(1R, 2R)-1, 2-diphenylethylenediamine grafted metal-organic framework catalyst, and preparation method and applications thereof
A technology of diphenylethylenediamine and metal-organic framework, which is applied to the metal-organic framework structure catalyst and its preparation of grafted-1,2-diphenylethylenediamine, asymmetric catalytic synthesis of (S)-warfarin In the field of application, it can solve the problems of difficult catalyst recovery and poor selectivity, and achieve good thermal and chemical stability, high efficiency and stability, and high specific surface area.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0029] Dehydrate 0.5g of MIL-101 in vacuum at 150°C for 12h. After cooling to room temperature, quickly pour the dehydrated MIL-101 into anhydrous toluene (30mL), 3.0mmol of 1,2-diphenylethyl The diamine was refluxed and stirred at 110oC for 12h, filtered and washed with ethanol, and dried at room temperature to obtain the grafted catalyst CDMIL.
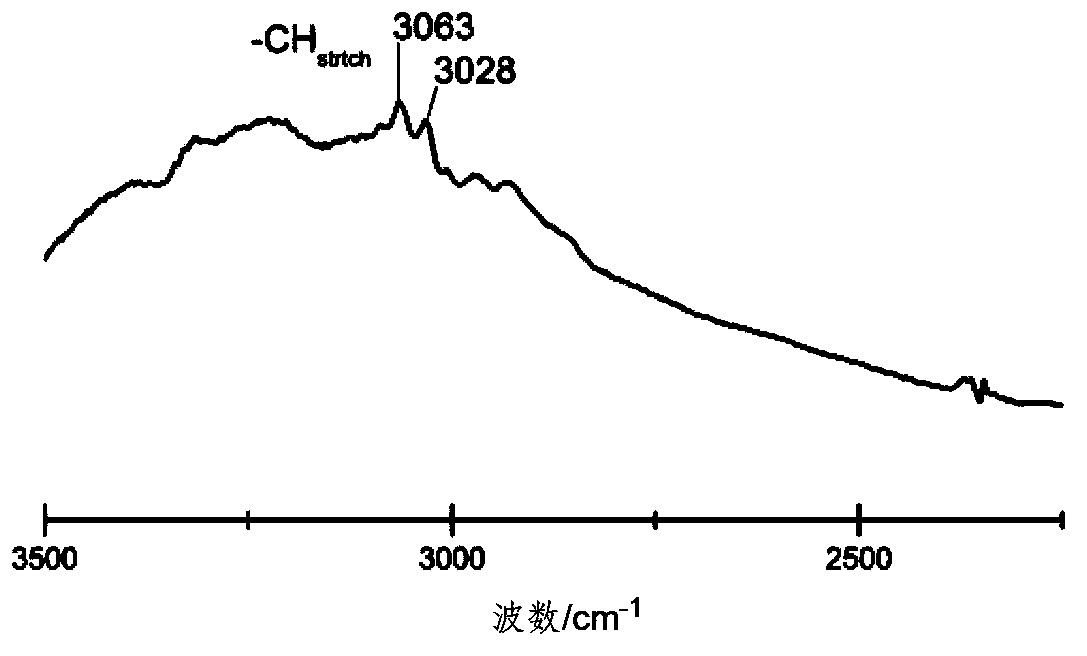
[0030] figure 1 Shown is the infrared spectrum of CDMIL. It can be seen from the figure that there are 3023cm -1 ,3028cm -1 Amino absorption peak.
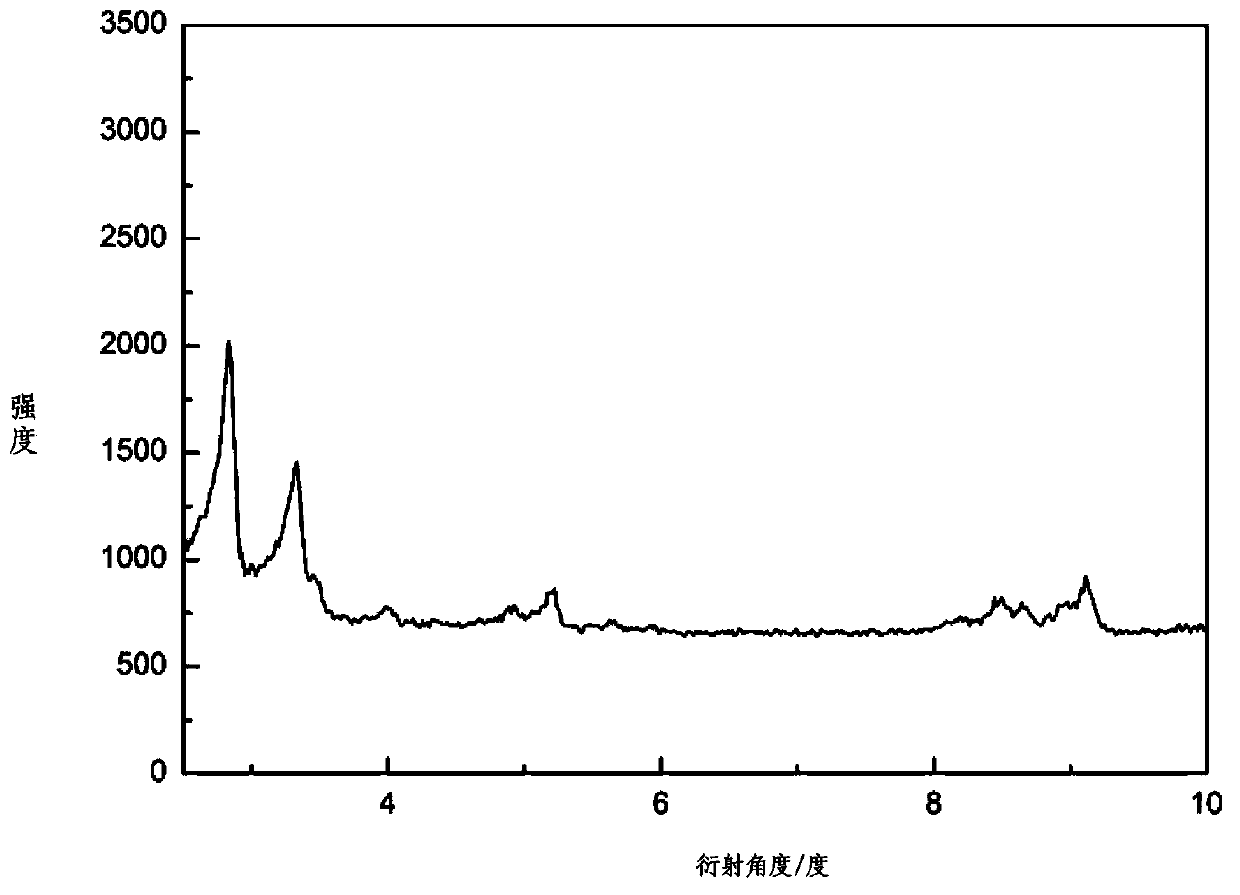
[0031] figure 2Shown is the CDMIL powder diffraction pattern. It can be seen from the figure that compared with the material MIL-101 material, the basic skeleton of the material exists.
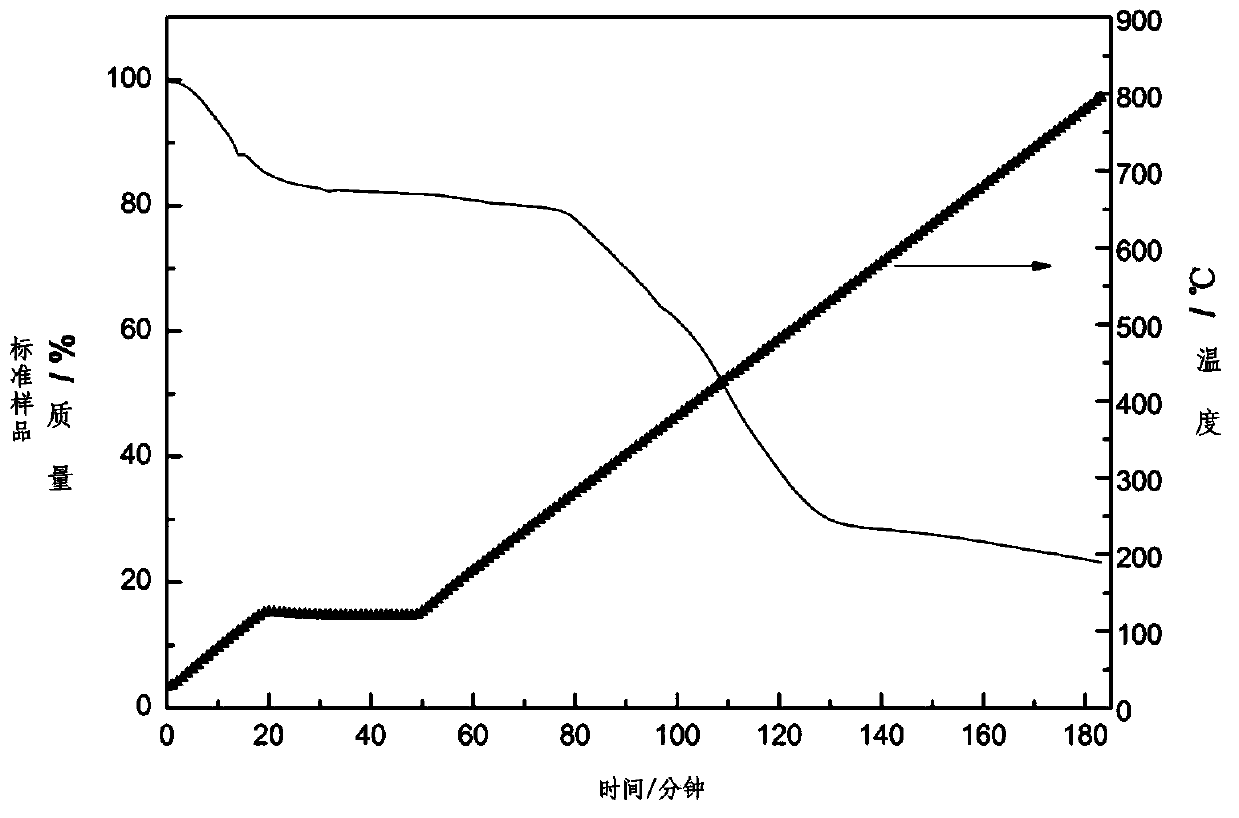
[0032] image 3 Shown is the CDMIL thermogravimetric analysis plot. It can be seen from the figure that the material is relatively stable at 0-250°C.
[0033] Figure 4 Shown is the SEM image of CDMIL. It can be seen from the figure that the morphology of the material is relatively regular....
Embodiment 2
[0035]
[0036] Put 4-hydroxycoumarin (1.62g, 10mmol), benzylidene acetone (2.20g, 15mmol), catalyst CDMIL (1.20g) and 50mL tetrahydrofuran into a 100mL test tube, and stir at 10°C for 96h. The catalyst was separated by centrifugation (400r / min, 10min), and after removing THF under reduced pressure, the column layer was carried out with a mixture of petroleum ether and ethyl acetate (the volume ratio of petroleum ether and ethyl acetate was 3:1) Analysis gave 2.8g of (S)-warfarin 3aa. Compared with traditional methods, there is no literature report that can produce gram-scale warfarin in the (S) configuration.
[0037] The structure of chiral warfarin 3aa obtained above was identified by nuclear magnetic resonance and high-resolution mass spectrometry (as shown in Figure 5(a), and Figure 5(b)). Proton NMR spectrum: (400MHz, CDCl 3 )δ7.97–7.86(m,1H),7.84–7.76(m,1H),7.60–7.52(m,1H),7.51–7.44(m,1H),7.38–7.16(m,14H),4.71( d,J=9.7Hz,0.2H),4.41–4.22(m,1H),4.15(td,J=11.1,7.1Hz,...
Embodiment 3
[0040] The first catalysis: put 4-hydroxycoumarin (1.62g, 10mmol), benzylidene acetone (2.20g, 15mmol), catalyst CDMIL (1.20g) and 50mL tetrahydrofuran in a 100mL test tube, and stir at 10°C for 96h. The catalyst was separated by centrifugation (400r / min, 10min), and after removing THF under reduced pressure, the column layer was carried out with a mixture of petroleum ether and ethyl acetate (the volume ratio of petroleum ether and ethyl acetate was 3:1) (S)-warfarin 3aa was obtained by analysis. The yield and enantioselectivity are shown in Table 1. The HPLC chromatogram is shown in Fig. 6(c).
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com