Production method of epoxy chloropropane by hydrogen peroxide method
A technology of epichlorohydrin and hydrogen peroxide, applied in organic chemistry and other directions, can solve the problems of increased side reactions, large surface tension, large energy consumption for separation, etc., and achieves the maintenance of activity and selectivity, high effective utilization, and high extraction efficiency. Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1 to Embodiment 7
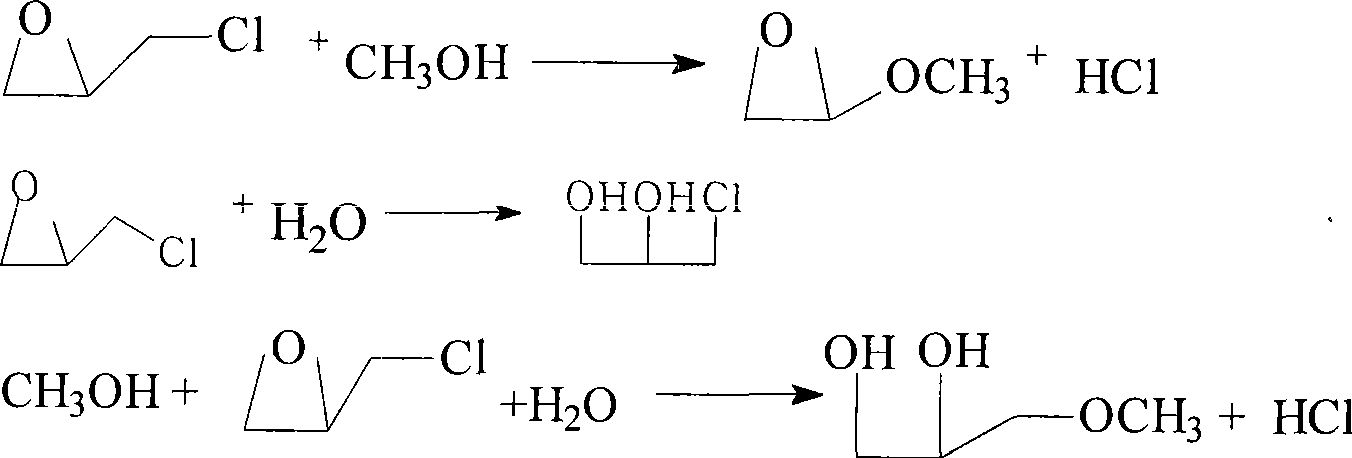
[0020] A 500mL four-necked flask is equipped with a mechanical stirrer, a thermometer, and a condenser tube. Add catalyst, methanol, and allyl chloride to the flask in a certain molar ratio, start stirring, and then dropwise add 35% H 2 o 2Raise the temperature to reflux for 1 to 1.5 hours; after the reaction is complete, let it stand and lower the temperature to ≤15°C, and separate layers. The catalyst is suspended in the water layer, the water layer is turbid and the oil layer is clear, the water layer and the catalyst therein are filtered together, the oil layer and the water layer are sampled and analyzed by gas chromatography.
[0021]
batch number n methanol: n chloropropane
ene n H2O2 h 2 o 2 Conversion rate
/ % Reservoir selectivity
/ % water layer selectivity
/ % Reaction yield
/ %
[0022] 1 2:2:1 98.8 98.5 92.5 95.8 2 2:3:1 98.8 98.6 92.2 95.8 3 2:4:1 98.8 98.9 91.8 95.9 ...
Embodiment 8 to Embodiment 13
[0028] Preparation method is with embodiment 1, and difference is: H 2 o 2 The concentration is 50%.
[0029]
Embodiment 14
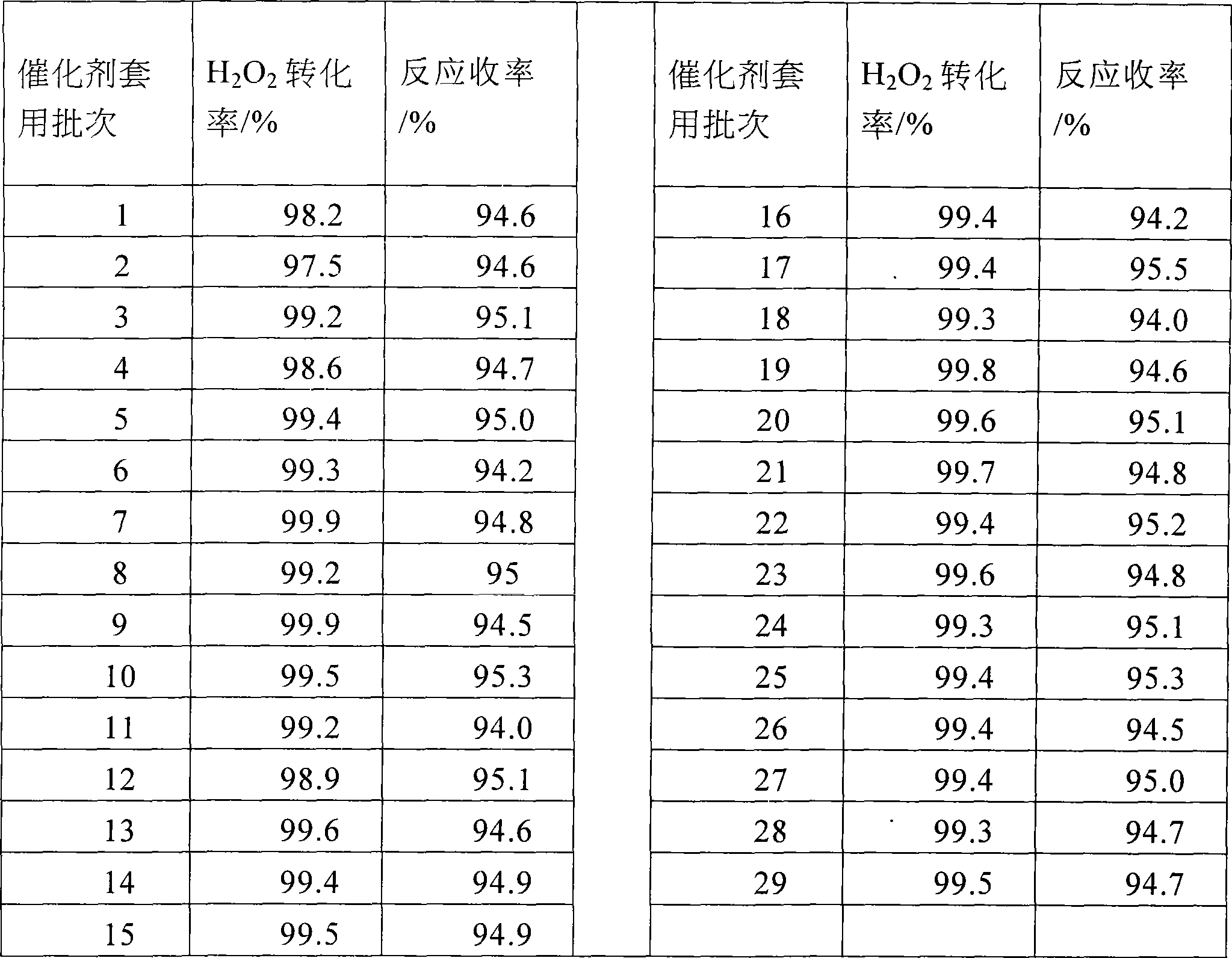
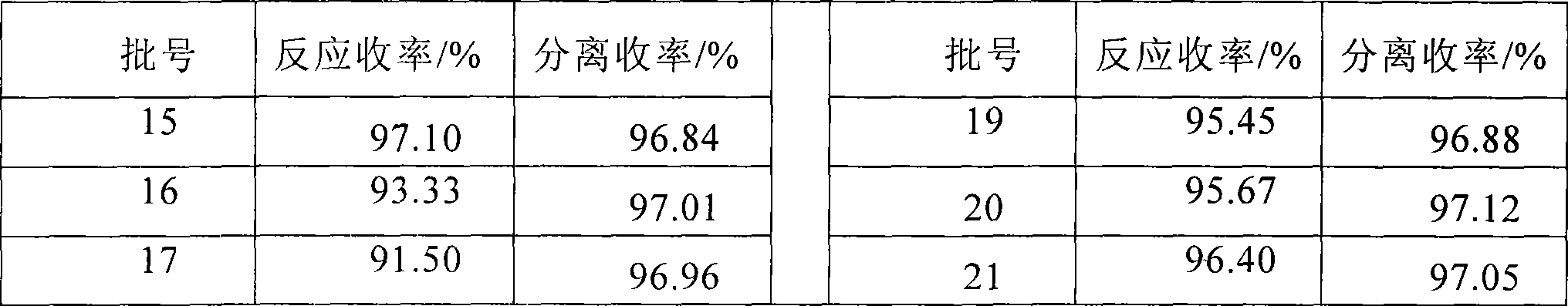
[0031] The preparation method is the same as in Example 1, except that the filter cake (wet catalyst) is directly applied mechanically after filtration or washed mechanically or applied mechanically to the next batch of reactions after regeneration, and 1% of the input amount is added when the catalyst is applied mechanically in each batch.
[0032]
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com