Synthetic method of daclatasvir
A synthesis method and technology of daclatasvir, which can be applied in the field of medicine and chemical industry, can solve the problems of high price and the like
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
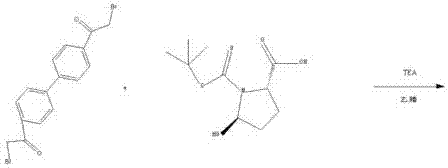
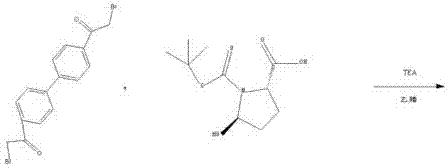
[0025] A preparation method of daclatasvir. Including the following steps:
[0026] 1-1 Synthesis of intermediate N-4: Add 600g of acetonitrile, 70g of bis(2-bromoacetyl)biphenyl, 23.5g of tert-butoxycarbonyl-L-proline, and 15.8g of triethylamine into a 1000ml three-neck flask, Stir the reaction at room temperature for 5 h, filter and evaporate the filtrate to dryness at 65°C to obtain intermediate N-4.
[0027] 1-2 Synthesis of intermediate N-3: Add 600 g of toluene and 32 g of ammonium acetate to 1-1, raise the temperature to 55° C. and stir for 18 hours, and a solid precipitates out. The solid obtained by filtration was dried at 70°C until the water content was less than 1.0%, to obtain intermediate N-3.
[0028] 1-3 Synthesis of intermediate N-2: Take the dried solid N-3, add 200g of toluene, 400g of methanol and 75g of 31% hydrochloric acid, heat up to 50°C for hydrolysis reaction for 10h, cool down to 20°C for 3h to crystallize, and filter to obtain The solid was vacu...
Embodiment 2
[0032] Example 1 was repeated, with the difference that 20 g of tert-butoxycarbonyl-L-proline was added in 1-1, and 102.3 g of daclatasvir was finally obtained, with a yield of 78.3%.
Embodiment 3
[0034] Repeat Example 1, the difference is that 30 g of MOC-L-valine was added in 1-4, and finally 100.7 g of daclatasvir was obtained, with a yield of 77.2%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com