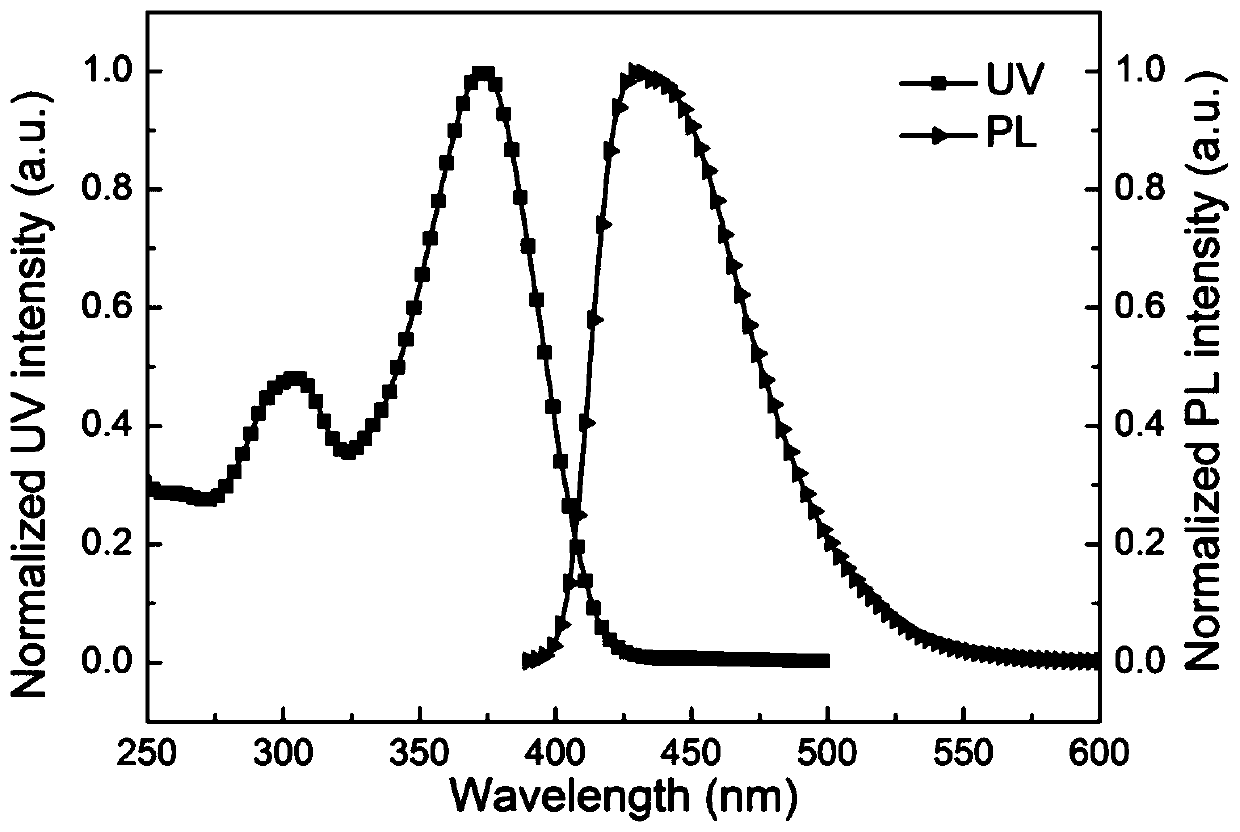
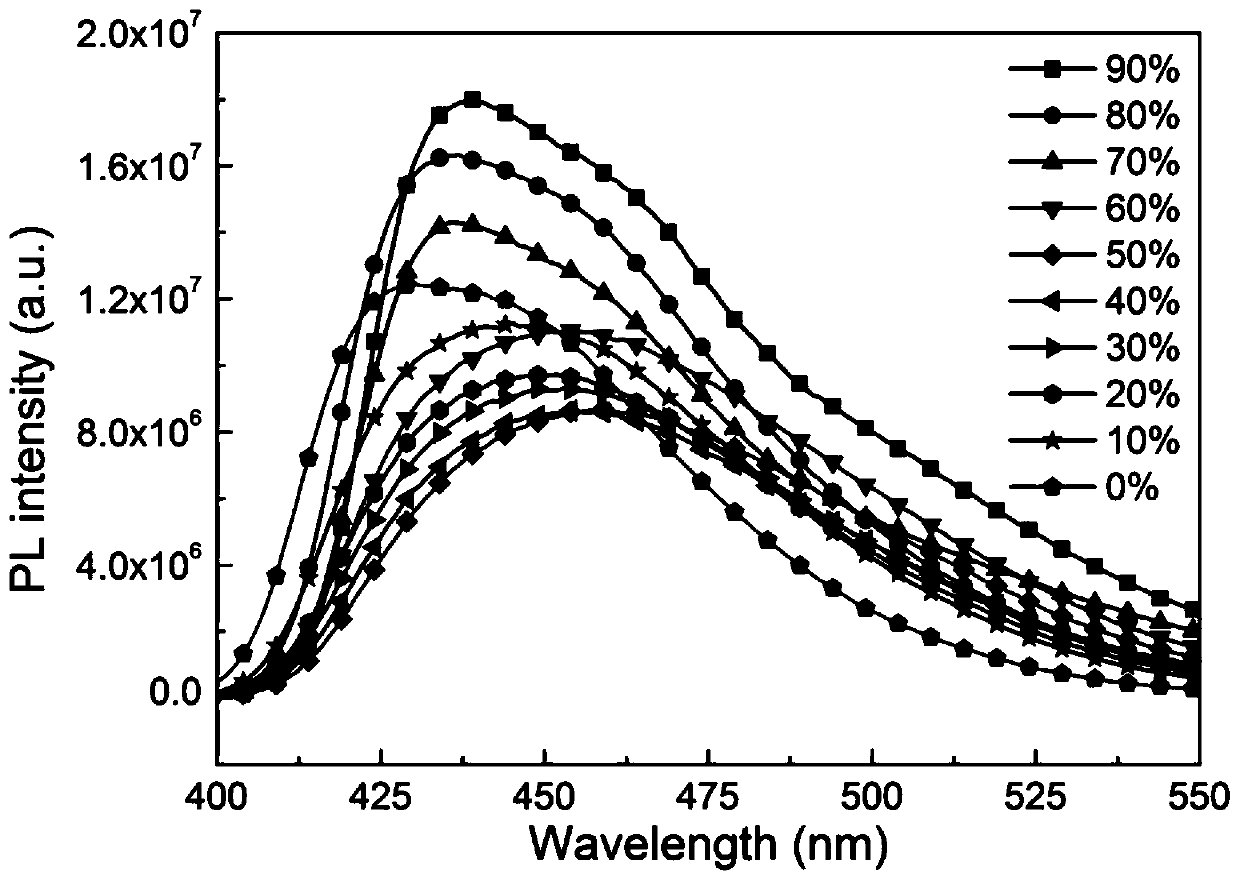
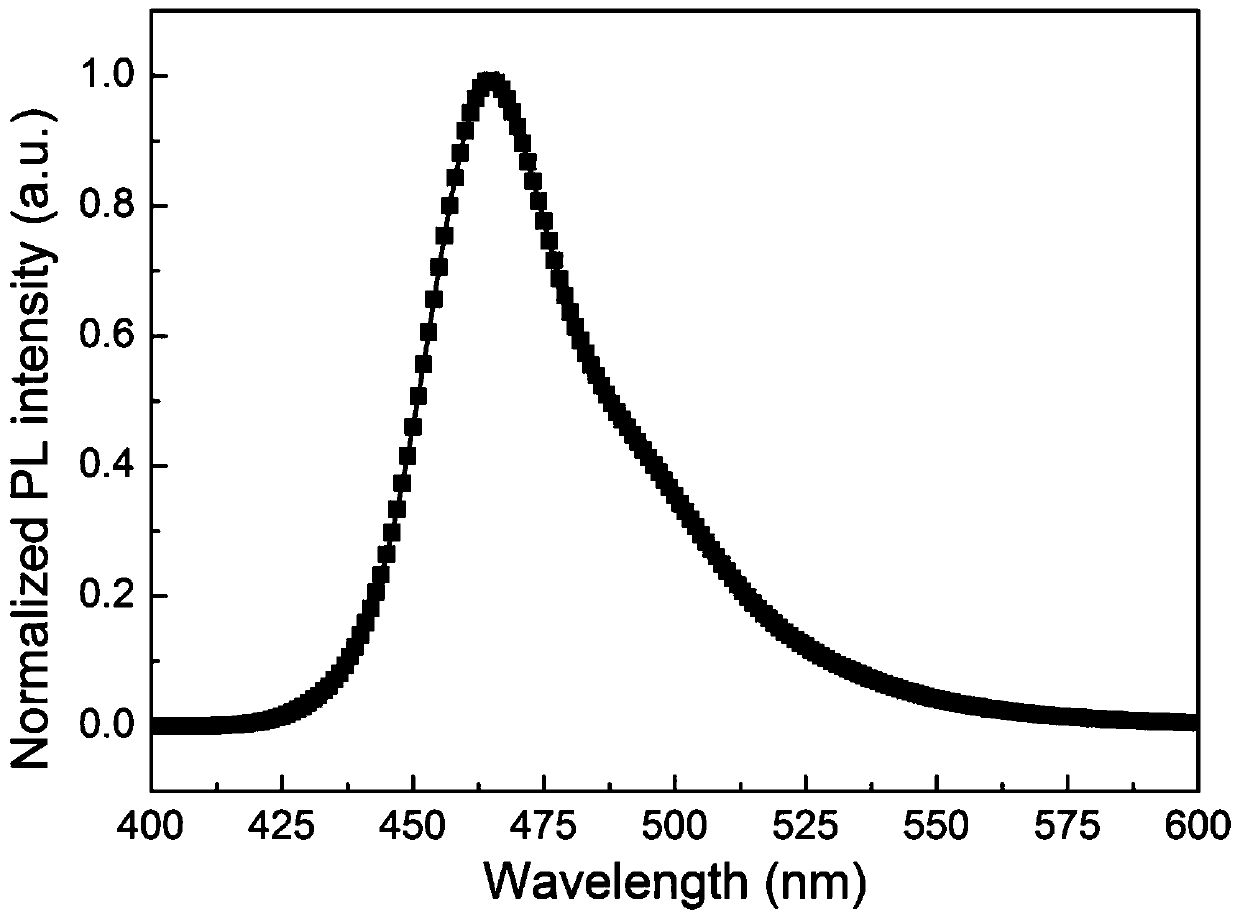
Organelle-targeted aggregation-induced emission material and preparation method thereof
A technology of aggregation-induced luminescence and organelles, which is applied in luminescence materials, chemical instruments and methods, and material analysis through optical means, can solve the problems of poor luminescence performance in the aggregated state, achieve good specific imaging performance, low preparation cost, The effect of simple synthesis and purification process
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0033] The organelle-targeted aggregation-induced luminescent material of this embodiment has the following structural formula (I):
[0034]
[0035] The preparation method of the aggregation-induced luminescent material of this embodiment includes the following steps:
[0036] Step 1: Using 4-boronate triphenylamine and 2,5-dibromopyridine as raw materials, fully dissolved in toluene solution, adding potassium carbonate solution and ethanol solvent, and using tetrakistriphenylphosphine palladium as the reaction catalyst, After reacting for 20 hours under nitrogen protection at 90°C, the target compound (I) was obtained after quenching with water, extraction with organic solution, washing, solvent removal and column chromatography purification.
[0037] The molar ratio between the 4-boronate triphenylamine and 2,5-dibromopyridine is 2.5:1, the molar ratio of potassium carbonate to 2,5-dibromopyridine is 6:1, tetratriphenyl The molar ratio of phosphine palladium to 2,5-dibr...
Embodiment 2
[0044] The organelle-targeted aggregation-induced luminescent material of this embodiment has the following structural formula (II):
[0045]
[0046] The preparation method of the aggregation-induced luminescent material of this embodiment includes the following steps:
[0047] Step 1: Using 4-boronate-4',4'-dimethoxytriphenylamine and 4,7-dibromo-2,1,3-benzothiadiazole as raw materials, fully dissolve in toluene solution Afterwards, potassium carbonate solution and ethanol solvent were added, and tetrakistriphenylphosphine palladium was used as the reaction catalyst. After reacting for 24 hours under nitrogen protection conditions at 85°C, it was quenched by adding water, extracted with organic solution, washed, removed solvent and column chromatography After purification, the target compound (II) is obtained.
[0048] The molar ratio between the 4-boronate-4',4'-dimethoxytriphenylamine and 4,7-dibromo-2,1,3-benzothiadiazole is 2.5:1, The molar ratio of potassium carbon...
Embodiment 3
[0055] The organelle-targeted aggregation-induced luminescent material of this embodiment has the following structural formula (III):
[0056]
[0057] The preparation method of the aggregation-induced luminescent material of this embodiment includes the following steps:
[0058] Using the product of Example 1 and methyl iodide as raw materials, add toluene to fully dissolve it, and heat at 88°C for 18 hours. After removing the toluene solvent, sodium hexafluorophosphate was added and fully dissolved in acetonitrile. After stirring at room temperature for 18 hours, washing with water, removing the solvent under reduced pressure and recrystallization, the target fluorescent product (III) was obtained.
[0059] The molar ratio between the product in Example 1 and methyl iodide is 1:2.5; the molar ratio between the product in Example 1 and sodium hexafluorophosphate is 1:10.
[0060] The reaction formula is:
[0061]
[0062] The specific operation is: add the product of...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com