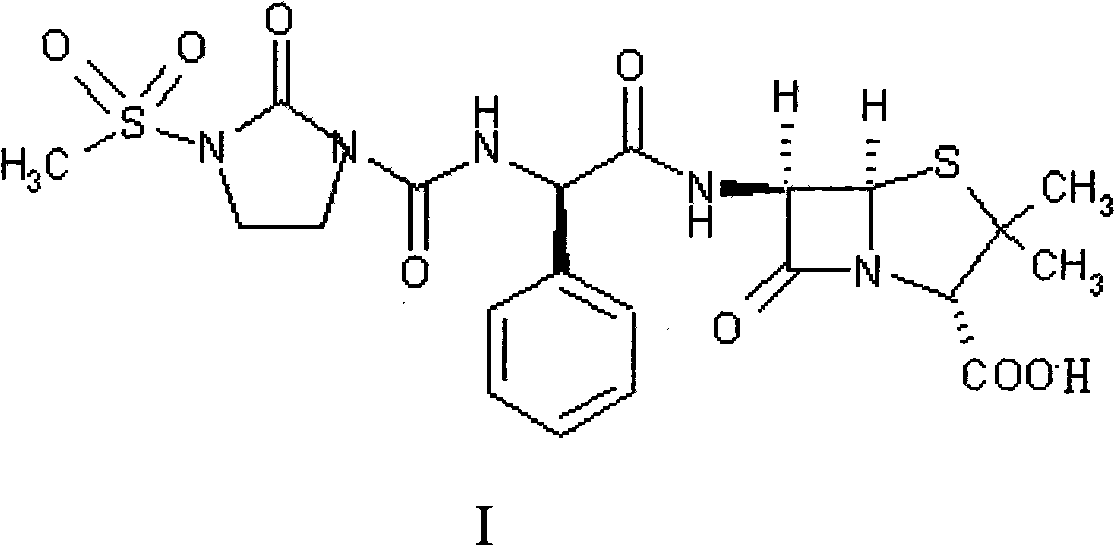
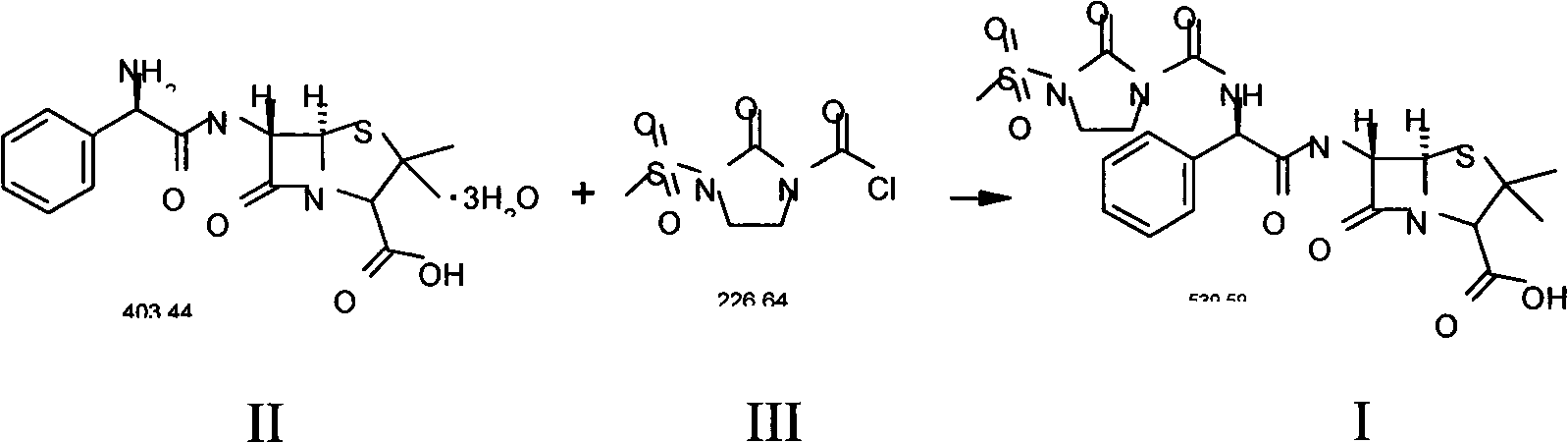
Preparation process of Mezlocillin
A technology of mezlocillin and ampicillin trihydrate, applied in the direction of organic chemistry, etc., can solve the problems of high solvent residue in ethyl acetate and unreported solvent residue in ethyl acetate, etc., and achieve the reduction of solvent residue, obvious economic benefits and Social benefit, quality improvement effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0054] Add 10kg of ampicillin trihydrate (II), 150L of purified water to the cleaned reactor, stir, cool to 0-5°C, add 4% sodium hydroxide solution dropwise until all ampicillin trihydrate is dissolved, add slowly 5.9kg 1-chloroformyl-3-methanesulfonyl-2-imidazolidinone (III) was subjected to condensation reaction, and the reaction temperature was kept at 0-5°C, while 4% sodium hydroxide solution was added dropwise to control the pH of the solution. Between 7.0-7.5; the condensation reaction is over, the reaction product is filtered, 40L of acetone is added to the filtrate, the temperature is controlled at 20-25℃, 1N hydrochloric acid is added dropwise, and solids are precipitated. When the pH value is 2.0, stop adding 1N hydrochloric acid and continue Stir for about 30 minutes; the crystalline product is separated by filtration, and the solid is washed three times with a 20% acetone aqueous solution (volume ratio, acetone:water=1:4) in a conventional amount, filtered to dryness, ...
Embodiment 2
[0057] Add 10kg of ampicillin trihydrate (II), 150L of purified water to the cleaned reactor, stir, cool to 0-5°C, add 4% sodium hydroxide solution dropwise until all ampicillin trihydrate is dissolved, add slowly 5.9kg 1-chloroformyl-3-methanesulfonyl-2-imidazolidinone (III), continue to maintain the reaction temperature at 0-5 ℃, while adding 4% sodium hydroxide solution to control the pH of the solution at 7.0-7.5 Between; the reaction product was filtered, 60L of acetone was added to the filtered filtrate, the temperature was controlled at 20-25 ℃, 1N hydrochloric acid was added dropwise to precipitate solids, when the pH value was 2.0, stop adding 1N hydrochloric acid, and continue to stir for about 30 minutes The crystalline product was separated by filtration, and the solid was washed three times with 20% acetone aqueous solution (volume ratio, acetone:water=1:4), filtered to dryness, and dried to obtain (I). The quality situation is as follows:
[0058]
Embodiment 3
[0060] Add 10kg of ampicillin trihydrate (II), 150L of purified water to the cleaned reactor, stir, cool to 0-5°C, add 4% sodium hydroxide solution dropwise until all ampicillin trihydrate is dissolved, add slowly 5.9kg 1-chloroformyl-3-methanesulfonyl-2-imidazolidinone (III), continue to maintain the reaction temperature at 0-5 ℃, while adding 4% sodium hydroxide solution to control the pH of the solution at 7.0-7.5 Between; the reaction product is filtered, 55L ethanol is added to the filtered filtrate, the temperature is controlled at 20-25 ℃, 1N hydrochloric acid is added dropwise to precipitate solids, when the pH value is 2.0, stop adding 1N hydrochloric acid, continue to stir for about 30 minutes The crystalline product is separated by filtration, and the solid is washed three times with a conventional 20% ethanol aqueous solution (volume ratio, ethanol: water=1:4), filtered to dryness, and dried to obtain (I). The quality situation is as follows:
[0061]
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com