Patents
Literature
72 results about "Sodium tetrafluoroborate" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
Sodium tetrafluoroborate is an inorganic compound with formula NaBF₄. It is a salt that forms colorless or white water-soluble rhombic crystals and is soluble in water (108 g/100 mL) but less soluble in organic solvents.
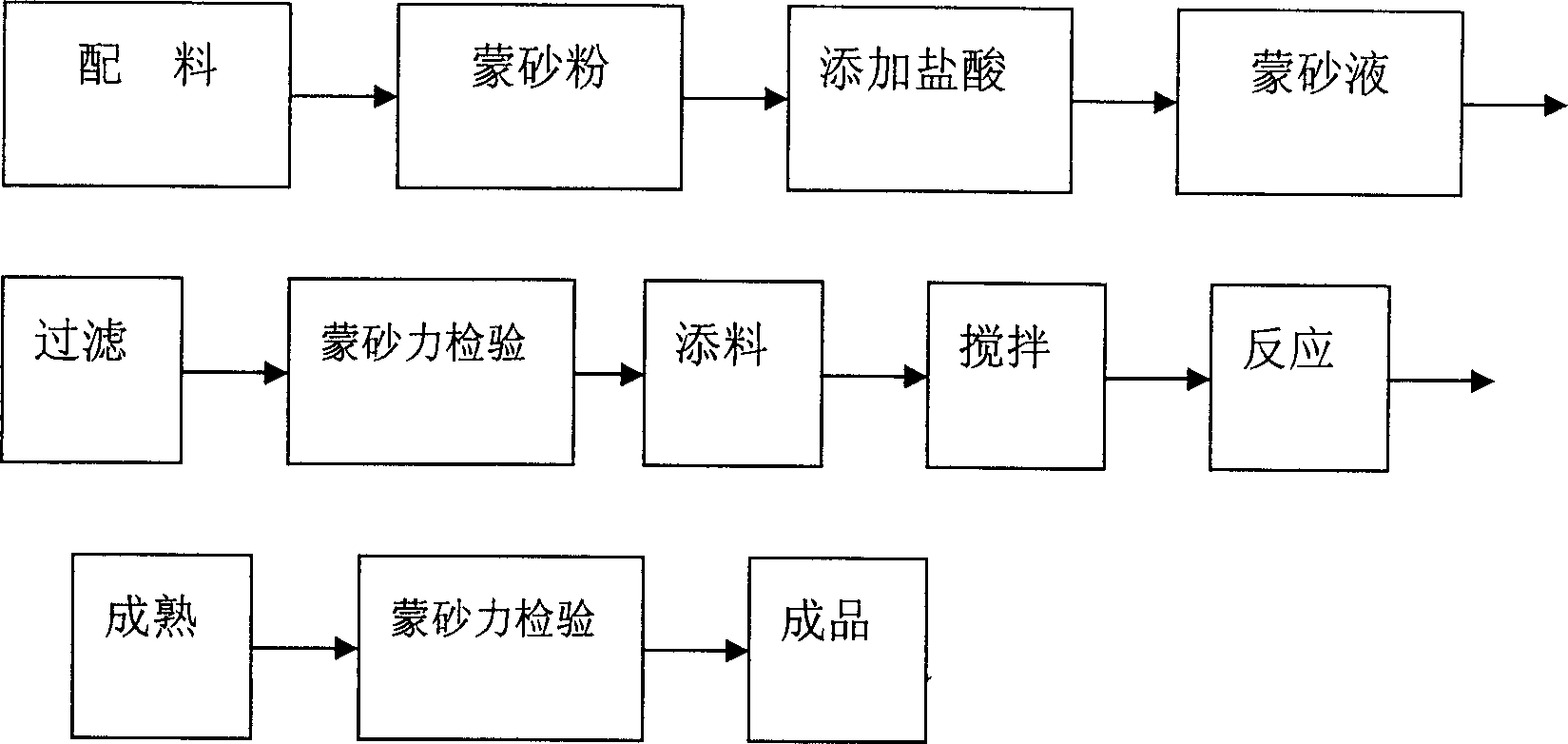
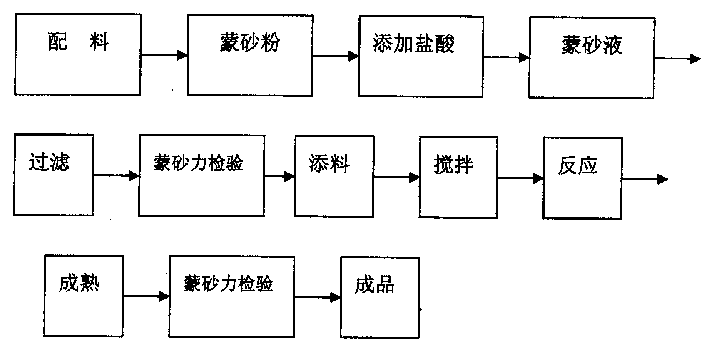
Sodium ion battery negative electrode sheet and sodium ion battery
InactiveCN104966813AEnhanced insertion siteIncrease profitCell electrodesSecondary cellsElectrolytic agentSodium tetrafluoroborate
The present invention discloses a sodium ion battery negative electrode sheet, the negative electrode sheet is a porous graphite film structure, the diameter of the pores is from 2 to 30 microns, the distance between the centers of the circles of the pores is 5 to 50 microns, mass ratio of carbon atoms in the porous graphite film is greater than 99%, the negative electrode sheet may be used directly as the sodium ion battery negative electrode sheet, can avoid the use of a conductive agent, a binder and a metal collector, and has high capacity, corrosion resistance and good conductivity. The present invention also discloses a sodium ion battery using the negative electrode sheet, the sodium ion battery comprises a positive electrode sheet, a negative electrode sheet, a separator and an electrolyte, the sodium ion battery electrolyte solvent is one or more than one of diethanol dimethyl ether, dimethyl ether tetraethanol, and tetrahydrofuran, the electrolyte is one of sodium perchlorate, sodium hexafluorophosphate, sodium tetrafluoroborate and sodium trifluoromethanesulfonate, and the sodium ion battery is simple in production process and good in charging and discharging cycle stability, and has good prospects in the new energy field.
Owner:QINGDAO INST OF BIOENERGY & BIOPROCESS TECH CHINESE ACADEMY OF SCI
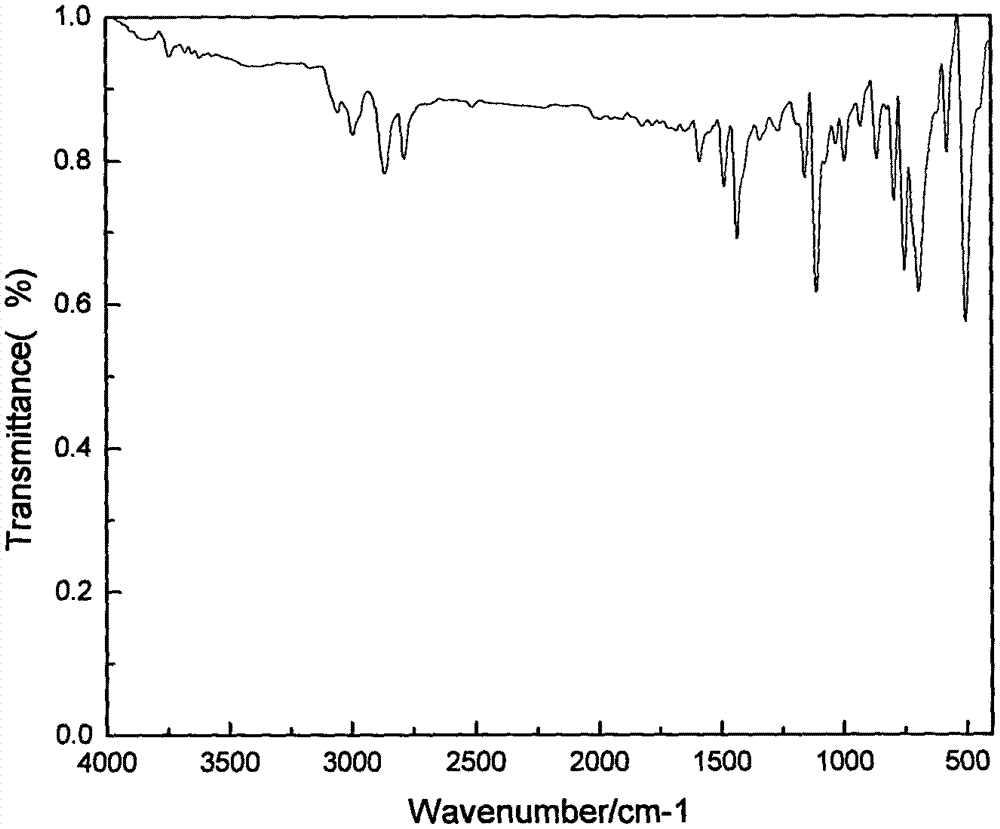
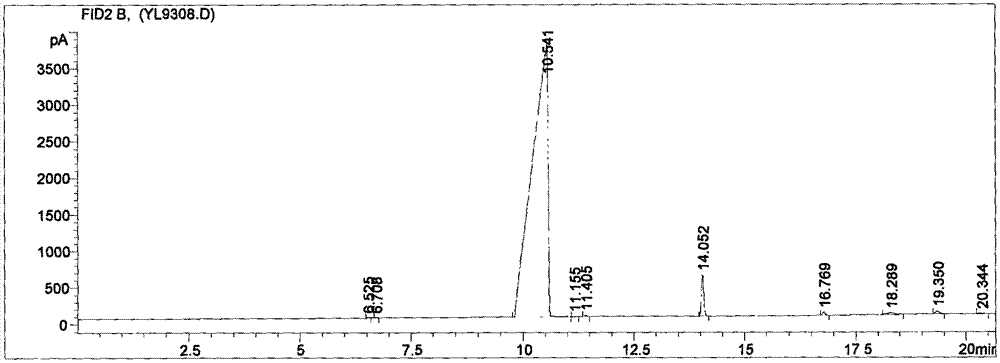
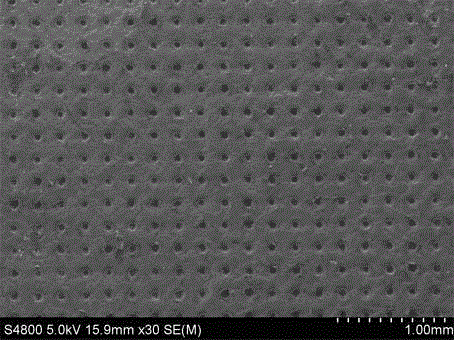
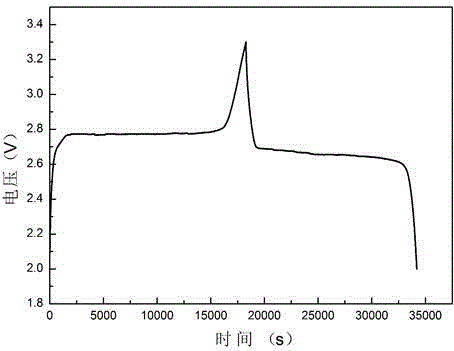
AM/AA/N-beta-CD polymer-ionic liquid [bquin]BF4 composite clay stabilizer and synthesis method thereof
ActiveCN103897121AHas inclusion functionHigh viscosityDrilling compositionSolubilitySodium Bentonite
The invention relates to a polymer-ionic liquid composite clay stabilizer used for restraining clay hydration expansion in petroleum industry and a synthesis method of the polymer-ionic liquid composite clay stabilizer. The synthesis method comprises the steps of: reacting single 6-allyl amino beta-cyclodextrin, acrylamide and acrylic acid under initiation of ammonium persulfate and sodium hydrogen sulfite in an aqueous solution under the condition of pH of 7 and the temperature of 35 DEG C, purifying by aqueous solution after 8 hours of reaction and drying to obtain a polymer; refluxing quinoline and n-butyl bromide for 48 hours at 60 DEG C to obtain brominated 1-normal-butyl-quinoline salt, stirring for 3 days with sodium fluoborate in acetonitrile, filtering, carrying out rotary evaporation and drying to obtain tetrafluoroborate 1-normal-butyl-quinoline salt ([bquin]BF4); refluxing the polymer and the [bquin]BF4 for 4 hours at 50 DEG C to obtain a polymer-ionic liquid compound. The polymer-ionic liquid compound has good water solubility, stability and clay expansion restraining power and the antiswelling rate to bentonite can reach 90.3%.
Owner:SOUTHWEST PETROLEUM UNIV
Organic promotive phosphating liquor as well as preparation method and use thereof
InactiveCN101457355AImprove protectionWide range of processingMetallic material coating processesSodium tetrafluoroboratePhosphoric acid
The invention provides an organic promoting phosphating solution, a preparation method thereof and the application thereof; wherein, the phosphating solution comprises basic solution and an accelerant which have the weight ratio of 1.5-2.5:1; the basic solution is the mixture of iron powders, phosphoric acid, zinc oxide, hydrofluoric acid, carbonate, fluosilicic acid and water; the accelerant is the mixture of sodium fluoborate, tartaric acid, sodium chlorate, caustic soda, trinitrobenzene sulfonic acid and water. The phosphating solution has wide phosphating process, can be used within the temperature of 5-65 DEG C, is suitable for the treatment before coating of automobiles, electric apparatuses, furniture and mechanical equipment, has simple production and use methods and meets the requirement of environmental protection.
Owner:DONGGUAN YINGMING CHEM ENG
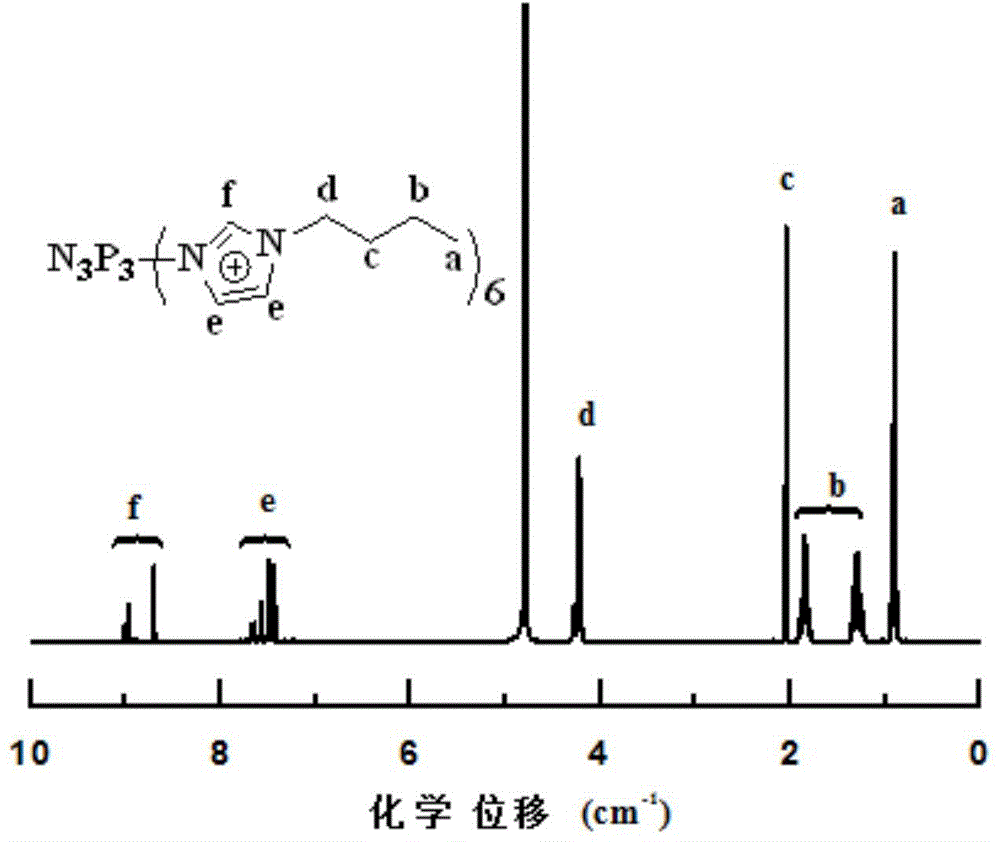
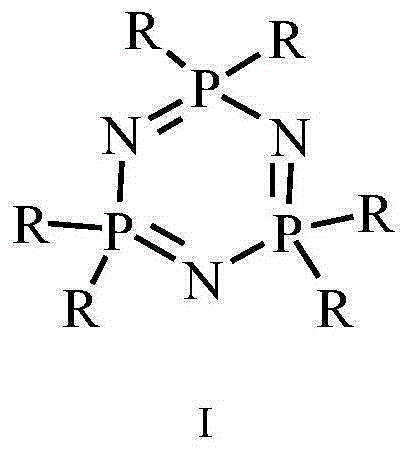
Hyperbranched ionic liquid based on HCCP and application of hyperbranched ionic liquid as fire retardant
ActiveCN104558046ASimple processLow equipment requirementsGroup 5/15 element organic compoundsPotassium hexafluorophosphateLithium
The invention provides a method for synthesizing a hyperbranched ionic liquid fire retardant based on HCCP. According to the method, HCCP reacts with trialkylamin, trialkyl phosphorus, and N-alkyl imidazole to realize ionization, salts such as sodium tetrafluoroborate, potassium hexafluorophosphate and bistrifluoromethanesulfonimide lithium, which contain different anions, are used for ion exchange, so that a hyperbranched ionic liquid fire retardant containing different anions is obtained, and synergistic flame retardance of HCCP and the ionic liquid is realized. The synthesized hyperbranched ionic liquid based on HCCP can be used as the fire retardant in various polymers.
Owner:ZHEJIANG UNIV OF TECH
Preparing process of Ru(II) polypyridine complex
InactiveCN1986555AGroup 8/9/10/18 element organic compoundsMicrobiological testing/measurementSodium tetrafluoroboratePhenanthroline
The preparation process of Ru(II)-polypyridine complex includes the following steps: preparing 1,10-phenanthroline-5,6-diquinone, pyridine [3, 2-a:2', 3'-c] phenazine as ligand I, and 7,8-dimethyl pyridine [3, 2-a: 2', 3'-c] phenazine as ligand II, Ru(bipy)2Cl2.2H2O (bipy=2, 2'-dipyridyl) as precursor I and Ru(phen)2Cl2.2H2O as precursor II; adding certain amount of Ru(bipy)2Cl2.2H2O / Ru(phen)2Cl2.2H2O, ligand I / ligand II, and mixture liquid of methanol and water, heating reflux for certain time, heating to concentrate, adding water and boiling, freezing, adding sodium tetrafluoborate, filtering and separating out precipitate, re-crystallization in alcohol, and stoving under infrared lamp to obtain the Ru(II)-polypyridine complex. The Ru(II)-polypyridine complex is used as nucleic acid recognizing probe for the analysis and detection of nucleic acid, and has high stability, high sensitivity, high selectivity and other advantages.
Owner:WUHAN UNIV
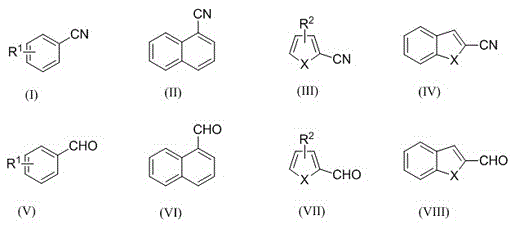
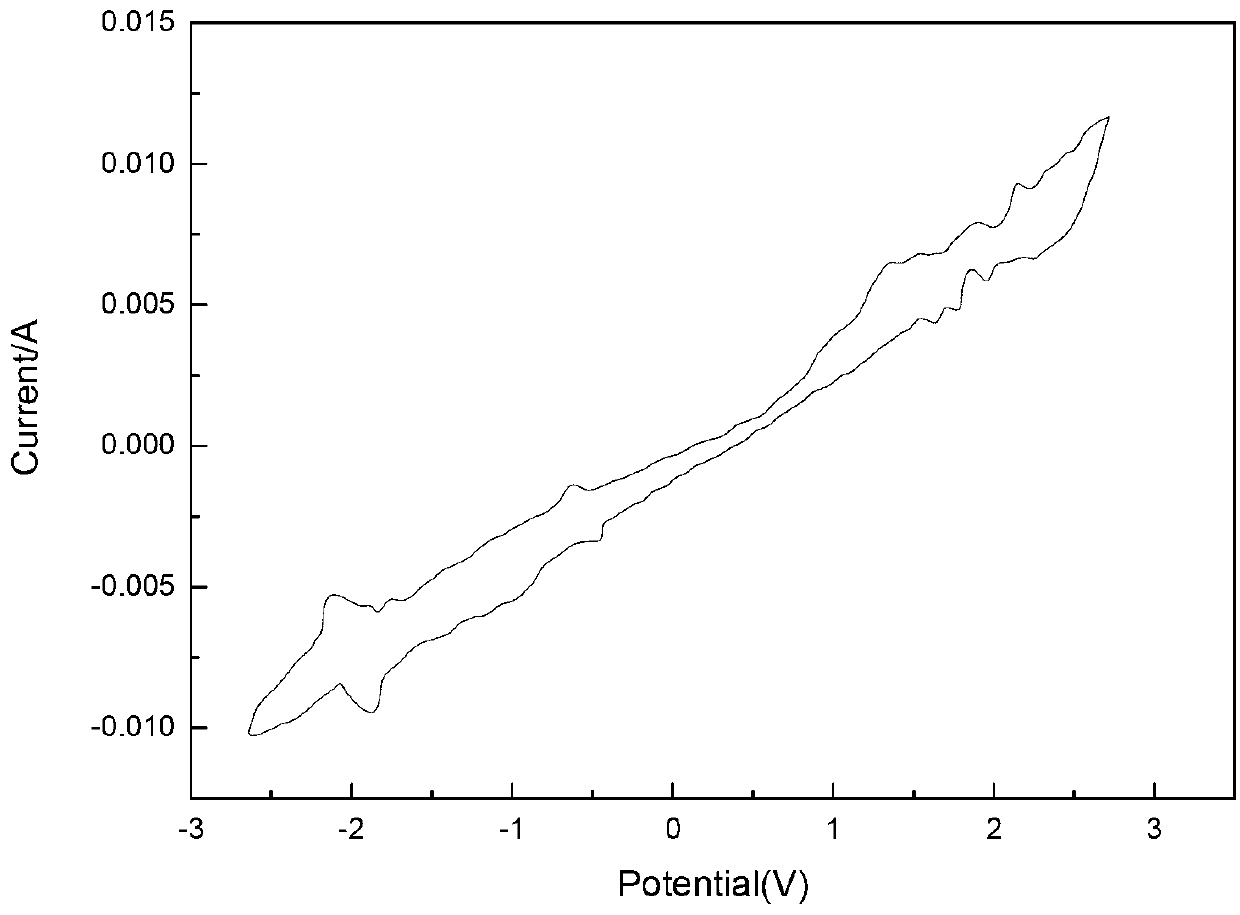
Electrochemical catalytic synthesis method of aromaticnitrile
ActiveCN105543886ASimple and safe operationMild reaction conditionsElectrolysis componentsElectrolytic organic productionElectrolysisSodium tetrafluoroborate
The invention discloses an electrochemical catalytic synthesis method of aromaticnitrile. Aromatic aldehyde is used as a raw material, HMDS (hexamethyldisilazane) is used as a nitrogen source, a three-electrode system is adopted, a negative electrode and a positive electrode adopt platinum electrodes, and a 0.1mol / l silver nitrate acetonitrile solution is used as a reference electrode; aromatic aldehyde, HMDS, TEMPO (2,2,6,6-tetramethyl-1-piperidine-N-oxyl free radicals) and acetic acid are added to an electrolyte acetonitrile solution with certain concentration, the mixture is stirred for an electrolytic reaction at the temperature of 5-40 DEG C and under the constant voltage of 0.5-8.0 V for 5-30 h, a reaction liquid is subjected to aftertreatment, and a product, namely, aromaticnitrile, is obtained; an electrolyte refers to sodium perchlorate, sodium periodate or sodium tetrafluoroborate. The method is simple and safe to operate and easy to implement.
Owner:SHANGHAI LINKCHEM TECH CO LTD
Extreme-pressure anti-wear lubricating agent
InactiveCN104450143ALow high temperature coefficient of frictionImprove the lubrication effectLubricant compositionCelluloseSodium tetrafluoroborate
The invention provides an extreme-pressure anti-wear lubricating agent, and relates to the technical field of high-temperature lubricating agents. The extreme-pressure anti-wear lubricating agent comprises components in weight ratio as follows: 0.1%-3% of a component A, 8%-35% of a component B, 2%-6% of a component C, 0.5%-5% of a component D, 0.5%-4% of a component E, 0.5%-4% of a component F, 0.8%-4% of a component G, 0.2%-1% of a component H and the balance of water, wherein the component A is one of or a mixture of sodium carboxymethylcellulose and xanthan gum; the component B is one of or a mixture of talc powder and bentonite; the component C adopts sodium silicate; the component D adopts one of or a mixture of two or three of sodium fluoborate, borax decahydrate and calcium metaborate; the component E adopts one of zinc stearate and lithium stearate; the component F adopts one of nano fullerene and alkaline sulfonate; the component G adopts sodium polyacrylate; and the component H adopts a water-based defoaming agent. The high-temperature friction coefficient value is very low, the lubricating property is excellent, and the lubricating requirement of current equipment under the high-temperature and high-load conditions can be well met.
Owner:NANHUA UNIV +1
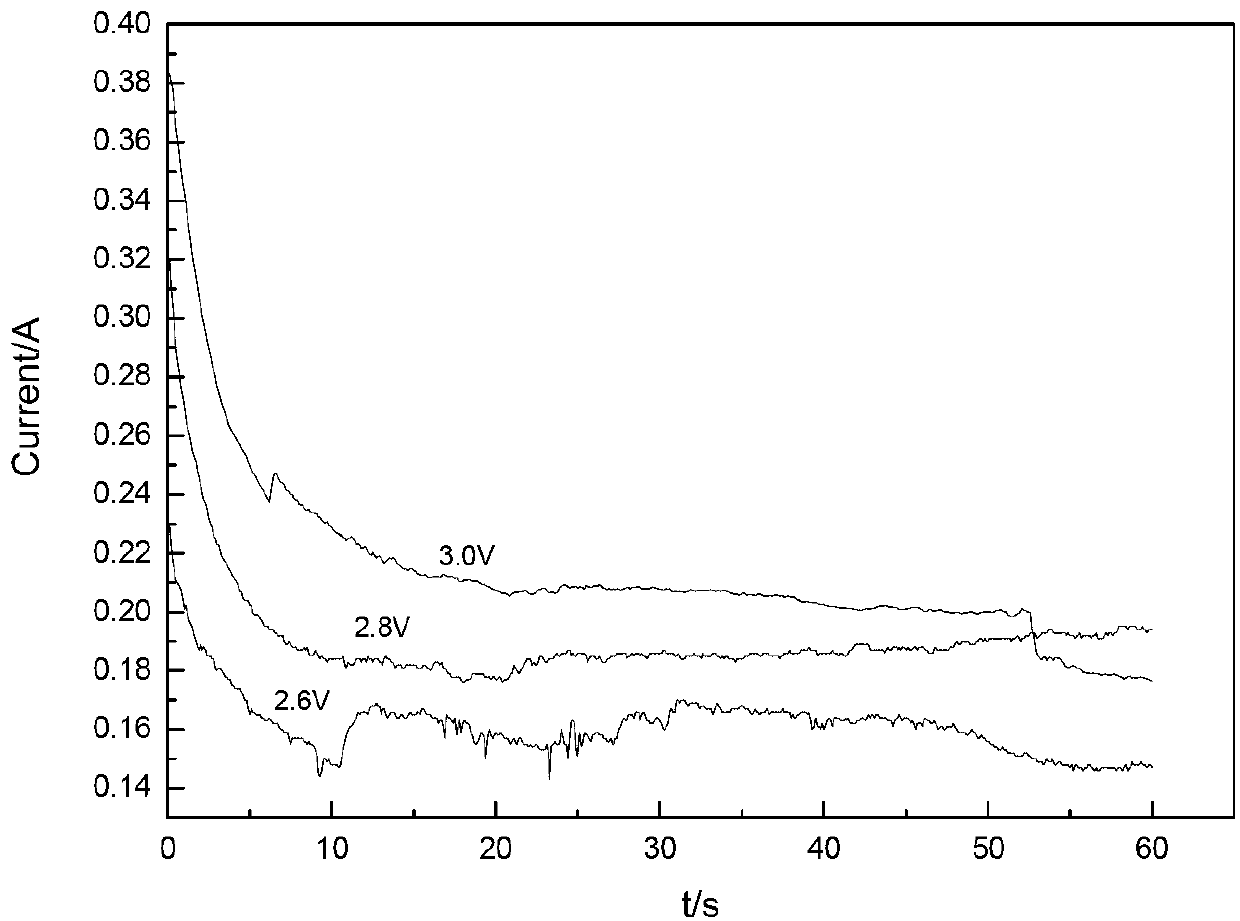
Method for preparing metal titanium by taking ionic liquid as electrolyte and performing electro-deoxidization at room temperature
The invention relates to a method for preparing metal titanium by electro-deoxidization and specifically relates to the method for preparing the metal titanium by taking ionic liquid as an electrolyte and performing the electro-deoxidization at room temperature. The method comprises the following steps: performing electrolysis at the room temperature by taking the ionic liquid as the electrolyte, taking graphite as an anode and taking a prepared cathode as a cathode, wherein the ionic liquid is prepared by firstly preparing an intermediate of the ionic liquid from N-methylimidazole and halogenated alkane by adopting a light wave-ultrasonic wave synthesis method and further reacting the intermediate of the ionic liquid with sodium tetrafluoroborate for preparation. The method disclosed by the invention has the advantages of convenience in operation, high reaction speed, high efficiency and high yield, as well as high product purity and capability of reducing production period, so that the method is an environment-friendly, green and low-energy-consumption new method for preparing metal titanium by electro-deoxidization.
Owner:SHANDONG UNIV OF TECH
Process for preparing etching cream of glass fibre
An etching cream for silk screen printing on surface of glass is prepared through preparing etching powder from 10 raw materials including ammonium hydrogen fluoride, ammonium fluoride, starch, barium sulfate, etc. stirring in sealed state, proportionally mixing it with industrial hydrochloric acid, reaction, filtering to obtain etching liquid, mixing it with organic solvent, polyacrylic resin, polyose compound, barium salt and lipomatic compound, and stirring.
Owner:上海多林玻璃技术有限公司
One alkyl triphenyl substituted group based phosphonium salt preparation method and application
InactiveCN107129511AImprove bindingHigh catalytic activityOrganic compound preparationOrganic-compounds/hydrides/coordination-complexes catalystsPhosphonium saltSodium tetrafluoroborate
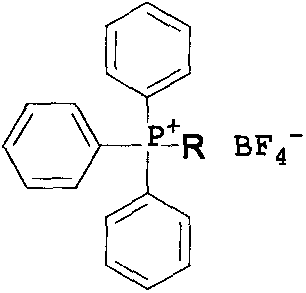
The invention discloses a one alkyl triphenyl substituted group based phosphonium salt preparation method and application. The preparation method includes that a one alkyl triphenyl phosphonium salt halide I is obtained through reaction of several alkyl halides and triphenylphosphine in a DMF (dimethyl formamide) solvent through nucleophilic substitution, and an aqueous solution of a compound I and a sodium tetrafluoroborate aqueous solution are subjected to ion exchange to prepare the phosphonium salt through high yield of recrystallization. The phosphonium salt preparation method and application has the advantages of low cost and easy obtaining of raw materials and low cost, one alkyl triphenyl substituted group containing phosphonium salt II serving as a phase transfer catalyst has the advantages of high catalytic activity, less required dose, long activity time, high thermal stability and low toxicity, high reaction recovery and the like in catalytic fluorine chlorine exchange reaction, and wide commercial application prospect is achieved.
Owner:WUHAN UNIV OF SCI & TECH
Sodium fluoborate compound, sodium fluoborate birefringent crystal as well as preparation method and application
ActiveCN108070902ASmall sizePolycrystalline material growthBy pulling from meltSpace groupBeam splitter
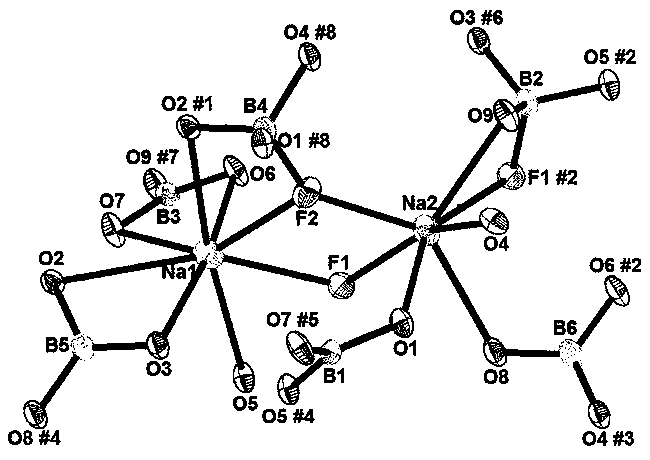
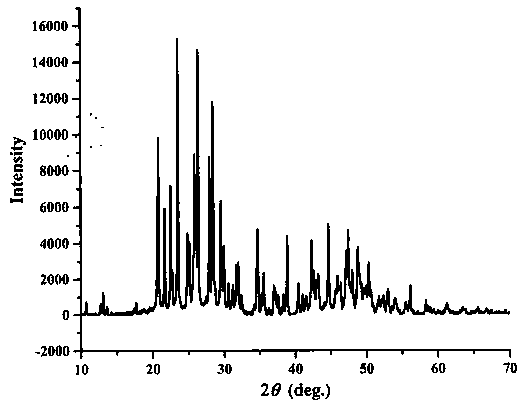
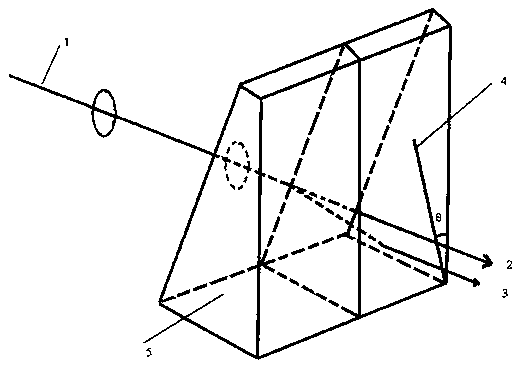
The invention relates to a sodium fluoborate compound, a sodium fluoborate birefringent crystal as well as a preparation method and an application. The compound has the chemical formula of Na2B6O9F2 and molecular weight of 292.84 and is prepared with a solid phase method. The sodium fluoborate birefringent crystal has the chemical formula of Na2B6O9F2 and molecular weight of 292.84 and belongs toa monoclinic system, the space group is P21 / c and the cell parameters are a=8.1964(12) angstrom, b=13.0005(19) angstrom, c=7.8955(11) angstrom, Z=4 and V=841.3(2) angstrom<3>, and the crystal is grownwith a flux method. The crystal is the birefringent crystal having centimeter-level large size and belonging to the monoclinic system, has birefringence of 0.080 at 589.3 nm and ultraviolet transmitting waveband cut-off edge of 169 nm and is suitable for the birefringent crystal. The crystal has the advantages that the operation is simple, the cost is low, used reagents are inorganic raw materials, the toxicity is low, the growth cycle is short, physical and chemical properties are stable and the like in the growth process. The crystal has important applications in preparation of Glan prisms,Wollaston prisms, Rochon prisms or polarizing beam splitter prisms, optoisolators, circulators and beam displacers.
Owner:XINJIANG TECHN INST OF PHYSICS & CHEM CHINESE ACAD OF SCI
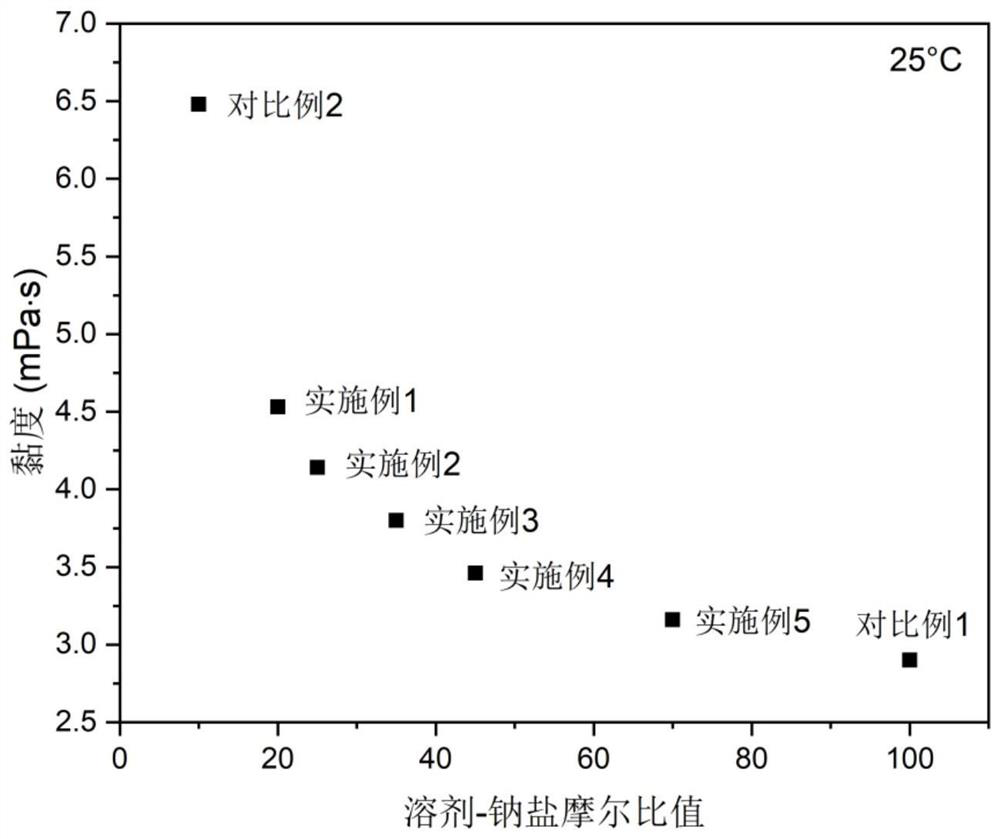
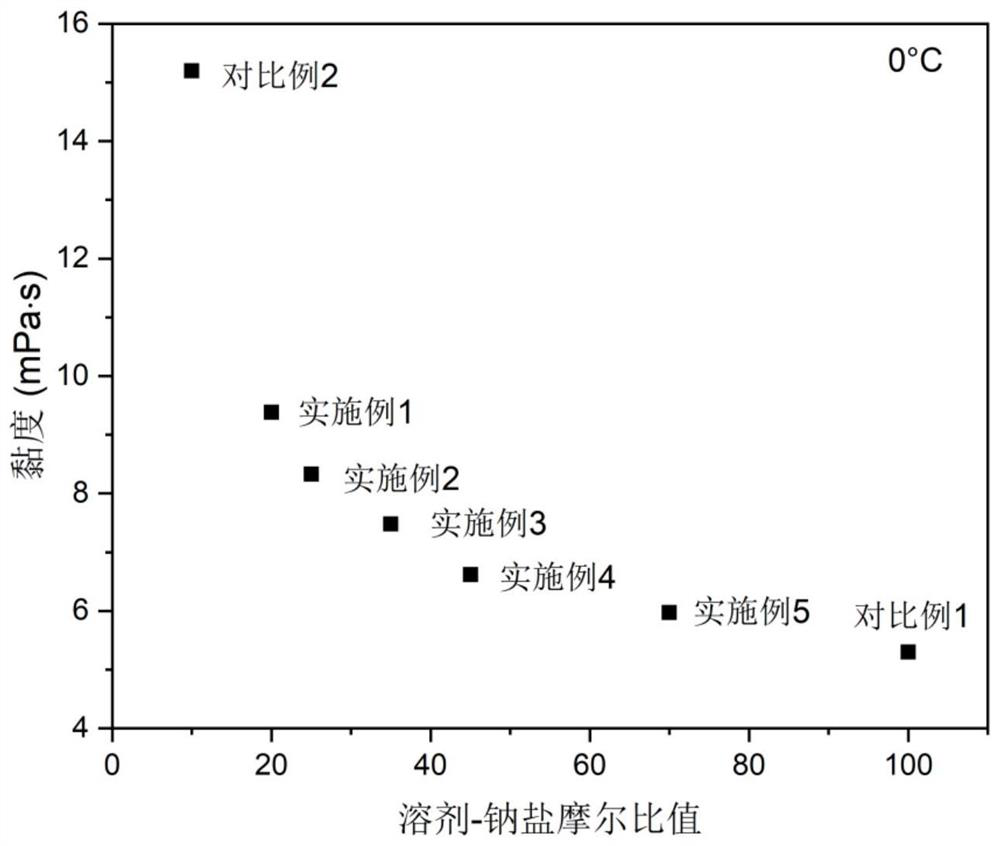
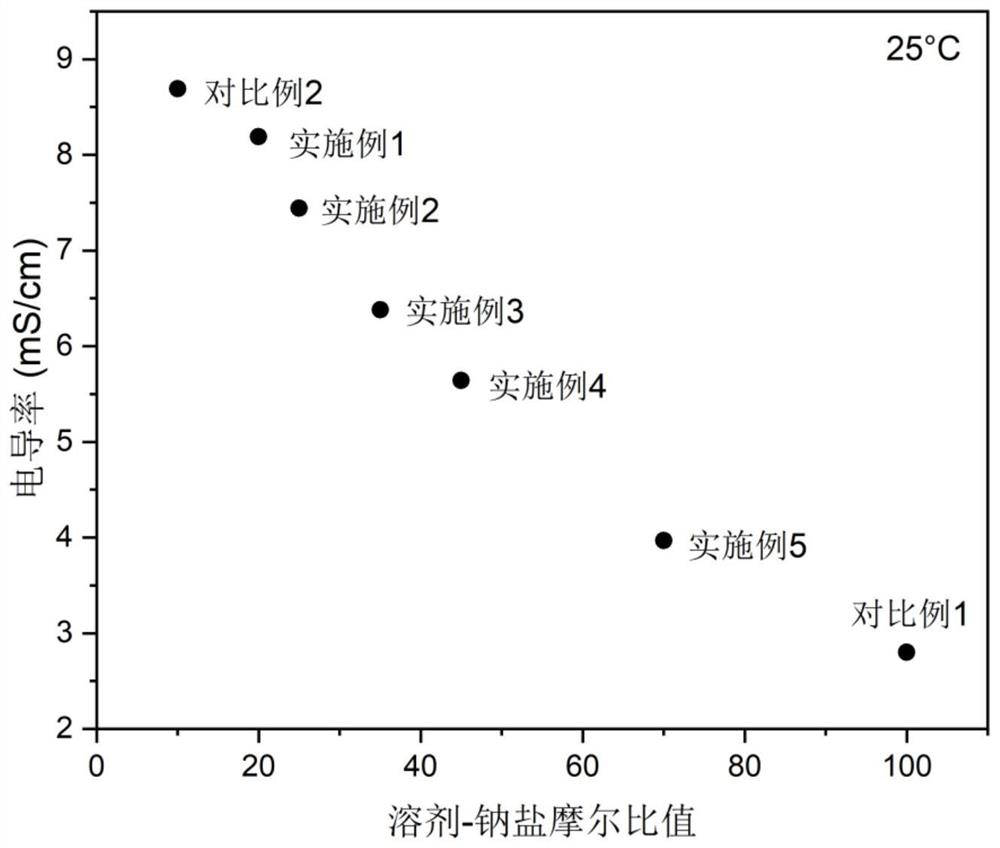
Electrolyte with high solvent-sodium salt ratio and sodium ion battery
PendingCN113299976AReduce viscosityImprove wettabilitySecondary cellsElectrolytic agentSodium tetrafluoroborate
The invention discloses an electrolyte with a high solvent-sodium salt ratio and a sodium ion battery. The electrolyte comprises sodium salt, a solvent and an additive, wherein the molar ratio of the solvent to the sodium salt is (20: 1)-(70: 1), and the addition amount of the additive accounts for 0.5%-5% of the mass of the electrolyte; the sodium salt comprises one or more of sodium perchlorate (NaClO4), sodium tetrafluoroborate (NaBF4), sodium hexafluorophosphate (NaPF6), sodium hexafluoroarsenate (NaAsF6), sodium trifluoroacetate (CF3COONa), sodium tetraphenylborate (NaB(C6H5)4), sodium trifluoromethanesulfonate (NaSO3CF3), sodium bis (fluorosulfonyl) imide (Na[(FSO2)2N]) or sodium bis (trifluoromethanesulfonyl) imide (Na[(CF3SO2)2N]).
Owner:INST OF PHYSICS - CHINESE ACAD OF SCI
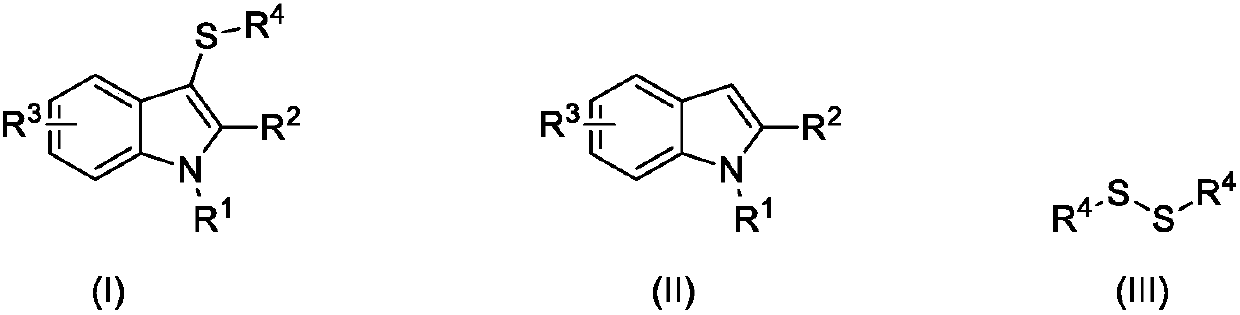
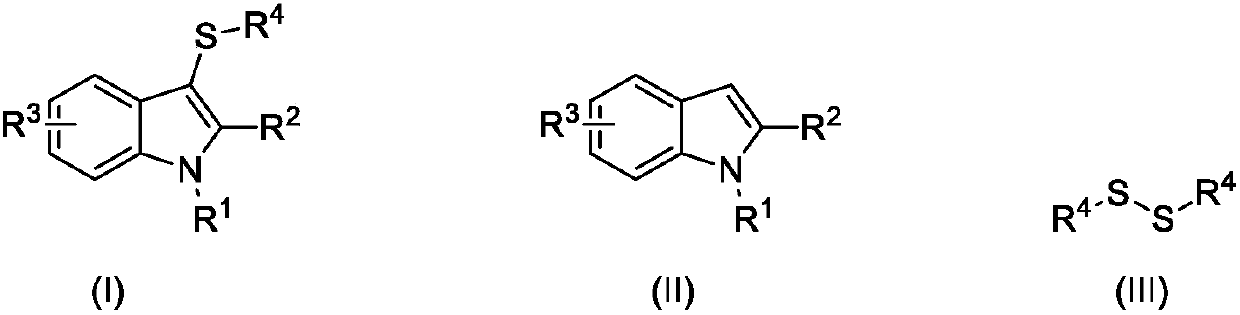
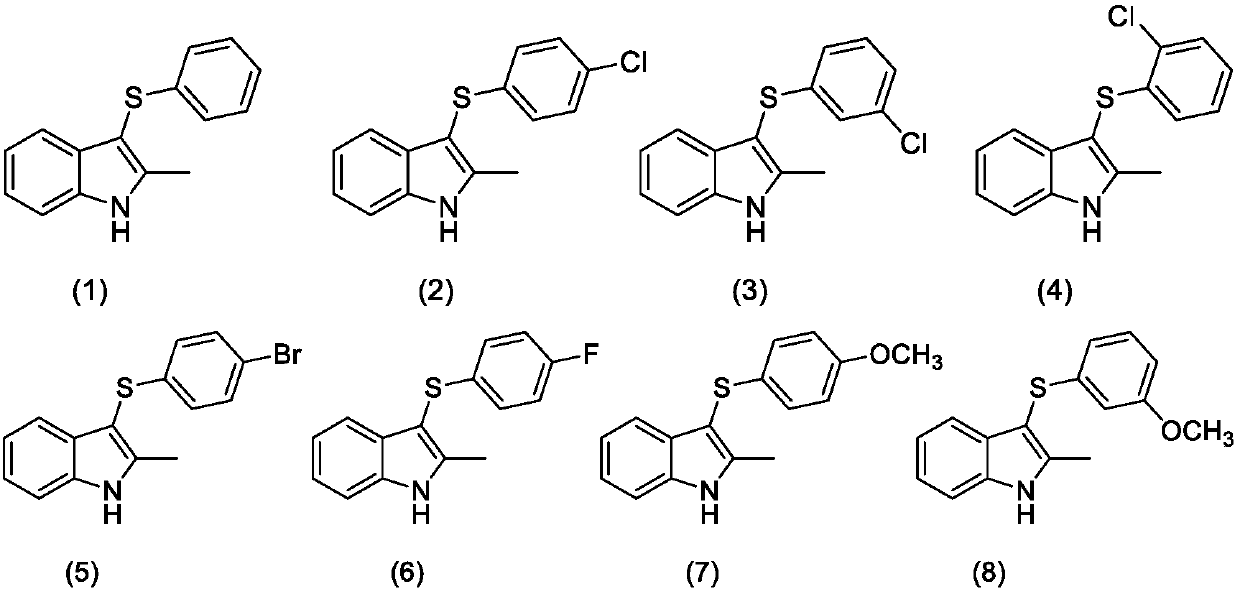
Synthesizing method for 3-mercapto indole compound through electrochemical catalytic oxidation
ActiveCN107620088AHigh yieldMild reaction conditionsElectrolysis componentsElectrolytic organic productionElectrolysisSodium tetrafluoroborate
The invention discloses a synthesizing method for a 3-mercapto indole compound through electrochemical catalytic oxidation. According to the method, a three-electrode system is used, a cathode and ananode are graphite electrodes, and a silver nitrate acetonitrile solution of 0.1mol / L is used as a reference electrode; and the indole compounds, disulfide and potassium iodide are added in a sodium tetrafluoroborate acetonitrile solution, stirring and electrolytic reaction are conducted for 3-24h under the condition of the temperature of 45-75 DEG C and 0.2-0.6 V constant voltage, a reaction solution is post-processed, and the product 3-mercapto indole compound is obtained. By means of the synthesizing method, operation is simple, convenient and safe, the yield of the product 3-mercapto indole compound is high, the reaction condition is mild, clean electrical energy is used as a redox agent, and the environmental cost is greatly reduced.
Owner:ZHEJIANG UNIV OF TECH
Room-temperature zinc slag-free phosphating agent and production method
InactiveCN110735134AReduce the impactReduce pollutionMetallic material coating processesEthylene diamine tetra aceticEthylene diamine
The invention provides a room-temperature zinc slag-free phosphating agent and a production method. The phosphating agent comprises the components including, by weight, 15-25 parts of zinc oxide, 45-65 parts of nitric acid, 15-30 parts of phosphoric acid, 0.1-0.4 part of fluorochemical, 0.1-1.5 parts of an accelerant, 5-10 parts of a complexing agent, 0.1-0.5 part of a surfactant, 0.05-0.12 part of an additive and 70-120 parts of water, wherein the fluorochemical is preferably sodium fluoborite, the accelerant is preferably copper nitrate, the complexing agent is preferably ethylene diamine tetraacetic acid, and the surfactant is preferably a compound of a nonionic surfactant and an ampholytic surfactant. The phosphating agent provided by the invention does not use harmful substances suchas nickel-containing compounds, the product stability is uniform, and a generated phosphating film is uniform and compact.
Owner:宁波际超新材料科技有限公司
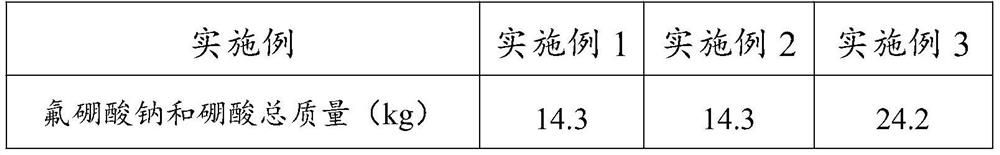
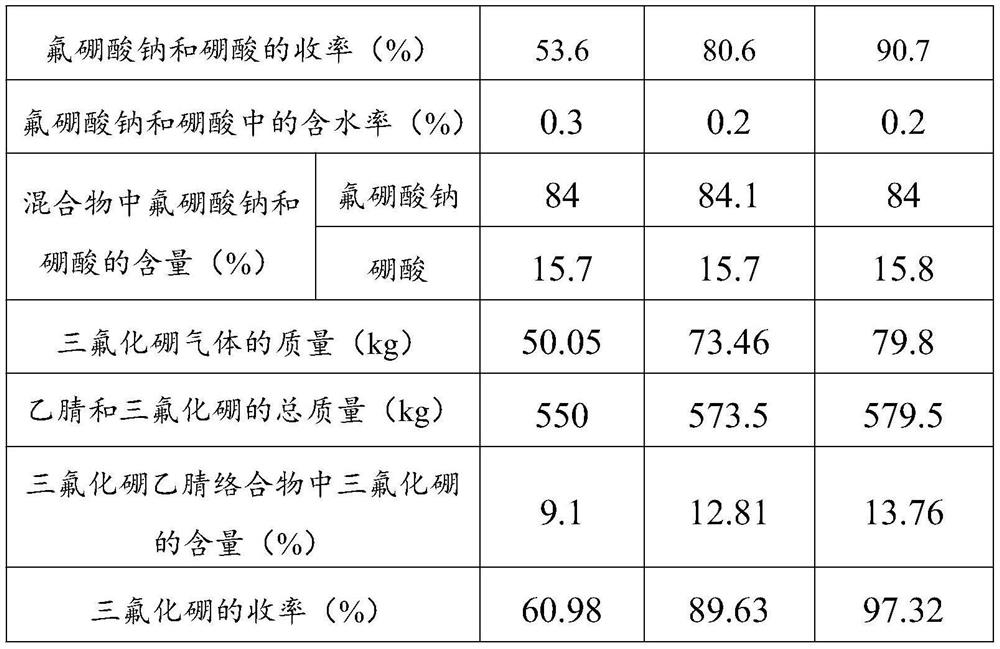
Preparation method of high-purity enriched 11B boron trifluoride gas
ActiveCN102115093AEasy to integrateHigh densityBoron halogen compoundsBoron trifluorideSodium tetrafluoroborate
The invention relates to a high-purity enriched 11B boron trifluoride gas and a preparation method thereof. The preparation method comprises the step of enriching the 11B in a double-tower in-series high-efficiency rectifying tower by a chemical exchange method by using a mass of 11B boron trifluoride methyl ether generated during producing the enriched 11B and taking the part of a byproduct as a raw material. The abundance of the 11B is higher than 95% (at.), and the purity is higher than 99wt%. Therefore, the 11B boron trifluoride methyl ether coordination compound is directly reacted with the sodium fluoride to obtain the 11B sodium fluoborate, so that the technical process is simple, the product purity is high, and the yield of the 11B sodium fluoborate is greatly improved. The 11B sodium fluoborate is directly heated at 600-700DEG C to be thermally decomposed so as to obtain the 11B boron trifluoride gas. The abundance of the prepared boron trifluoride gas 11B is higher than 95% (at.), and the purity is higher than 99.99vol%.
Owner:DALIAN BORONTEN SCI & TECH
Preparation method of oil injection pump plunger surface phosphating liquid
InactiveCN104250762AExtended service lifeLow process temperatureMetallic material coating processesSodium tetrafluoroborateCopper nitrate
The invention discloses a preparation method of an oil injection pump plunger surface phosphating liquid. The preparation method of the phosphating liquid comprises the steps: (1) taking an appropriate amount of water to blend zinc oxide into a paste; (2) taking an appropriate amount of water again, adding phosphoric acid and nitric acid to obtain a mixed solution, adding the pasty zinc oxide of the step (1), and then putting into a reaction kettle; and (3) successively adding nickel nitrate, ferric nitrate, copper nitrate, sodium citrate, tartaric acid, sodium fluoroborate, sodium chlorate, hydrogen peroxide and sodium carbonate into the reaction kettle by a way of adding the next component after the former component is dissolved, and after all the components are dissolved, adjusting the pH to 2-3.
Owner:WUXI LUOSHE TECH VENTURE
Oxidation-resistant antibacterial leather paint and preparation method thereof
InactiveCN106380987ALow water absorptionImprove water resistanceAntifouling/underwater paintsPaints with biocidesZinc Acetate DihydrateAmmonium sulfate
The invention discloses an oxidation-resistant antibacterial leather paint which is prepared from the following raw materials in parts by weight: 1-2 parts of aluminum dihydrogen phosphate, 0.7-1 part of zinc dialkyl dithiophosphate, 0.1-0.3 part of 2-bromo-4-methylphenol, 0.1-0.2 part of trimethyl hydroxyethyl ethylenediamine, 1.7-2 parts of polyvinylpyrrolidone, 0.8-1 part of polyacrylamide, 1.6-2 parts of stannous chloride, 0.1-0.13 part of triethanolamine, 190-200 parts of methyl methacrylate, 3-4 parts of lauryl sodium sulfate, 0.1-0.2 part of urotropine, 0.2-0.3 part of ammonium persulfate, 16-20 parts of zinc acetate, 4-7 parts of silver nitrate, 0.8-1 part of sodium fluorborate, 0.7-1 part of polyoxyethylene oleate, 0.1-0.2 part of n-salicylanilide and 6-7 parts of 20-25% formaldehyde solution. The paint has the advantages of favorable antibacterial property, excellent water vapor permeability and excellent water resistance. The leather coated by the obtained emulsion has favorable antibacterial property and excellent hygienic property.
Owner:WUHU HONGKUN AUTO PARTS
Preparation method of graphene conductive paper with controllable conductivity
InactiveCN103539107AReduce conductivityConductivity controllableGrapheneCable/conductor manufactureIce waterFiltration
The invention relates to a preparation method of a graphene conductive paper with controllable conductivity. The preparation method comprises the following steps of: (1) a pretreatment process; (2) an oxidation process; (3) a reduction process: diluting oxidized graphene dispersion liquid by using distilled water, then regulating a pH value by using ammonia, and adding hydrazine hydrate to prepare graphene dispersion liquid; (4) a modifying process: dissolving phenylamine into a solution prepared from concentrated hydrochloric acid and distilled water, dropping a sodium nitrite solution, filtering to obtain filter liquor, adding sodium tetrafluoroborate to the filter liquor, successively flushing obtained diazonium salt by using ice water and cold ether, and mixing the graphene dispersion liquid and the diazonium salt for reaction; (5) a film preparation measuring process: carrying out suction filtration on a reacted mixed solution through a water-phase film, drying to obtain a graphene film, measuring the sheet resistance of the film by using a four-probe system, and converting conductivity. The preparation method disclosed by the invention achieves the purpose of controlling the graphene conductivity by quantificationally destroying the conjugated structure of graphene by utilizing the diazonium salt and has wide application prospect in the fields of electric devices, electrodes, and the like.
Owner:QINGDAO HUAGAO GRAPHENE CORP LTD +1
Method for synthesizing N-aryl formamide compound
InactiveCN108752155AImprove toleranceExpensive gramsCarboxylic acid nitrile preparationOrganic compound preparationDimethylaniline N-oxideSodium tetrafluoroborate
A method for synthesizing an N-aryl formamide compound comprises the following steps: putting an N, N-dimethylaniline compound, cuprous chloride, sodium tetrafluoroborate and salicylic acid in an organic solvent in an oxygen atmosphere, reacting for 0.5-48 hours at 30-60 DEG C, and separating and purifying a product to obtain the N-aryl formamide compound, wherein the using amount of the organic solvent is as follows: the amount-of-substance concentration of the N, N-dimethylaniline compound, the cuprous chloride, the sodium tetrafluoroborate and the salicylic acid is 0.5-1mol / L. The method has the advantages as follows: the operation is simple, a used catalyst is cheap, a reaction condition is mild, the product yield is high, the defects of expensive raw material reagents, a harsh condition, cumbersome synthesis steps, not high total yield and the like in the prior art are overcome, and a good application prospect is achieved.
Owner:XIJING UNIV
Preparation method of grafted quaternary phosphonate ionic liquid
InactiveCN102580471ACumulative specific surface areaCumulative specific surface area is largeDispersed particle separationAir quality improvementPolymer scienceSodium tetrafluoroborate
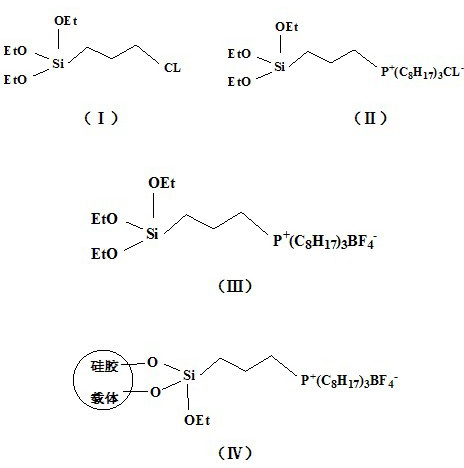
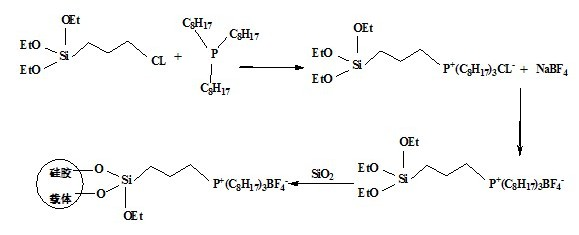
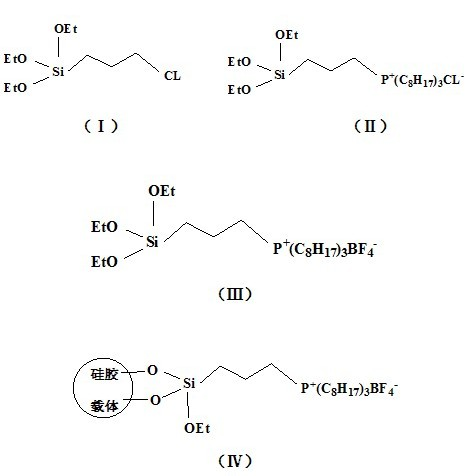
The invention relates to a preparation method of a grafted quaternary phosphonate ionic liquid. The preparation method comprises the following steps of: using 3-(chloropropyl)triethoxysilane and trioctylphosphine to react and generate a chlorinated quaternary phosphonate ionic liquid intermediate, then using the chlorinated ionic liquid intermediate and sodium fluoroborate to synthesize a tetrafluoroborate quaternary phosphonate ionic liquid intermediate, and finally using the tetrafluoroborate ionic liquid intermediate and silica to react and generate the grafted quaternary phosphonate ionic liquid. The grafted quaternary phosphonate ionic liquid provided by the preparation method of the grafted quaternary phosphonate ionic liquid can be used as the CO2 adsorbent alone and can also be used along with other adsorption materials; and compared with the silica gel supported 1-butyl-3-methylimidazolium tetrafluoroborate ionic liquid which is synthesized by the traditional immersion method and the silica gel supported 1-butyl-3-methylimidazolium tetrafluoroborate ionic liquid which is synthesized by the sol-gel method, the grafted quaternary phosphonate ionic liquid has larger cumulative specific surface area and better CO2 adsorption property.
Owner:CHINA UNIV OF MINING & TECH
Non-aqueous electrolyte for inhibiting sodium-ion battery swelling as well as preparation method and application thereof
InactiveCN108288730ASuppress flatulenceImprove Coulombic efficiencyFinal product manufactureSecondary cells servicing/maintenanceSodium tetrafluoroborateSodium-ion battery
The invention discloses non-aqueous electrolyte for inhibiting sodium-ion battery swelling as well as a preparation method and application thereof. The non-aqueous electrolyte comprises a sodium salt,an organic solvent and a first functional additive, wherein the molecular formula of the first functional additive is R1R2R3C3H3O3S; the content of the first functional additive in the non-aqueous electrolyte is 0.001-30wt%; the sodium salt refers to one or more of sodium hexafluorophosphate, sodium tetrafluoroborate, sodium hexafluoroarsenate, sodium borate dioxalate, sodium ditrifluoromethyl sulfonamide, sodium trifluoromethanesulfonate, sodium difluorosulfonimide or sodium perchlorate; the organic solvent refers to one or more of cyclic carbonate, chain linear carbonate, carboxylic ester or cyclic lactone.
Owner:INST OF PHYSICS - CHINESE ACAD OF SCI
Efficient decontaminating and cleaning shoe polish
InactiveCN104356952AImprove water resistanceGood flexibilitySurface-active non-soap compounds and soap mixture detergentsPolishing compositionsCelluloseNitrocellulose
The invention relates to an efficient decontaminating and cleaning shoe polish. The shoe polish is prepared from the following raw materials in parts by weight: 6-11 parts of nitrocellulose, 3-8 parts of sodium fluoroborate, 5-9 parts of ammonium citrate, 3-8 parts of dodecyl sodium sulphate, 5-7 parts of acetic acid dibutyl ester, 4-10 parts of neoprene, 3-5 parts of zinc oxide, 3-6 parts of n-hexane, 1-5 parts of sodium hydroxide, 4-8 parts of aluminum trichloride, 2-6 parts of stearic acid, 5-9 parts of cellulose, 6-10 parts of polyoxyethylene fatty acid, 2-5 parts of castor oil and 1-5 parts of paraffine. The efficient decontaminating and cleaning shoe polish disclosed by the invention has the benefits that the water resistance and the flexibility of leather shoes can be improved, and the corrosion resistance of the leather shoes can be improved, so that the service lives of the leather shoes are prolonged.
Owner:QINGDAO TOPLINK INFORMATION TECH
Extracting desulfurizing agent, preparation method thereof and desulfurizing refining method for biodiesel oil
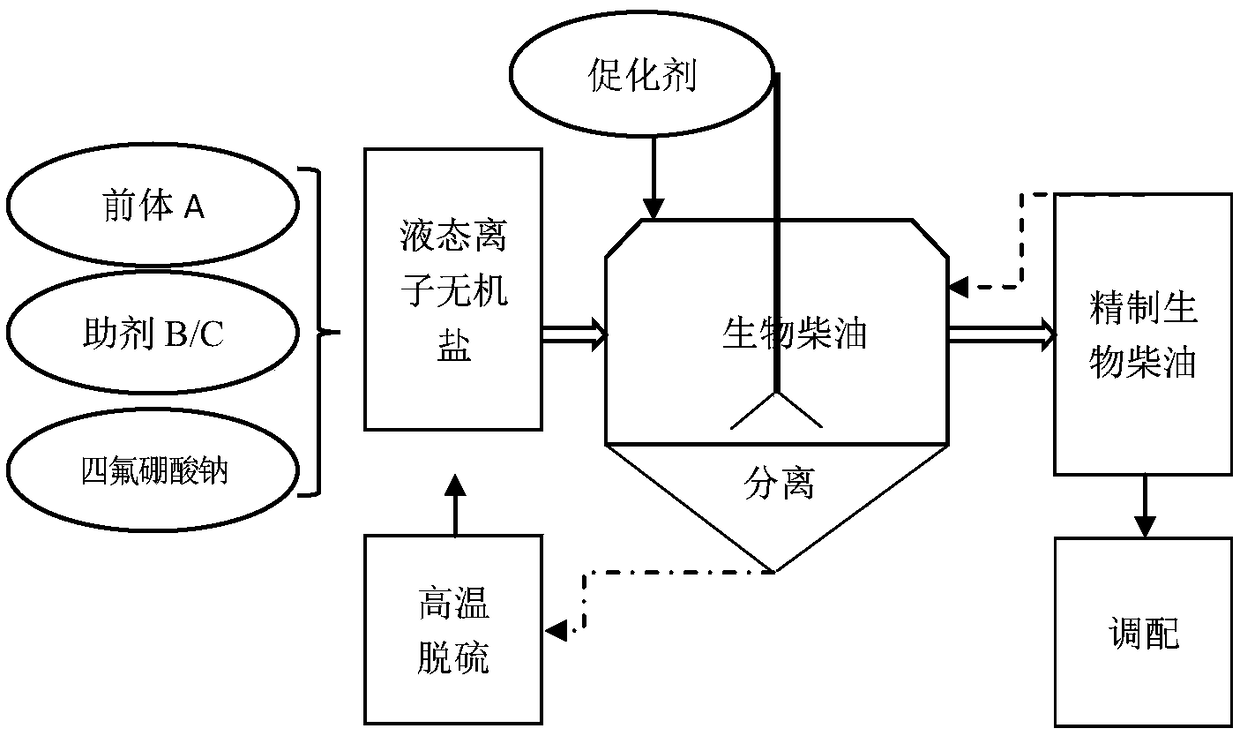
InactiveCN108822884AEffective removal of sulfur contentEasy to separateBiofuelsFatty-oils/fats refiningBiodieselSodium tetrafluoroborate
The invention belongs to the field of deep-processing of biomass energy liquid fuels and discloses an extracting desulfurizing agent, a preparation method thereof and a desulfurizing refining method for biodiesel oil. The extracting desulfurizing agent is liquid state ionic inorganic salt and is synthesized by carrying out a reaction on a precursor A and sodium tetrafluoroborate in a mass ratio of(40-50):(50-70), wherein the precursor A is 1-butyl-3-methylimidazole bromide or 1-propyl-3-methylimidazole bromide. The desulfurizing refining method for biodiesel oil comprises the following steps:adding the extracting desulfurizing agent and an accelerant of the extracting reaction into the biodiesel oil; and leaving the biodiesel oil to stand and layer after the reaction to separate the biodiesel oil. The liquid state ionic organic salt can remove sulfur in the biodiesel oil effectively, and the two phases are extremely easily separated, so that an emulsion phenomenon can be avoided effectively. The sulfur content of the biodiesel oil is reduced by extraction by means of the liquid state ionic organic salt. The sulfur content in the biodiesel oil can be reduced by 90% or above. The extracting desulfurizing agent has the advantages of low energy consumption, low refining consumption and no pollution.
Owner:HUNAN ACAD OF FORESTRY
Preparation method of high-purity enriched 11B boron trifluoride gas
ActiveCN102115093BEasy to integrateHigh densityBoron halogen compoundsBoron trifluorideSodium tetrafluoroborate
The invention relates to a high-purity enriched 11B boron trifluoride gas and a preparation method thereof. The preparation method comprises the step of enriching the 11B in a double-tower in-series high-efficiency rectifying tower by a chemical exchange method by using a mass of 11B boron trifluoride methyl ether generated during producing the enriched 11B and taking the part of a byproduct as araw material. The abundance of the 11B is higher than 95% (at.), and the purity is higher than 99wt%. Therefore, the 11B boron trifluoride methyl ether coordination compound is directly reacted with the sodium fluoride to obtain the 11B sodium fluoborate, so that the technical process is simple, the product purity is high, and the yield of the 11B sodium fluoborate is greatly improved. The 11B sodium fluoborate is directly heated at 600-700DEG C to be thermally decomposed so as to obtain the 11B boron trifluoride gas. The abundance of the prepared boron trifluoride gas 11B is higher than 95% (at.), and the purity is higher than 99.99vol%.
Owner:DALIAN BORONTEN SCI & TECH
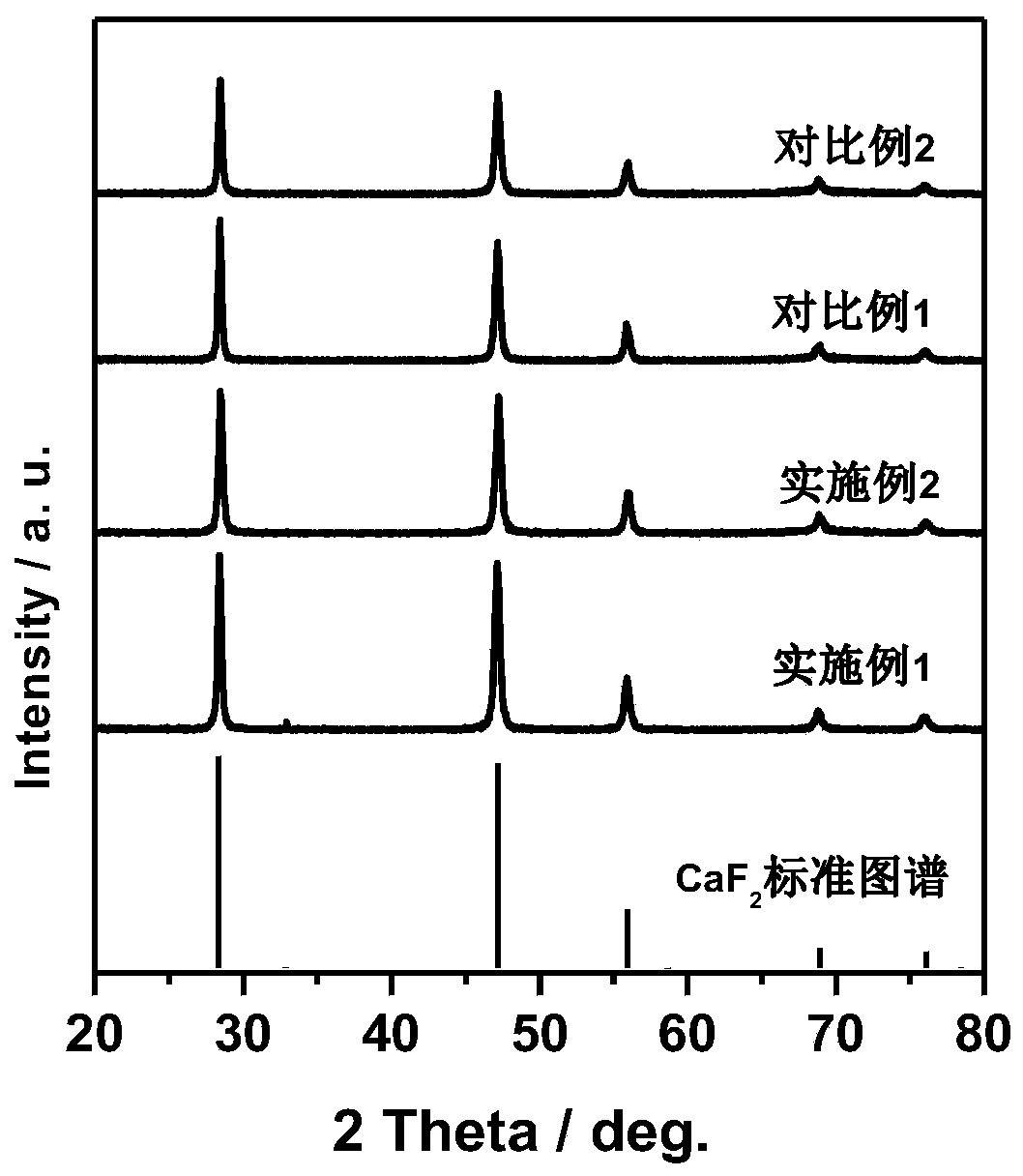
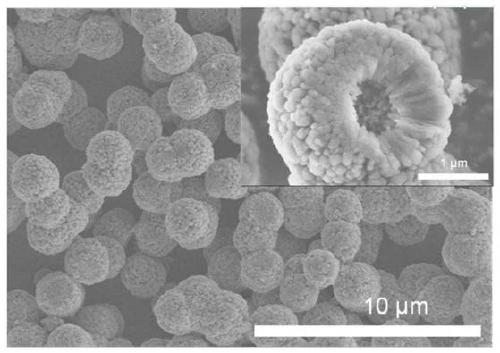
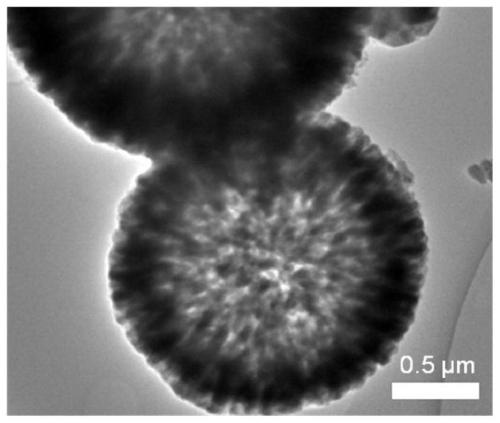
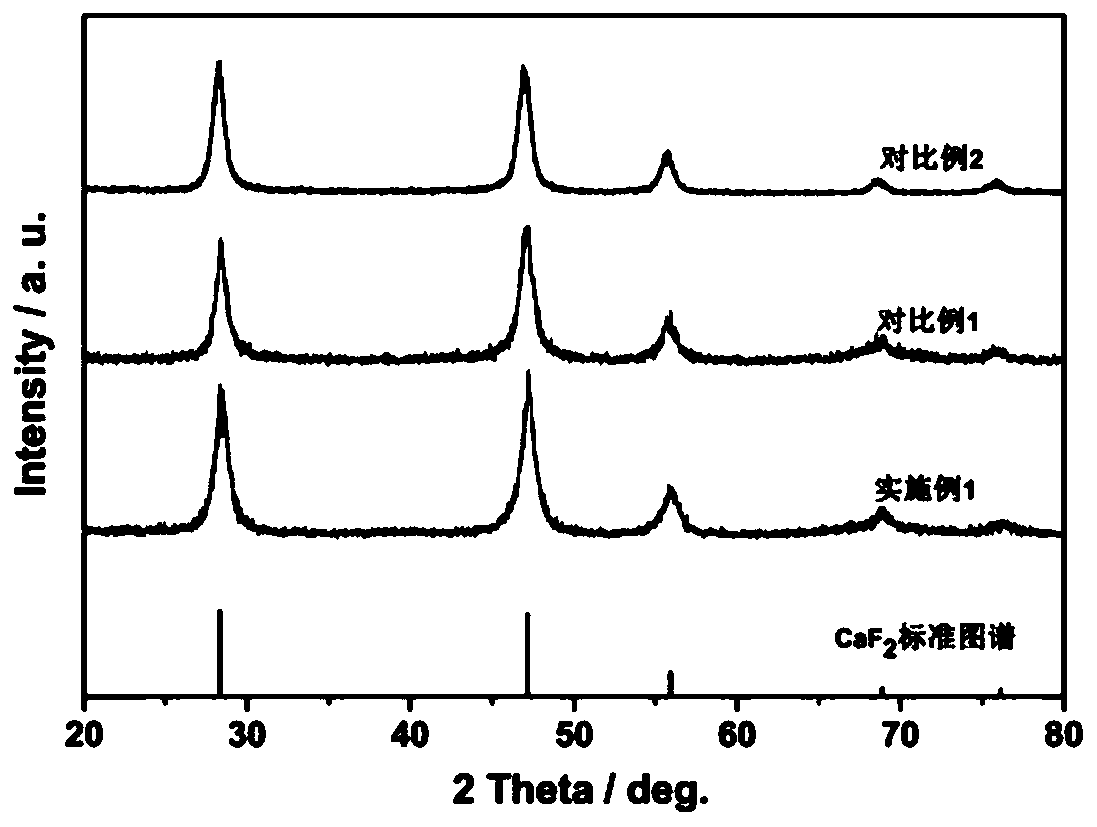
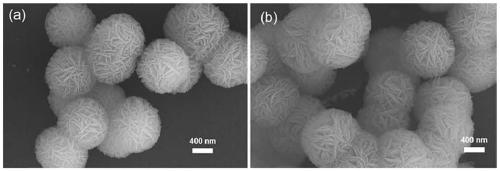
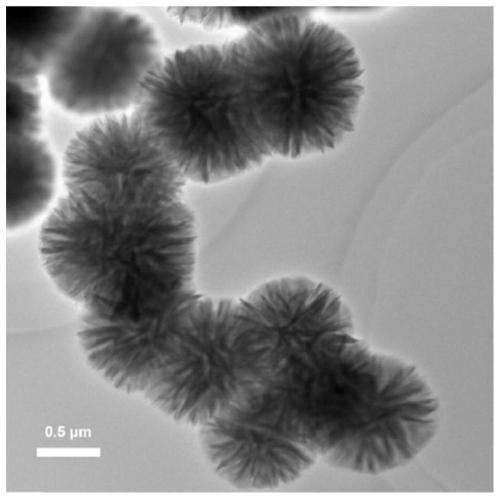
Preparation method of CaF2 hollow nanospheres
InactiveCN109734116AThe synthesis method is simpleConsistent shapeCalcium/strontium/barium fluoridesCitrinin hydrateSodium tetrafluoroborate
The invention relates to a preparation method of CaF2 hollow nanospheres. F127 is taken as surfactant, calcium nitrate tetrahydrate is taken as calcium source, sodium citrate dihydrate is taken as complexing agent, and sodium tetrafluoroborate is taken as fluorine source, and the CaF2 hollow nanospheres are synthesized by hydrothermal method. The synthesis method is simple, rapid and easy to repeat. The hollow spheres obtained are uniform in shape and size.
Owner:ZHEJIANG NORMAL UNIVERSITY
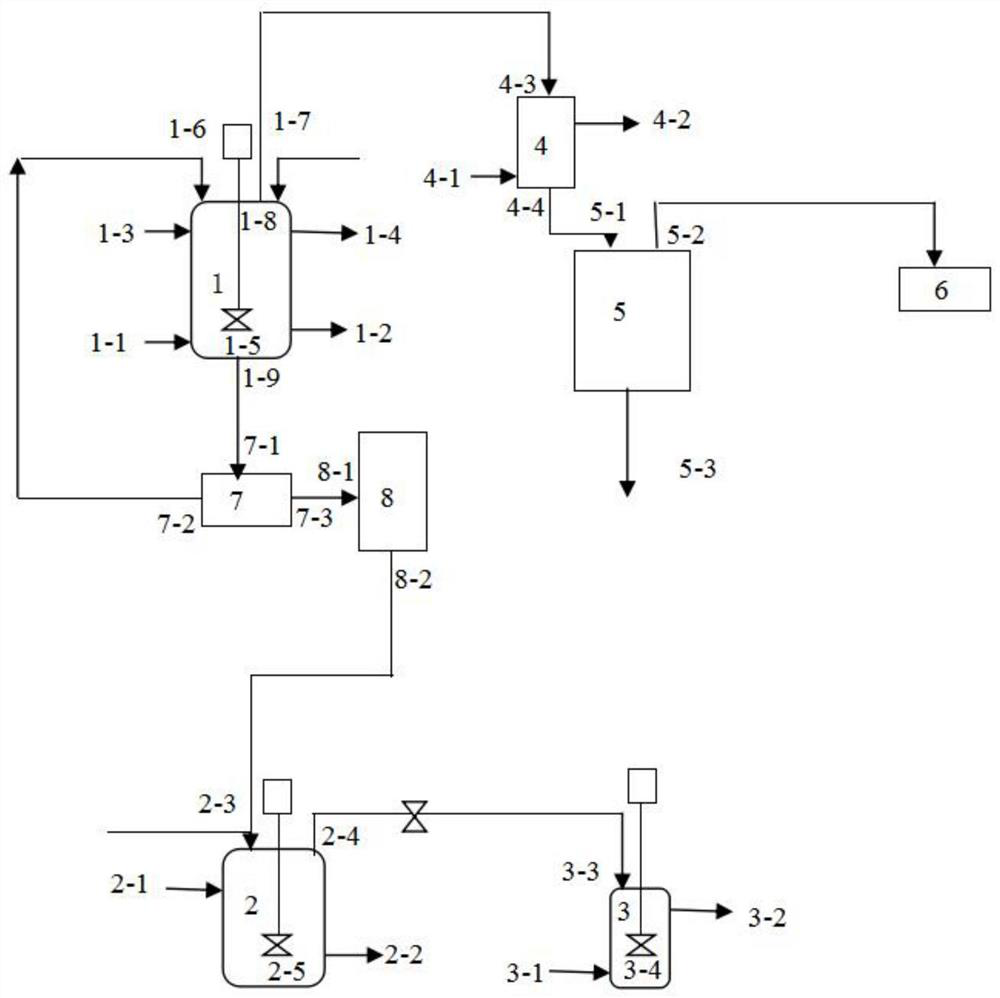
Method and device for recovering boron trifluoride in wastewater containing boron trifluoride
PendingCN112850731AReduce the impactReduce wasteBoron halidesGroup 3/13 element organic compoundsSodium tetrafluoroborateBoron trifluoride
The invention belongs to the technical field of wastewater treatment, and particularly relates to a method and device for recovering boron trifluoride in wastewater containing boron trifluoride. The recovery method provided by the invention comprises the following steps: mixing wastewater containing boron trifluoride with strong base to carry out acid-base neutralization reaction to obtain a neutralization reaction product, wherein the boron trifluoride contained in the wastewater is hydrolyzed into fluoboric acid and boric acid; and mixing the neutralization reaction product with fuming sulfuric acid, and carrying out a replacement reaction to obtain boron trifluoride gas. According to the method, by adding the strong base into the boron trifluoride-containing wastewater and carrying out acid-base neutralization reaction on the strong base and fluoboric acid in the wastewater, sodium fluoborate is obtained; and sodium fluoborate, boric acid and fuming sulfuric acid react to obtain the boron trifluoride gas. According to the method, boron trifluoride in the wastewater is recycled by utilizing a simple chemical reaction. The recovery method provided by the invention is simple and easy to implement; the influence of boron trifluoride on the environment and an existing wastewater treatment system is reduced; and the waste of resources is reduced.
Owner:SHANDONG HEYI GAS CO LTD DONGYING CITY
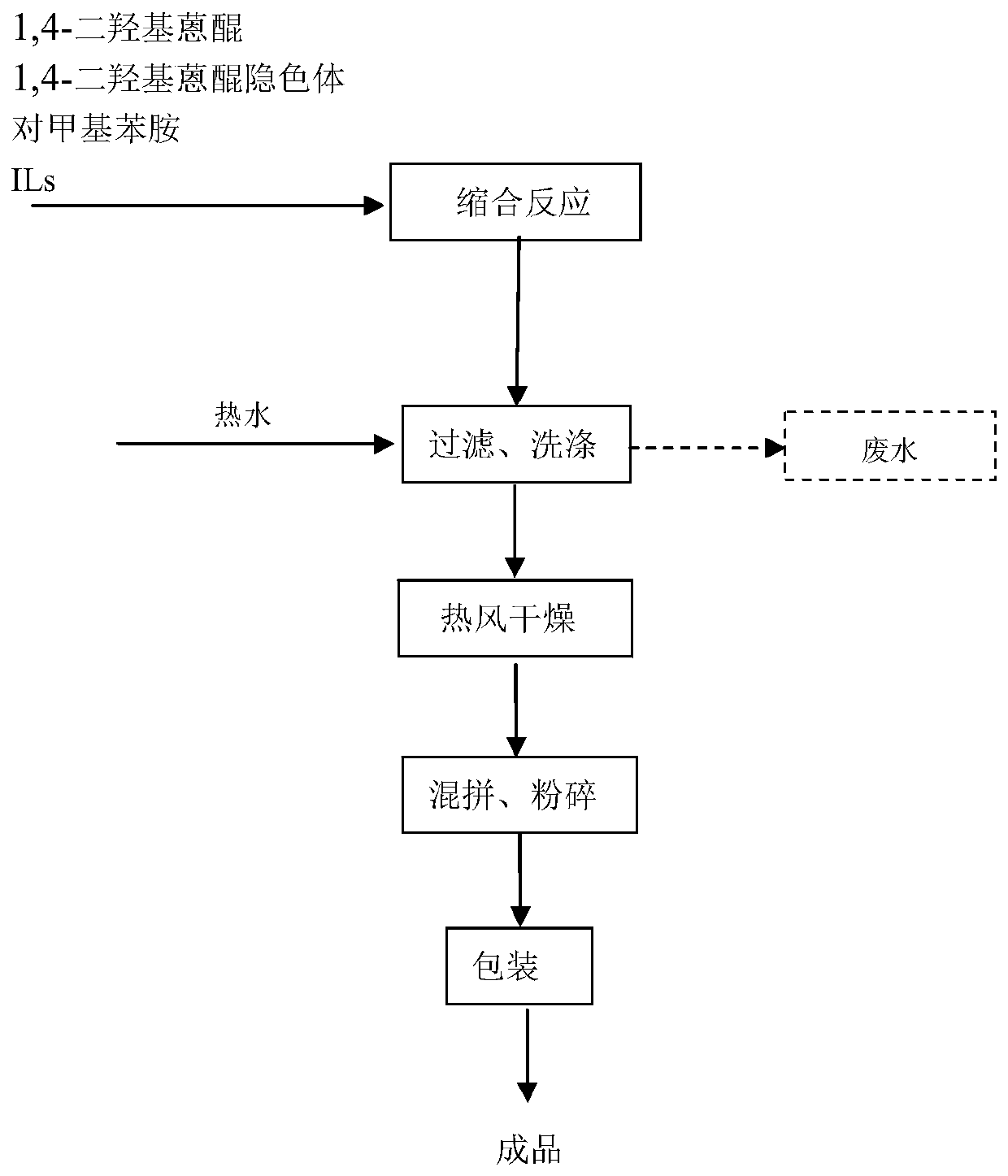
Synthetic method of low-toxicity low-harm environment-friendly solvent green 3
PendingCN110615741AImprove high temperature stabilityIncrease lossOrganic chemistryOrganic compound preparationSodium tetrafluoroborateSynthesis methods
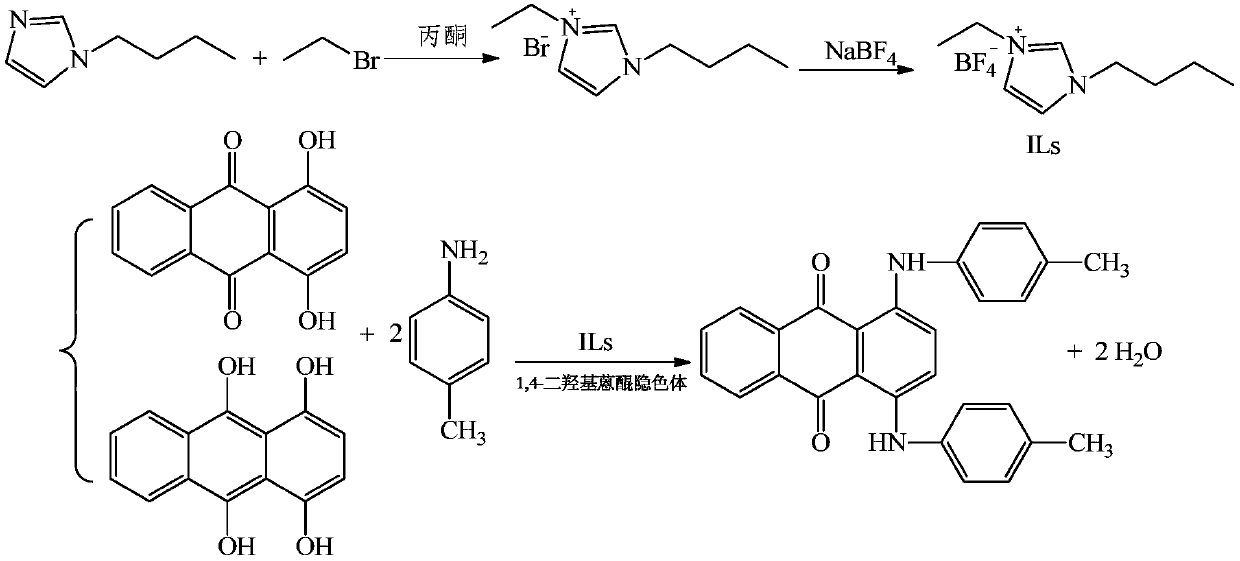
The invention relates to a synthesis method of a low-toxicity low-harm environment-friendly solvent green 3, which comprises the following steps: (a) adding N-butylimidazole and acetone into a reaction vessel, slowly dropwise adding bromoethane for reaction, adding sodium tetrafluoroborate for anion exchange, and continuing stirring the reactants to obtain an ionic liquid; (b) adding 1,4-dihydroxyanthraquinone, a 1,4-dihydroxy anthraquinone leuco body and p-toluidine into the ionic liquid, and carrying out condensation reaction to obtain a first mixed solution; and (c) cooling the first mixedsolution, carrying out suction filtration to obtain a filter cake and a filtrate, and carrying out washing post-treatment on the filter cake to obtain the solvent green 3. According to the method, the synthesized ionic liquid is used as a solvent, the condensation reaction is double condensation through model selection of organic cations and anions in the ionic liquid, the product purity is obviously improved to 98% or above, the yield reaches 95% or above, in addition, the ionic liquid can be recycled, the cost is greatly reduced, and the pollutant discharge amount is reduced; the performances of the solvent green 3 dye as shown as follows: the [delta]E is less than 0.5, [delta]C is brilliant, and the pressure value is less than 0.2.
Owner:安徽清科瑞洁新材料有限公司
Fluorinated gadolinium carbonate as well as preparation method and application thereof
ActiveCN113277545AThe preparation method is convenientEasy to operateInorganic material magnetismRare earth metal compounds preparation/treatmentMagnetic anisotropySodium tetrafluoroborate
The invention discloses fluorinated gadolinium carbonate as well as a preparation method and application thereof and belongs to rare earth gadolinium materials. The fluorinated gadolinium carbonate is a novel fluorine and carbonate coordinated rare earth gadolinium material, and the structural composition formula of the fluorinated gadolinium carbonate is [GdCO3F] n (1), the maximum magnetic entropy change value of the material is 69.9 J kg <-1 > K <-1 > at 2K and 7T. The preparation method comprises the following steps of: adding hydrated gadolinium carbonate as a raw material into water; adding sodium tetrafluoroborate; fully stirring an obtained mixed solution, and transferring the solution into a hydrothermal kettle for hydrothermal reaction; and performing washing and drying to obtain a target product. A synthesis device of the fluorinated gadolinium carbonate is simple, preparation is convenient and fast, operation is easy, and the fluorinated gadolinium carbonate can be applied to preparation of magnetic refrigeration materials. Rare earth Gd < 3 + > with a high spinning ground state and small magnetic anisotropy is selected as cations, CO3 < 2-> with small molecular weight is selected as a ligand, the mass ratio of the rare earth to the ligand is increased to improve the magnetic density, and meanwhile, a fluorine ligand is doped into the material, so that the magnetic refrigeration effect of the material is greatly improved.
Owner:XIAMEN UNIV
CaF2 nano material with high specific surface area and high thermal stability as well as preparation method and application thereof
PendingCN111514843AOther chemical processesCalcium/strontium/barium fluoridesSodium tetrafluoroborateEthylic acid
The invention relates to the field of adsorption separation, in particular to a CaF2 nano material with high specific surface area and high thermal stability as well as a preparation method and application thereof. The CaF2 nano material is a multilayer nanosheet flower-shaped CaF2 material synthesized by taking disodium ethylene diamine tetraacetate as a complexing agent, calcium acetate as a calcium source and sodium tetrafluoroborate as a fluorine source and adopting a hydrothermal method. According to the CaF2 nano-material provided by the invention, the pH value and crystallization time of the synthetic liquid are optimized, so that the morphology and size of the CaF2 nano-material are effectively controlled, and the highest thermal stability, the maximum specific surface area and theoptimal water absorption performance of the CaF2 nano-material are obtained. In addition, the CaF2 material prepared by the technical scheme of the invention can be used as a water absorbent to efficiently adsorb trace moisture in HF gas to prepare HF high-purity electronic special gas, and can be recycled after regeneration.
Owner:ZHEJIANG NORMAL UNIVERSITY
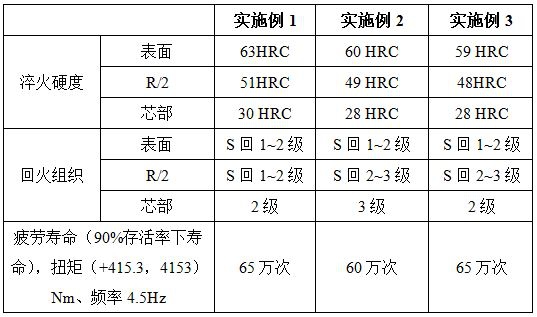
Surface carbonitriding treatment method for planet shaft for tractor axle
ActiveCN114000095AHigh strengthImprove toughnessSolid state diffusion coatingFurnace typesSodium tetrafluoroboratePhysical chemistry
The invention discloses a surface carbonitriding treatment method for a planet shaft for a tractor axle. The surface carbonitriding treatment method comprises the steps of infiltration-assisted pretreatment, carbonitriding treatment and quenching and tempering treatment. The infiltration-assisted pretreatment comprises steps of surface treatment, infiltration-assisted coating liquid preparation, drying and sintering; a carbonitriding agent used in carbonitriding is composed of sodium carbonate, barium carbonate, Mo3N2, sodium tetrafluoroborate, sodium chloride and potassium chloride according to the mass ratio of (30-46): (23-36): (28-33): (1-4): (5-9): (1-3). According to the planet shaft for the tractor axle obtained through the carbonitriding treatment method, the quenching hardness of the surface ranges from 59 HRC to 63 HRC, the quenching hardness of the R / 2 position ranges from 48 HRC to 51 HRC, and the quenching hardness of the core ranges from 28 HRC to 30 HRC; the surface of the tempered structure is S(back) 1-2 grade, R / 2 is S(back)1-2 grade, and the core part is 2-3 grade; and the fatigue life (under 90% of survival rate) is 60-65 million times.
Owner:SHANDONG GAHEAD DRIVE TECH
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com
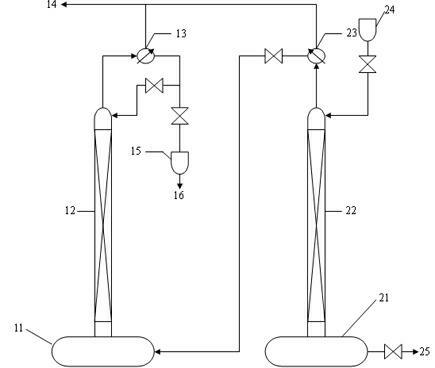
![AM/AA/N-beta-CD polymer-ionic liquid [bquin]BF4 composite clay stabilizer and synthesis method thereof AM/AA/N-beta-CD polymer-ionic liquid [bquin]BF4 composite clay stabilizer and synthesis method thereof](https://images-eureka.patsnap.com/patent_img/e15dbbf8-c72c-4ab6-bf51-8de551180619/140408101615.PNG)
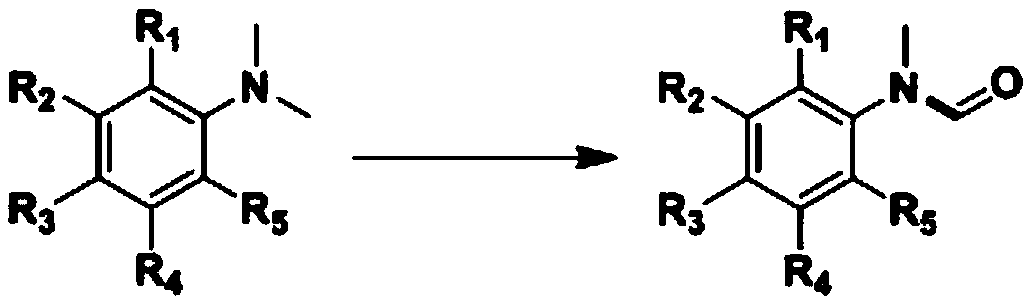
![AM/AA/N-beta-CD polymer-ionic liquid [bquin]BF4 composite clay stabilizer and synthesis method thereof AM/AA/N-beta-CD polymer-ionic liquid [bquin]BF4 composite clay stabilizer and synthesis method thereof](https://images-eureka.patsnap.com/patent_img/e15dbbf8-c72c-4ab6-bf51-8de551180619/140408101620.PNG)
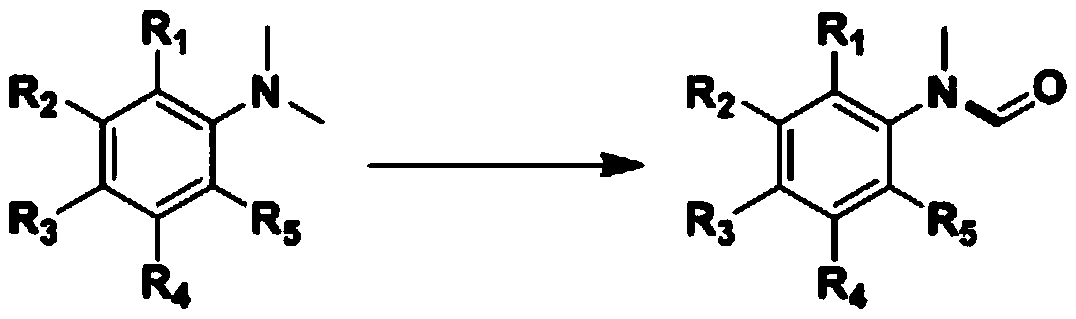
![AM/AA/N-beta-CD polymer-ionic liquid [bquin]BF4 composite clay stabilizer and synthesis method thereof AM/AA/N-beta-CD polymer-ionic liquid [bquin]BF4 composite clay stabilizer and synthesis method thereof](https://images-eureka.patsnap.com/patent_img/e15dbbf8-c72c-4ab6-bf51-8de551180619/140408101624.PNG)