A class of camphor-based imidazole type ionic liquid and its preparation method and application
An ionic liquid and imidazole-based technology, which is used in the application of catalyzed aldehyde-alcohol oxidative esterification, camphor-based imidazole-based ionic liquid and its preparation field, can solve the problem of tolerance of low-efficiency functional groups, severe reaction conditions, and atom economy Low-level problems, to achieve the effect of good practicability, high catalytic efficiency, safe and easy operation
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0032] The preparation of embodiment 1 camphor-based imidazole type ionic liquid catalyst
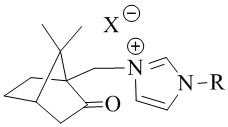
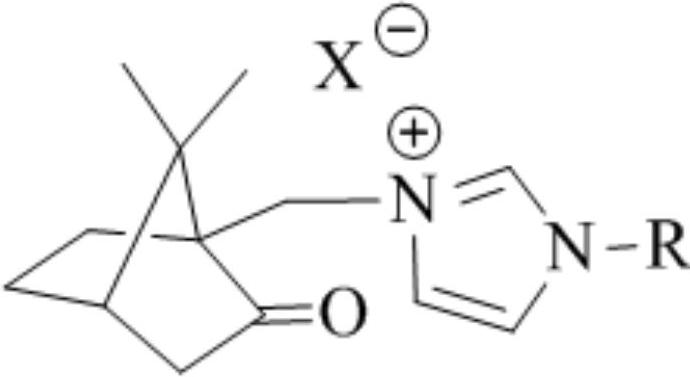
[0033] 1) The structural formula of the camphor-based imidazole type ionic liquid catalyst is:
[0034]
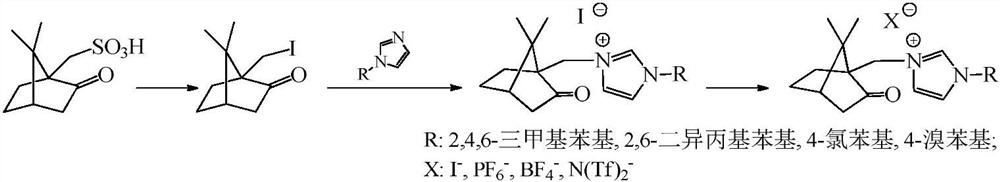
[0035] The preparation process is: 3-(7,7-dimethyl-2-oxobicyclo[2.2.1]hept-1-yl)methyl-1-(2,4,6-trimethyl)phenyl- Preparation of 1H-imidazole-3-iodide salt: 18.6g 1-(2,4,6-trimethyl)phenyl-1H-imidazole, 27.8g 10-iodocamphor and 50mLN,N-dimethylformazol Amides were sequentially added to a three-necked flask equipped with a stirrer, a thermometer, and a condensing reflux device, and reacted at 130°C for about 4 hours until one of the raw materials was completely reacted. After cooling, 50 mL of ethyl acetate was added to precipitate the product, and the camphor was obtained by filtration. The crude product of imidazolium iodide salt was recrystallized from ethanol to obtain the target compound. Product characterization data are as follows: yield 81%, 1 H NMR (400MHz, CDCl 3 )δ:...
Embodiment 2
[0060] Example 2 Using the camphor-based imidazole type ionic liquid catalyst prepared in Example 1 to catalyze aldehyde-alcohol oxidative esterification
[0061] 1) Preparation of ethyl benzoate: Accurately weigh 0.1mol benzaldehyde, add it to a 500mL round bottom flask, then add 0.5mol ethanol, 0.005mol 3-(7,7-dimethyl-2-oxobicyclo[ 2.2.1] Hept-1-yl)methyl-1-(2,4,6trimethyl)phenyl-1H-imidazole-3-iodide salt, 0.05mol of cesium carbonate, 150mL of toluene, covered with air Balloon, stirred at 60°C for 3h. After the reaction was completed, it was cooled to room temperature, washed with saturated brine until neutral, concentrated under reduced pressure, and purified by column chromatography to obtain ethyl benzoate with a yield of 98%. Product characterization data are as follows: 1 H NMR (400MHz, CDCl 3 )δ: 8.12-7.96(m, 2H), 7.55-7.46(m, 1H), 7.40(t, J=7.7Hz, 2H), 4.36(q, J=7.1Hz, 2H), 1.37(t, J =7.1Hz, 3H). 13 C NMR (101MHz, CDCl 3 )δ: 166.5, 132.7, 130.5, 129.5, 128.2, ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com