Daclatasvir film coating tablet preparation and preparation method thereof
A film coating, daclatasvir technology, applied in the directions of pill delivery, antiviral agents, sugar-coated pills, etc., can solve the problems of high cost, large side effects, complex preparation process, etc., to break the price monopoly, small individual differences, simple craftsmanship
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
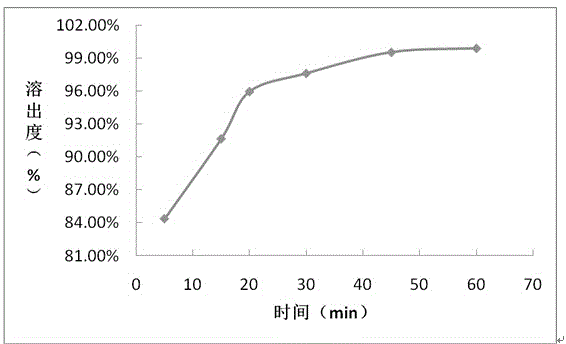
Embodiment 1
[0044] prescription:
[0045] Composition Prescription amount (mg / tablet)
[0046] Daclatasvir 60
[0047] Croscarmellose Sodium 21
[0048] Microcrystalline cellulose 273
[0050] According to above-mentioned prescription quantity, preparation process is as follows:
[0051] (1) Material pretreatment: sieve daclatasvir, croscarmellose sodium, microcrystalline cellulose, and magnesium stearate for later use;
[0052] (2) Mixing: Weigh the active drug daclatasvir, croscarmellose sodium, microcrystalline cellulose and magnesium stearate in the prescribed amount and mix them evenly;
[0053] (3) Place the mixed powder in (2) in a dry granulator for granulation;
[0054] (4), total mixing: uniformly mix the dry granules prepared in (3) with the prescribed amount of croscarmellose sodium and magnesium stearate;
[0055] (5) Press plain tablets: place the granules prepared in (4) in a high-speed rotary tablet press;
[0056] (6) ...
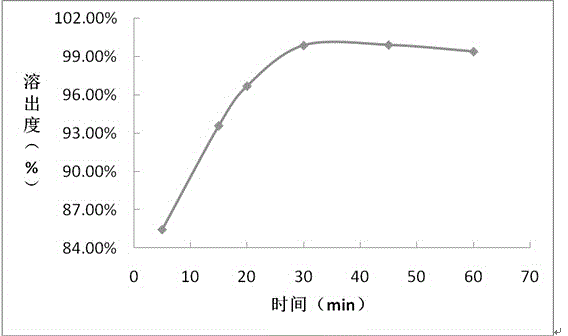
Embodiment 2
[0058] prescription:
[0059] Composition Prescription amount (mg / tablet)
[0060] Daclatasvir 60
[0061] Hypromellose 21
[0062] Microcrystalline Cellulose 270
[0064] According to above-mentioned prescription quantity, preparation process is as follows:
[0065] (1) Material pretreatment: sieve daclatasvir, hypromellose, microcrystalline cellulose, and magnesium stearate for later use;
[0066] (2) Mixing: Weigh the active drug daclatasvir, hypromellose, microcrystalline cellulose and magnesium stearate in the prescribed amount and mix them evenly;
[0067] (3) Place the mixed powder in (2) in a dry granulator for granulation;
[0068] (4), total mixing: uniformly mix the dry granules prepared in (3) with the prescribed amount of hypromellose and magnesium stearate;
[0069] (5) Press plain tablets: place the granules prepared in (4) in a high-speed rotary tablet press;
[0070] (6) Film coating: film-coat the qualified ...
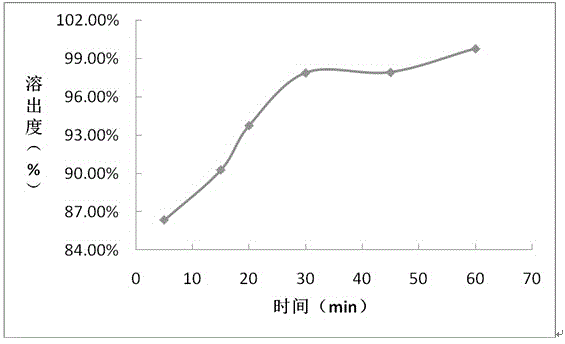
Embodiment 3
[0072] prescription:
[0073] Composition Prescription amount (mg / tablet)
[0074] Daclatasvir 60
[0075] Croscarmellose Sodium 21
[0078] According to above-mentioned prescription quantity, preparation process is as follows:
[0079] (1) Material pretreatment: sieve daclatasvir, croscarmellose sodium, anhydrous lactose, and magnesium stearate for later use;
[0080] (2) Mixing: Weigh the prescription amount of the active drug daclatasvir, croscarmellose sodium, anhydrous lactose, and magnesium stearate and mix evenly;
[0081] (3) Place the mixed powder in (2) in a dry granulator for granulation;
[0082] (4), total mixing: uniformly mix the dry granules prepared in (3) with the prescribed amount of croscarmellose sodium and magnesium stearate;
[0083] (5) Press plain tablets: place the granules prepared in (4) in a high-speed rotary tablet press;
[0084] (6) Film coating: film-coat the quali...
PUM
| Property | Measurement | Unit |
|---|---|---|
| particle diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com