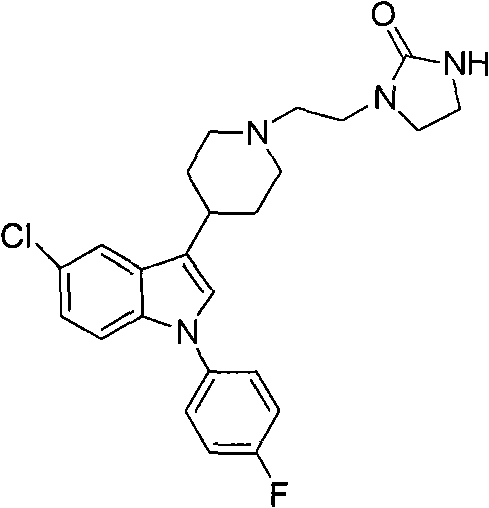
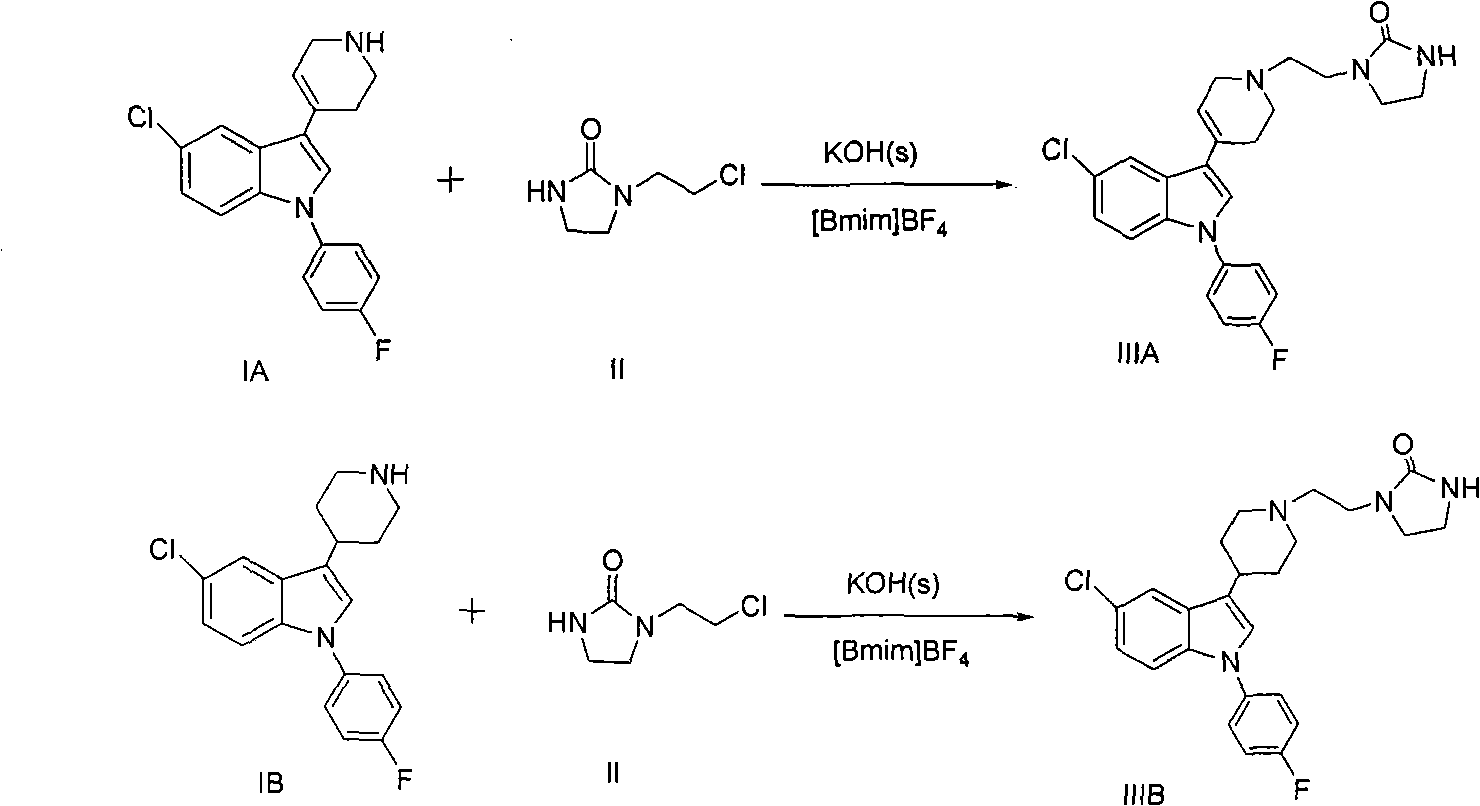
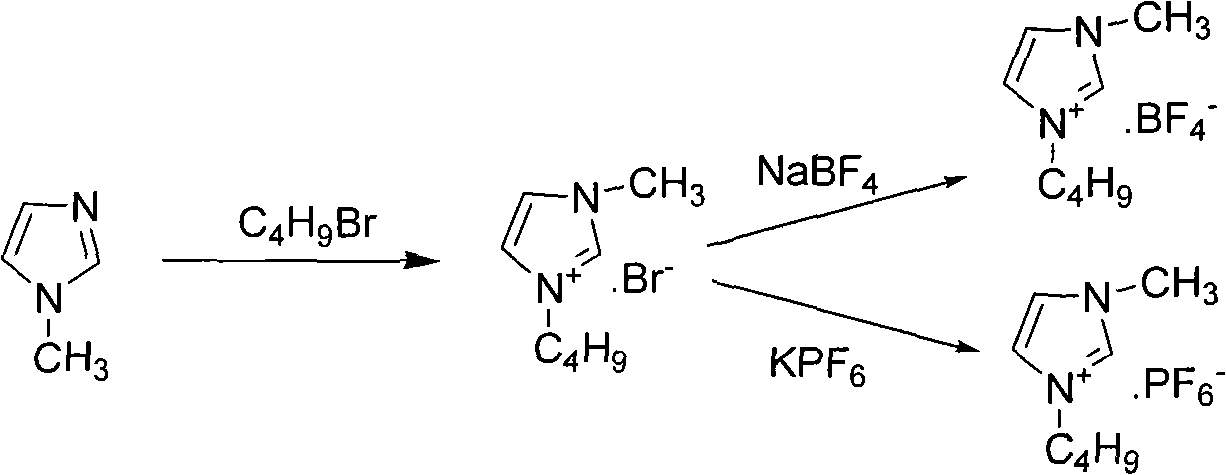
Method for preparing sertindole by using alkyl imidazole type ionic liquid as solvent
An ionic liquid solvent, alkyl imidazole type technology, applied in the field of preparation of serindole, can solve the problems of affecting product purity, consumption of large organic solvent, low reaction yield, etc., to avoid the use of volatile organic solvents, solve the Recovery and purification issues, effects of easy control of reaction conditions
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0029] 5-Chloro-1-(4-fluorophenyl)-3-(1,2,3,6-tetrahydropyridin-4-yl)-1 hydrogen-indole hydrochloride (1g, 0.0028mol) and 1-(2-Chloroethyl)-2-imidazolidinone (0.5g, 0.0034mol) was placed in a 100mL three-necked flask, and the ionic liquid 1-butyl-3-methylimidazolium tetrafluoroborate (6.7g , 0.03mol), and solid KOH (0.55g, 0.0098mol) were stirred evenly, and reacted at 110°C for 15h to obtain a dark yellow viscous liquid. Then add 10 mL of water to the reaction solution, stir for 5 min to obtain a light yellow liquid; use dichloromethane solvent to extract 3 times, wash the organic phase 2 times, dry over anhydrous sodium sulfate, filter, and evaporate to dryness to obtain 1.09 g of a light yellow solid, namely The purity of the target product is 99.5% (HPLC), and the yield is 89.1%. At the same time, the water phase after dichloromethane extraction is filtered to remove solid impurities, evaporated to remove water, filtered to remove inorganic salt impurities, and vacuum-dri...
Embodiment 2
[0031] 5-Chloro-1-(4-fluorophenyl)-3-(1,2,3,6-tetrahydropyridin-4-yl)-1 hydrogen-indole hydrochloride (1g, 0.0028mol) and 1-(2-Chloroethyl)-2-imidazolidinone (0.8g, 0.0056mol) was placed in a 100mL three-necked flask, and the ionic liquid 1-butyl-3-methylimidazolium hexafluorophosphate (8.9g, 0.031mol), solid Na 2 CO 3 (0.6g, 0.01mol) was stirred evenly, and reacted at 120°C for 10h to obtain a dark yellow viscous liquid. Then add 10 mL of water to the reaction solution, stir for 5 min to obtain a light yellow liquid; use dichloromethane solvent to extract 3 times, wash the organic phase 2 times, dry over anhydrous sodium sulfate, filter, and evaporate to dryness to obtain 1.04 g of a light yellow solid, namely It is the target product with a purity of 99.6% (HPLC) and a yield of 85.1%. At the same time, the water phase extracted by dichloromethane is filtered to remove solid impurities, evaporated to remove water, filtered to remove inorganic salt impurities, vacuum-dried ...
Embodiment 3
[0033] 5-Chloro-1-(4-fluorophenyl)-3-(1,2,3,6-tetrahydropyridin-4-yl)-1 hydrogen-indole hydrochloride (2g, 0.0055mol) and 1-(2-Chloroethyl)-2-imidazolidinone (1.1g, 0.0072mol) was placed in a 100mL three-necked flask, and the ionic liquid hydroxide 1-butyl-3-methylimidazole (8.9g, 0.057mol ), solid NaOH (1.12g, 0.02mol) was stirred evenly, and reacted at 150°C for 24h to obtain a brown viscous liquid. Then add 15 mL of water to the reaction solution, stir for 5 min to obtain a light yellow liquid; use ethyl acetate solvent extraction 3 times, wash the organic phase 2 times, dry over anhydrous sodium sulfate, filter, and evaporate to dryness to obtain 2.05 g of a light yellow solid, namely The purity of the target product is 99.5% (HPLC), and the yield is 85.2%. At the same time, the aqueous phase after ethyl acetate extraction is filtered to remove solid impurities, evaporated to remove water, filtered to remove inorganic salt impurities, vacuum-dried to recover the ionic liq...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com