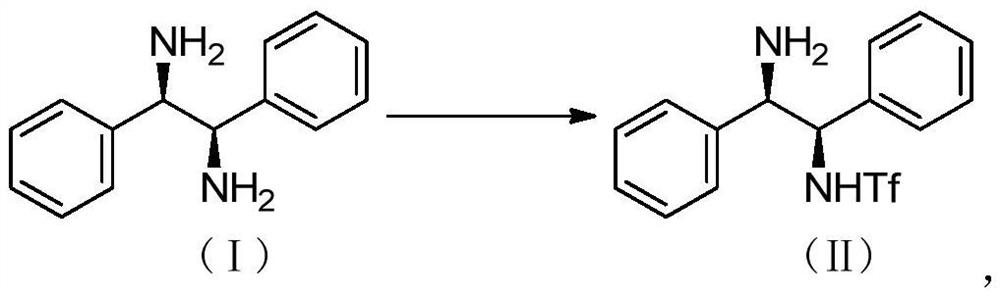
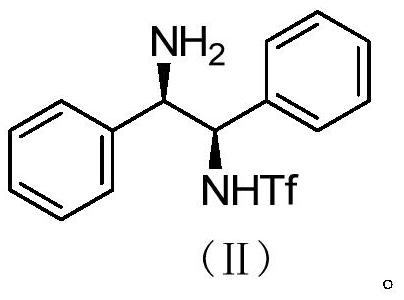
Preparation method of catalyst intermediate
A compound and time-fixed technology, applied in the field of preparation of catalyst intermediates, can solve the problems of unsuitable for pilot scale-up, weak selectivity, poor selectivity, etc., and achieve the effects of low cost, simple process, control and stability of ee value
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0031] The preparation of embodiment 1 compound (II)
[0032] Add 600ml of dichloromethane and 100g of compound (I) into a 2L four-neck flask, stir and cool down to -10°C, add 22.6g of methanesulfonic acid dropwise at one time, stir for 0.5 hours, control the temperature below 0°C and slowly add Tf 2 O in dichloromethane (Tf 2 O: 132.90g, dichloromethane: 300ml), the dropwise addition is completed, and the control is sampled after 1 hour of heat preservation reaction;
[0033] After the reaction is complete, add 200ml of drinking water, adjust the pH to 7-8 with 10% aqueous sodium hydroxide solution, raise the temperature to 15°C, let stand to separate layers, wash the organic layer once with 200ml of drinking water, and evaporate the organic layer to dryness under reduced pressure to obtain the crude product ;
[0034] Add 900ml of methyl tert-butyl ether to the crude product, heat up to 55°C, keep stirring for 0.5 hours, cool down to 25°C, filter, and dry in vacuo to obtai...
Embodiment 2
[0035] The preparation of embodiment 2 compound (II)
[0036] Add 600ml of dichloromethane and 100g of compound (I) into a 2L four-neck flask, stir and cool down to -10°C, add 45.27g of methanesulfonic acid dropwise at one time, stir for 0.5 hours, control the temperature below 0°C and slowly add Tf 2 O in dichloromethane (Tf 2 O: 132.90g, dichloromethane: 300ml), the dropwise addition is completed, and the control is sampled after 1 hour of heat preservation reaction;
[0037] After the reaction is complete, add 200ml of drinking water, adjust the pH to 7-8 with 10% aqueous sodium hydroxide solution, raise the temperature to 15°C, let stand to separate layers, wash the organic layer once with 200ml of drinking water, and evaporate the organic layer to dryness under reduced pressure to obtain the crude product ;
[0038]Add 900ml of methyl tert-butyl ether to the crude product, raise the temperature to 55°C, keep stirring for 0.5 hours, cool down to 25°C, filter, and dry in ...
Embodiment 3
[0039] The preparation of embodiment 3 compound (II)
[0040] Add 600ml of dichloromethane and 100g of compound (I) into a 2L four-neck flask, stir and cool down to -10°C, add 31.81g of trifluoromethanesulfonic acid dropwise at one time, stir for 0.5 hours, and slowly add dropwise while controlling the temperature not exceeding 0°C Tf 2 O in dichloromethane (Tf 2 O: 132.90g, dichloromethane: 300ml), the dropwise addition is completed, and the control is sampled after 1 hour of heat preservation reaction;
[0041] After the reaction is complete, add 200ml of drinking water, adjust the pH to 7-8 with 10% aqueous sodium hydroxide solution, raise the temperature to 15°C, let stand to separate layers, wash the organic layer once with 200ml of drinking water, and evaporate the organic layer to dryness under reduced pressure to obtain the crude product ;
[0042] Add 900ml of methyl tert-butyl ether to the crude product, heat up to 55°C, keep stirring for 0.5 hours, cool down to 2...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com