Preparation process of mezlocillin sodium
A mezlocillin sodium and preparation process technology, applied in the field of compound preparation, can solve the problems of no crystal growth process, unstable quality, low product content, etc., and achieve the effect of easy dissolution, stable quality and high purity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
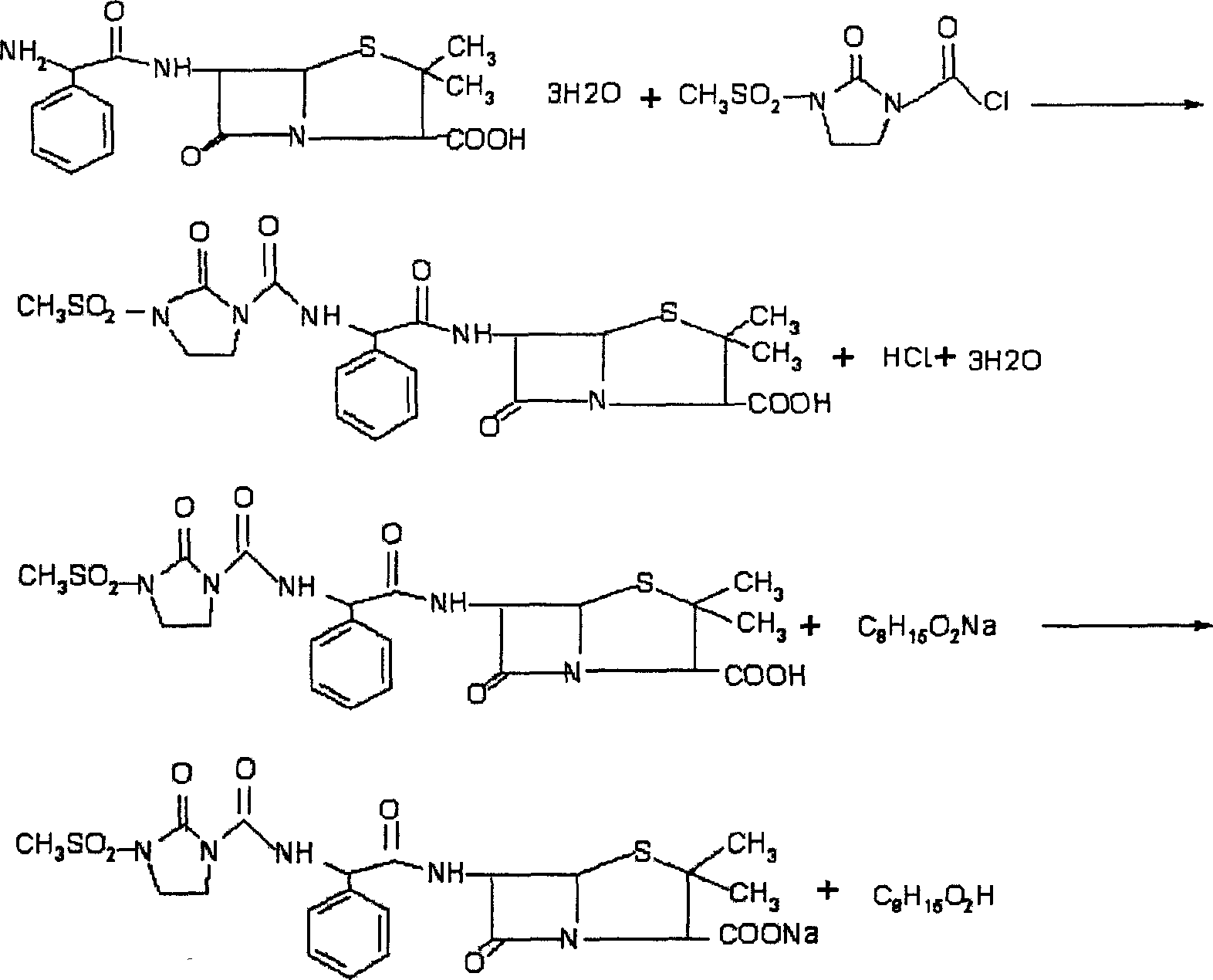
[0033] The preparation technology of mezlocillin sodium of the present invention is as follows:
[0034] Acylation: Add 10 g of ampicillin trihydrate and 75 ml of water into a clean and dry three-necked flask at room temperature, and cool down to 16°C. After the stirring was started, 11.8kg of 1-chloroformyl-3-methanesulfonyl-2-imidazolidinone was added to the reaction solution in stages, stirred rapidly to make it react, and the pH value was adjusted to 6.8 with sodium bicarbonate. After the addition, the reaction was continued for 20 minutes, and the pH was measured to be 6.84.
[0035] Acidification: Add 100ml of ethyl acetate to the above reaction solution, add 1.2M hydrochloric acid dropwise under stirring until the pH of the bottom aqueous solution is 1.86, complete the acidification, separate the layers, and take the ester layer. The aqueous layer was washed with 20 ml of ethyl acetate, the ethyl acetate solution was combined, and 20 ml of methanol was added to pass th...
Embodiment 2
[0039] The preparation technology of mezlocillin sodium of the present invention is as follows:
[0040] Acylation: Add 10 g of ampicillin trihydrate and 75 ml of water into a clean and dry three-necked flask at room temperature, and cool down to 18°C. After the stirring was started, 11.8 kg of 1-chloroformyl-3-methanesulfonyl-2-imidazolidinone was added to the reaction solution in stages, stirred rapidly to make it react, and the pH value was adjusted to 6.6 with sodium bicarbonate. After the addition, the reaction was continued for 20 minutes, and the measured pH was 6.7.
[0041] Acidification: Add 100ml of ethyl acetate to the above reaction solution, add 1.2M hydrochloric acid dropwise under stirring until the pH of the bottom aqueous solution is 2, after the acidification is completed, separate the layers, and take the ester layer. The aqueous layer was washed with 20 ml of ethyl acetate, the ethyl acetate solution was combined, and 25 ml of methanol was added to pass t...
Embodiment 3
[0046] The preparation technology of mezlocillin sodium of the present invention is as follows:
[0047]Acylation: Add 10g of ampicillin trihydrate and 75ml of water into a clean and dry three-necked flask at room temperature, and cool down to 15°C. After the stirring was started, 11.8 kg of 1-chloroformyl-3-methanesulfonyl-2-imidazolidinone was added to the reaction solution in stages, stirred rapidly to make it react, and the pH value was adjusted to 6.7 with sodium bicarbonate. After the addition, the reaction was continued for 20 minutes, and the measured pH was 6.7.
[0048] Acidification: Add 100ml of ethyl acetate to the above reaction solution, add 1.2M hydrochloric acid dropwise under stirring until the pH of the bottom aqueous solution is 1.9, after the acidification is completed, separate the layers, and take the ester layer. The aqueous layer was washed with 20 ml of ethyl acetate, combined with the ethyl acetate solution, and 23 ml of methanol was added to pass t...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com