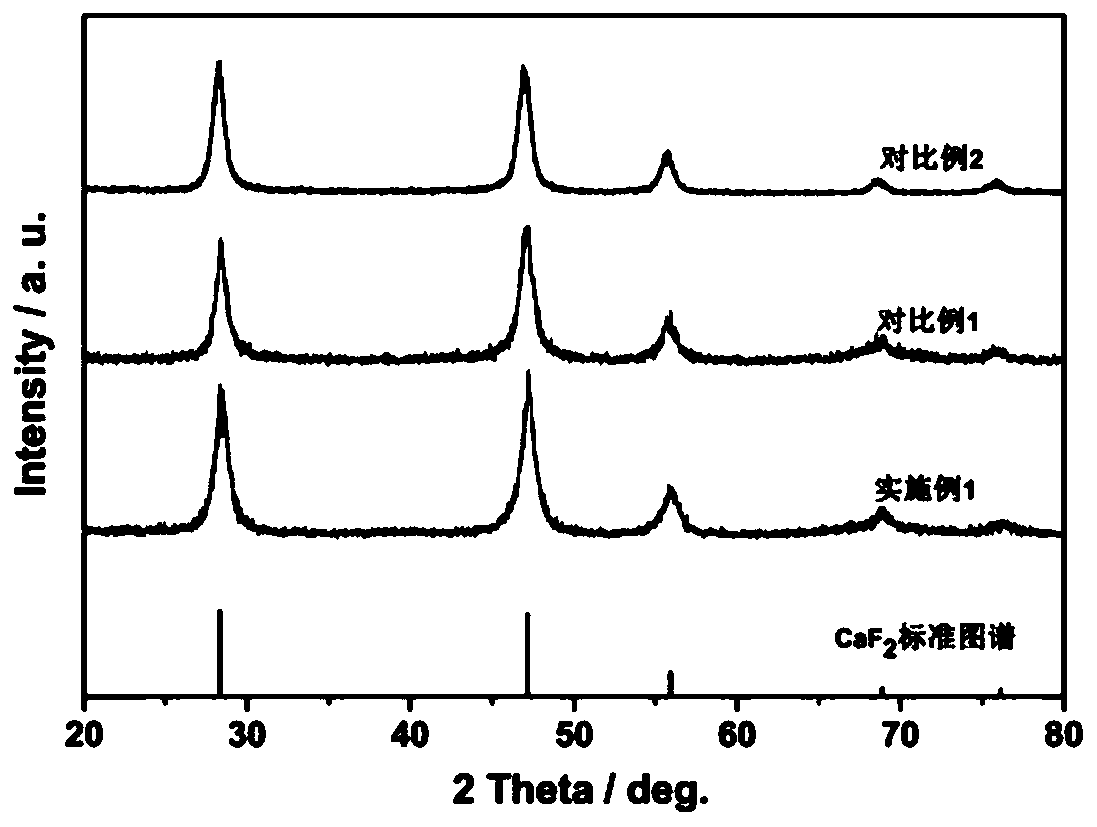
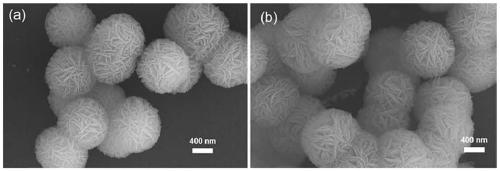
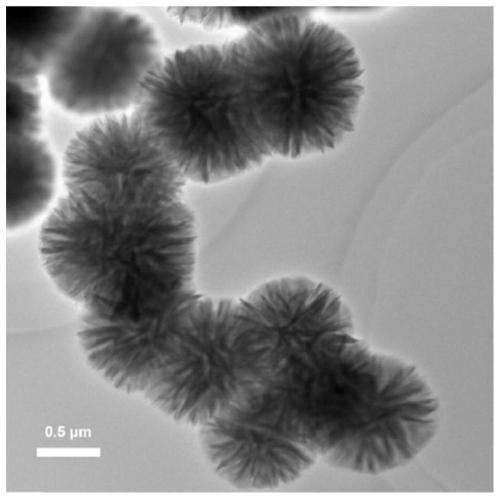
CaF2 nano material with high specific surface area and high thermal stability as well as preparation method and application thereof
A technology with high thermal stability and high specific surface area, used in chemical instruments and methods, separation methods, fluorine/hydrogen fluoride, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0046] According to the molar ratio of each material, Ca(CH 3 COO) 2 :Na 2 EDTA:NaBF 4 :H 2 O=1:5:2:1676.6, the 1mmolCa (CH 3 COO) 2 Dissolve in 30mL distilled water, after stirring and dissolving, add 5mmol Na 2 EDTA·2H 2 O, magnetically stirred for 30min to obtain mixed solution A, then in mixed solution A, add 2mmol NaBF 4 At the same time, acetic acid was added dropwise to adjust the pH value of the solution to 3.9; the mixed solution B was obtained after magnetic stirring for 10 minutes, and the mixed solution B was transferred to a hydrothermal stainless steel reactor lined with polytetrafluoroethylene, and placed in a 433K oven for crystallization After 1 h; cool to room temperature, alternately centrifuge and wash with distilled water and absolute ethanol, repeat 3 times, and finally place the obtained sample in a 333K oven to dry overnight to obtain a white powdery solid. Heat treatment at 573K and vacuum condition for 7h to obtain the sample after heat treatm...
PUM
| Property | Measurement | Unit |
|---|---|---|
| water absorption | aaaaa | aaaaa |
| length | aaaaa | aaaaa |
| diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com