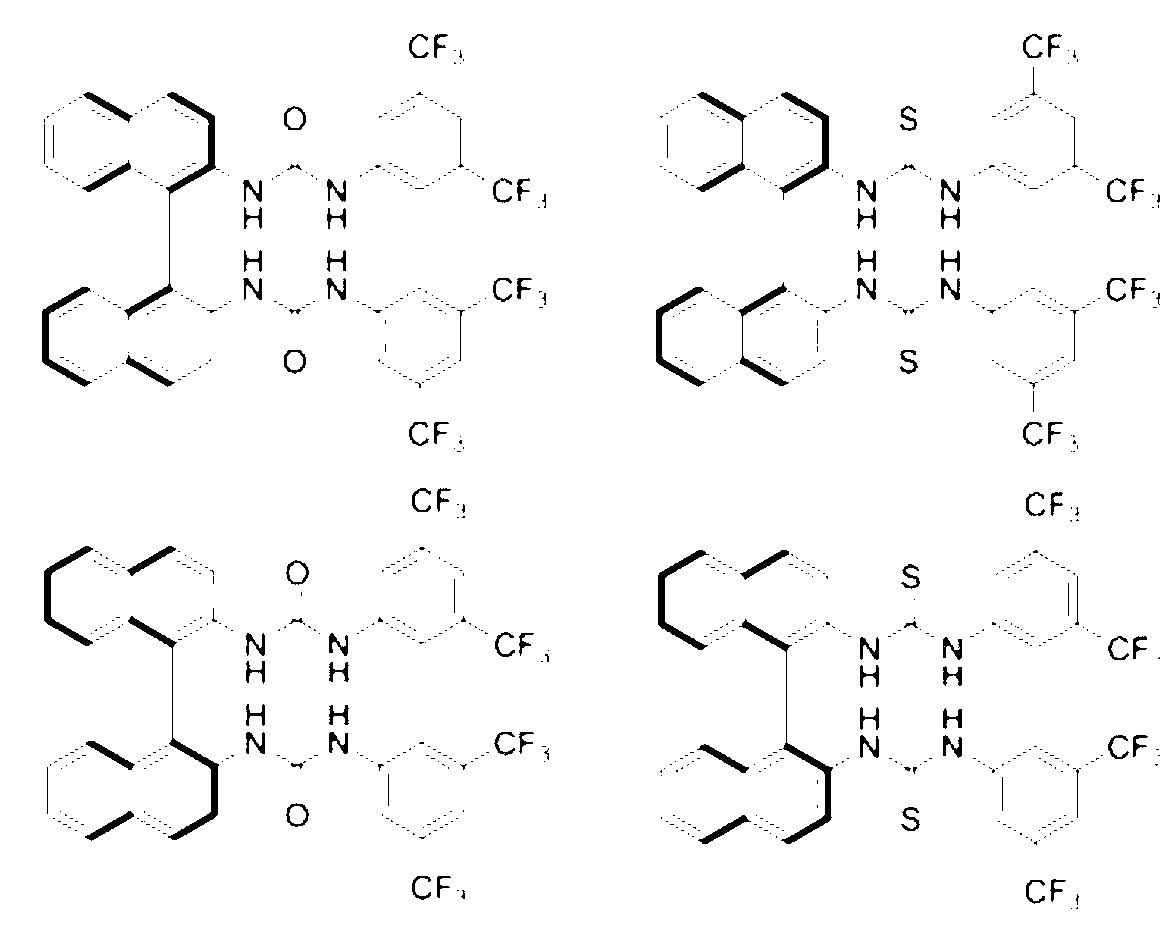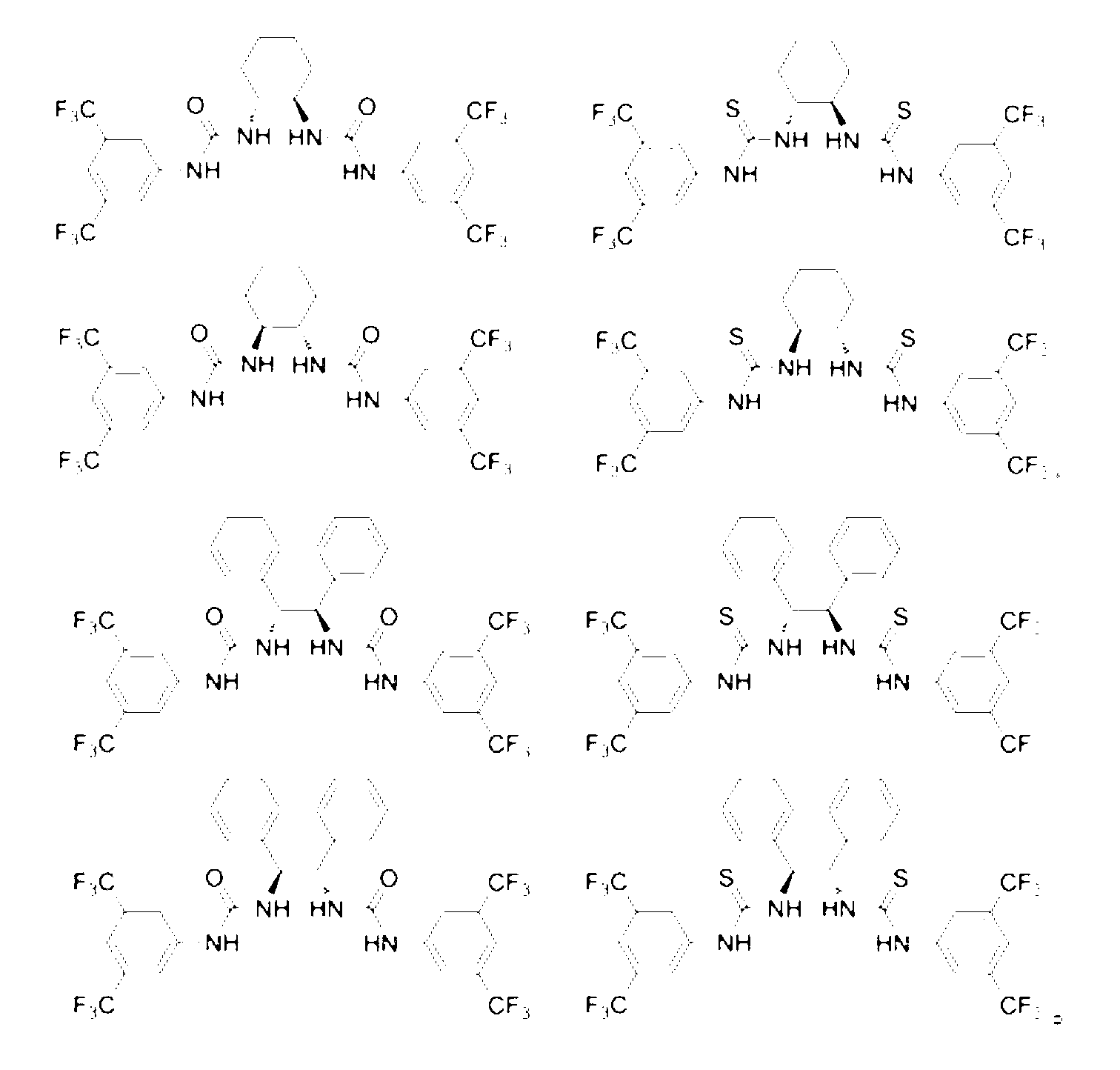Method for measuring optical purity of chiral carboxylic acid
A measurement method and optical purity technology, which is applied in the field of rapid determination of the optical purity of chiral carboxylic acids, can solve problems such as difficult synthesis, narrow application range, and inability to achieve baseline separation, and achieve good repeatability and wide application range.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
example 1
[0023] Example 1 Determination of Optical Purity of Mandelic Acid Mixed Configuration Sample
[0024] Take racemic mandelic acid, optically pure R-mandelic acid, mandelic acid sample to be tested, N,N-dimethyl-4-aminopyridine, chiral shift reagent (S,S)-1,2-di[( 3,5-bistrifluoromethylphenyl)thioureido]-1,2-diphenylethane were prepared into 15 mMol / L deuterated chloroform solution for later use. First, take 0.2 mL of the prepared racemic mandelic acid deuterated chloroform solution and add 0.4 mL of deuterated chloroform, mix well, put it into a 400 MHz nuclear magnetic resonance instrument and record at room temperature 1 H NMR signal, in (δ H = 0) as the internal standard to obtain the chemical shift of mandelic acid α-H as δ= 5.26; each take 0.2 mL of prepared racemic mandelic acid and N, N-dimethyl-4-aminopyridine, chiral shift reagent After adding the same NMR tube and mixing, put it into a 400 MHz NMR instrument and record at room temperature 1 H NMR signal, in (δ H ...
example 2
[0026] Example 2 Determination of Optical Purity of 2-Phenylpropionic Acid Mixed Configuration Sample
[0027] Take racemic 2-phenylpropionic acid, optically pure R-2-phenylpropionic acid, 2-phenylpropionic acid sample to be tested, N,N-dimethyl-4-aminopyridine, chiral shift reagent ( S, S)-1,2-bis[(3,5-bistrifluoromethylphenyl)thioureido]-1,2-diphenylethane were prepared in 15 mMol / L deuterated chloroform solution spare. First, take 0.2 mL of the prepared racemic 2-phenylpropionic acid deuterated chloroform solution and add 0.4 mL of deuterated chloroform, mix well, put it into a 400 MHz nuclear magnetic resonance instrument and record at room temperature 1 H NMR signal, in (δ H = 0) as internal standard to obtain 2-phenylpropanoic acid CH 3 The chemical shift of -H is δ= 1.53; take 0.2 mL of prepared racemic 2-phenylpropionic acid and N, N-dimethyl-4-aminopyridine, and chiral shift reagent into the same NMR tube, mix and release recorded in a 400 MHz NMR instrument at r...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com