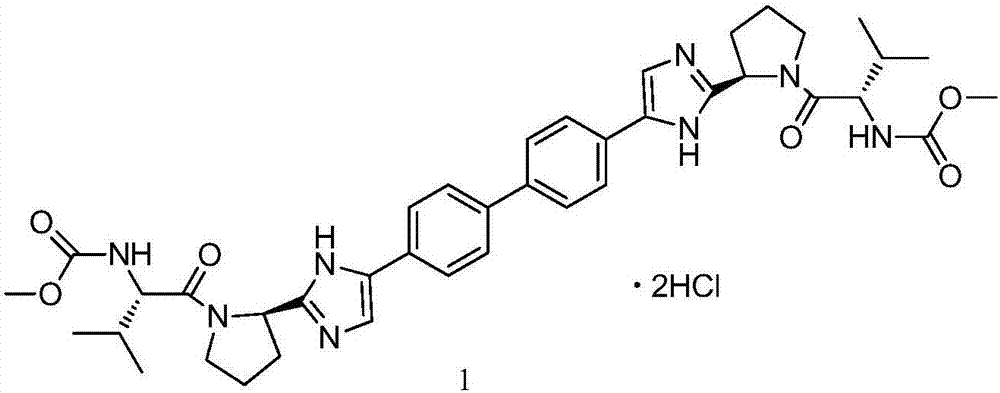
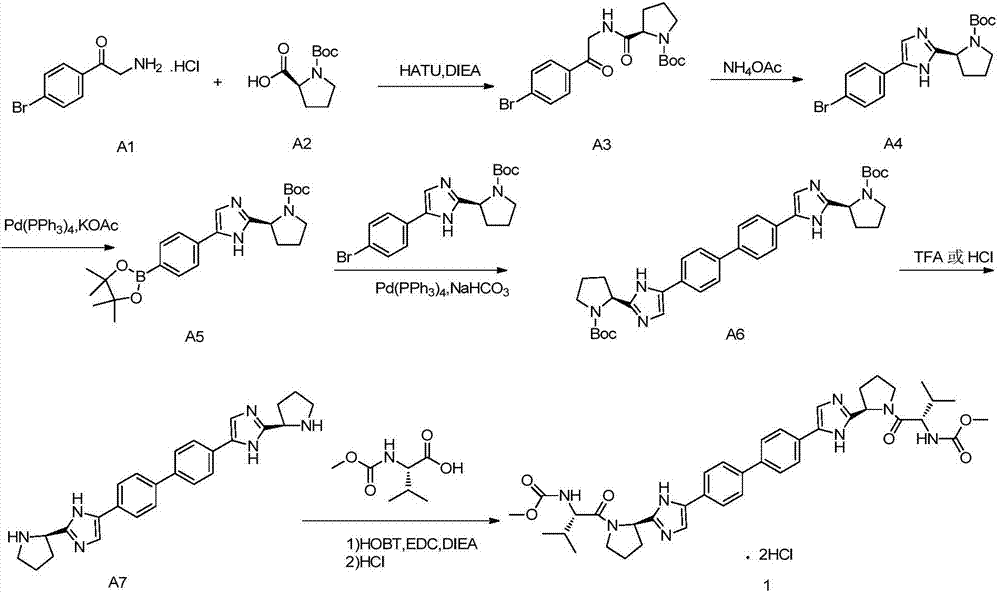
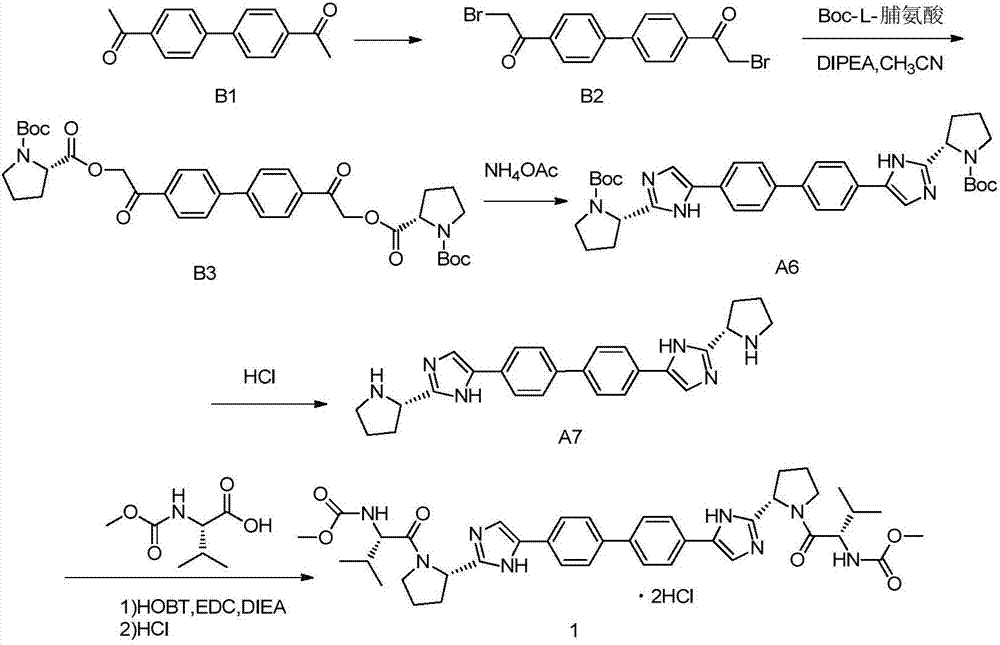
Preparation method for daclatasvir
A technology for daclatasvir and a compound is applied in the field of preparation of daclatasvir, and can solve the problems of cumbersome steps, low economy, low production efficiency and the like
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0077] Take L-proline (2g, 0.017mol) in a single-necked bottle, add 20mL of methanol, control the temperature below 0°C, and slowly add SOCl 2 (2.48g, 0.021mol), gradually returned to room temperature and reacted overnight, and TLC detected that the reaction was complete. Concentrate under reduced pressure to obtain an oily substance, which is compound D1, which is directly used for the next reaction.
Embodiment 2
[0079] Take Moc-L-valine (3.04g, 17.4mmol), EDC.HCl (4.01g, 20.9mmol), HOBt (3.19g, 20.9mmol) and acetonitrile (30ml) in a reaction flask, and stir for 1h. Compound D1 prepared in Example 1 was added, and triethylamine (4.39 g, 43.4 mmol) was slowly added dropwise under ice-cooling, and reacted for 18 h, and the reaction was complete as detected by TLC. Add brine (3ml), stir at 50°C for 5h, cool to room temperature, extract with EA and water, and the organic layer is sequentially washed with dilute NaHCO 3 Solution, water and dilute brine were washed, dried over anhydrous sodium sulfate, concentrated under reduced pressure, and purified by column to obtain 4.803 g of compound D3, with a two-step yield of 96.5%.
Embodiment 3
[0081] Get 4.8g of compound D3 prepared in Example 2 in a 500mL eggplant-shaped bottle, add 50mL THF, dissolve the product in 5mL water, add 1.27g LiOH·H 2 O. The reaction was carried out at room temperature overnight, and TLC detected that the reaction was complete. Extracted with EA and water, the organic layer was washed successively with dilute HCl solution, water and dilute brine, dried over anhydrous sodium sulfate, and concentrated under reduced pressure to obtain compound D8, which was put into the next step reaction.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com