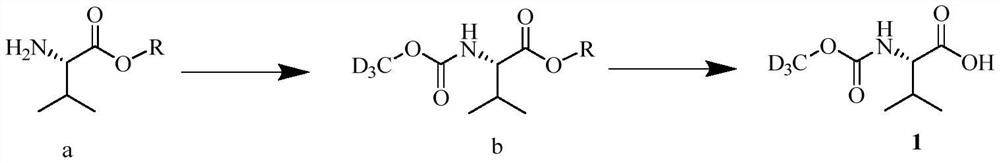
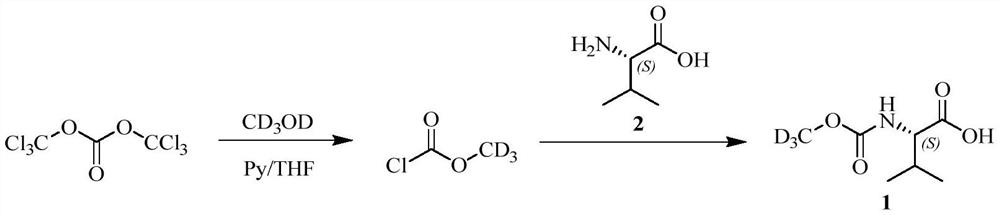
Preparation method of deuterated daclatasvir intermediate
A technology of compound and carboxyl protecting group, which is applied in the field of drug synthesis and can solve problems such as large environmental pollution
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0060]
[0061] Synthesis of N-tert-butoxycarbonyl-L-valine p-bromobenzyl ester (4):
[0062] Dissolve N-tert-butoxycarbonyl-L-valine (3) (21.73g, 0.1mol) in NMP (150mL), add p-bromobenzyl bromide (27.49g, 0.11mol), Cs 2 CO 3 (19.55g, 0.06mol), stirred, reacted at 25°C for 3h, TLC showed that the reaction was complete, added 0.8L ice water to the reaction solution, extracted with EtOAc (200mL×3), washed the organic phase with 500mL saturated NaCl aqueous solution, anhydrous sodium sulfate Dry, filter, and spin dry the solvent under reduced pressure to obtain 44.40 g of crude compound 4, which can be directly put into the next step reaction. It can also be separated and purified by silica gel column chromatography (PE:EtOAc 50: 1~20:1) to obtain light yellow syrup (4) with a yield of 99%.
[0063] 1 H NMR (500MHz, CDCl 3 ), δ: 0.87 (d, J=7.0Hz, 3H, CH 3 ),0.96(d,J=6.5Hz,3H,CH 3 ),1.46(s,9H, (CH 3 ) 3 C),2.15(m,1H,CH(CH 3 ) 2 ), 4.27 (dd, J = 4.5, 9.0Hz, 1H, NHCH), ...
Embodiment 2
[0088] Compound 5' and CD of different molar ratios of embodiment 2 3 OD prepared compound 6'
[0089] Taking NO.2 as an example, the experimental operation is as follows: CDI (3.73g, 0.023mol), CD 3 OD (0.83g, 0.023mol) and KOH (44.9 mg, 0.8mmol) were added to toluene (25mL), heated to 70°C, and after 3h of reaction, L-valine methyl ester (5') (1.31g , 0.01mol), the reaction was continued at 70°C for 20h. The peak area ratio of the product detected by HPLC was 90%.
[0090] The experimental operation of NO.1 and NO.3-4 is the same as that of NO.2, only compound 5' and CD 3 The molar ratio of OD is different, and the specific ratio is shown in the table below.
[0091]
Embodiment 3
[0092] Embodiment 3 prepares compound 6' under different alkaline conditions
[0093] CDI (3.73g, 0.023mol), CD 3 OD (0.83g, 0.023mol) and NaOH (32.0mg, 0.8mmol) were added to toluene (25mL), heated to 70°C, and after 3h of reaction, L-valine methyl ester (5') (1.31g , 0.01mol), the reaction was continued at 70°C for 20h. The peak area ratio of the product detected by HPLC was 85%.
[0094]
[0095] The HPLC detection condition of embodiment 2-3 is: chromatographic column is Waters Sunfire C18, 250mm * 4.6mm, 5 μm; With 0.01% trifluoroacetic acid solution as mobile phase A, acetonitrile is mobile phase B, flow rate is 1.0ml per minute, Carry out linear gradient elution according to the table below; column temperature is 35°C; detection wavelength is 200nm. Calculated by peak area normalization method.
[0096]
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com