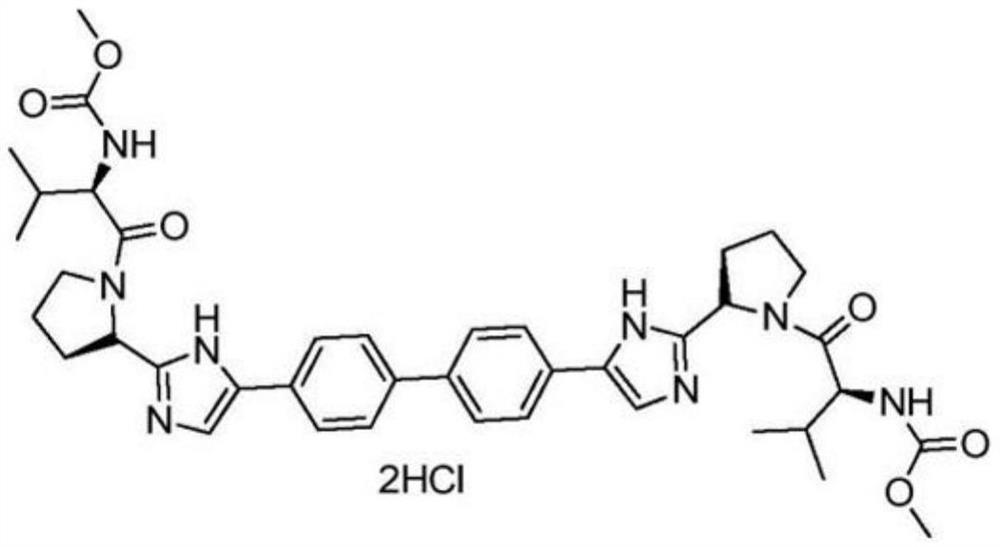
A kind of synthetic method of daclatasvir starting material
A daclatasvir and synthetic method technology, applied in the field of industrial synthesis of intermediate raw materials, can solve the problems of harsh reaction conditions, complex process routes, unfavorable industrialization, etc., and achieve less by-products, simple process, and easy operation Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
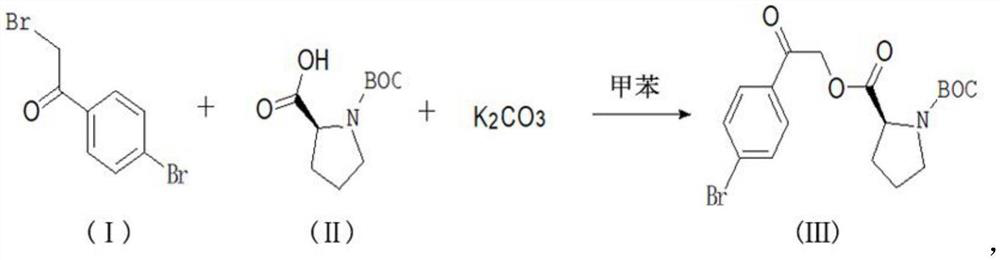
[0021] The synthesis method of the daclatasvir starting material of this embodiment comprises the following steps:
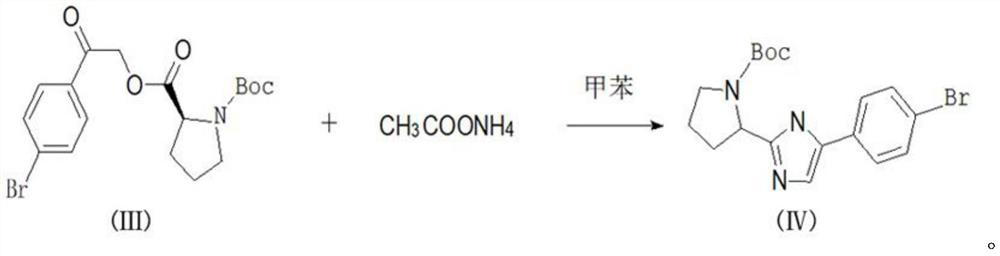
[0022] Add 27.8g of 2,4'-dibromoacetophenone, 21.6g of L-BOC-proline, 27.6g of potassium carbonate, and 300g of toluene into a 500mL reaction flask, stir mechanically, raise the temperature in a water bath, and control the temperature React at 28°C for 8 hours, take a sample for detection, after the reaction is over, add water to quench, stir for 20 minutes, stand to separate layers, collect the upper toluene layer, discard the lower water layer, add 1L of pure water to the toluene layer again, and stir After 20 minutes, the layers were left to stand, and the upper toluene layer was collected. The temperature of the toluene layer was raised to reflux, and the reflux was kept for 60 minutes after water separation. The reflux was stopped, and the reaction solution was collected for the next reaction.
[0023] Ammonium acetate was added to the above reaction soluti...
Embodiment 2
[0028] The synthesis method of the daclatasvir starting material of this embodiment comprises the following steps:
[0029] Add 27.8g of 2,4'-dibromoacetophenone, 22g of L-BOC-proline, 28g of potassium carbonate, and 280g of toluene into a 500mL reaction flask, stir mechanically, raise the temperature in a water bath, and control the temperature at 30 ℃, react for 7 hours, take a sample for detection, after the reaction is completed, add water to quench, stir for 18 minutes, let stand to separate layers, collect the upper toluene layer, discard the lower water layer, add 1L of pure water to the toluene layer again, and stir for 18 minutes Finally, let stand to separate layers, collect the upper toluene layer, heat up the toluene layer to reflux, keep the reflux and divide water for 45 minutes, then stop the reflux, and collect the reaction solution to be used in the next reaction.
[0030] Ammonium acetate was added to the above reaction solution, heated to reflux and stirred ...
Embodiment 3
[0032] The synthesis method of the daclatasvir starting material of this embodiment comprises the following steps:
[0033] Add 27.8g of 2,4'-dibromoacetophenone, 21.6g of L-BOC-proline, 27.6g of potassium carbonate, and 350g of toluene into a 500mL reaction flask, stir mechanically, raise the temperature in a water bath, and control the temperature React at 25°C for 9 hours, take a sample for testing, after the reaction is over, add water to quench, stir for 15 minutes, let stand to separate layers, collect the upper toluene layer, discard the lower water layer, add 1L of pure water to the toluene layer again, stir After 15 minutes, the layers were left to stand, and the upper toluene layer was collected. The temperature of the toluene layer was raised to reflux, and the reflux was maintained for 30 minutes after water separation. The reflux was stopped, and the reaction solution was collected for the next reaction.
[0034] Ammonium acetate was added to the above reaction so...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com