Method and device for recovering boron trifluoride in wastewater containing boron trifluoride
A boron trifluoride and recovery method technology, applied in boron halide compounds, chemical instruments and methods, organic chemistry, etc., can solve problems such as toxicity, damage to sewage treatment systems, and no degradation and conversion of fluorine and boron, so as to reduce the impact, The effect of reducing waste
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
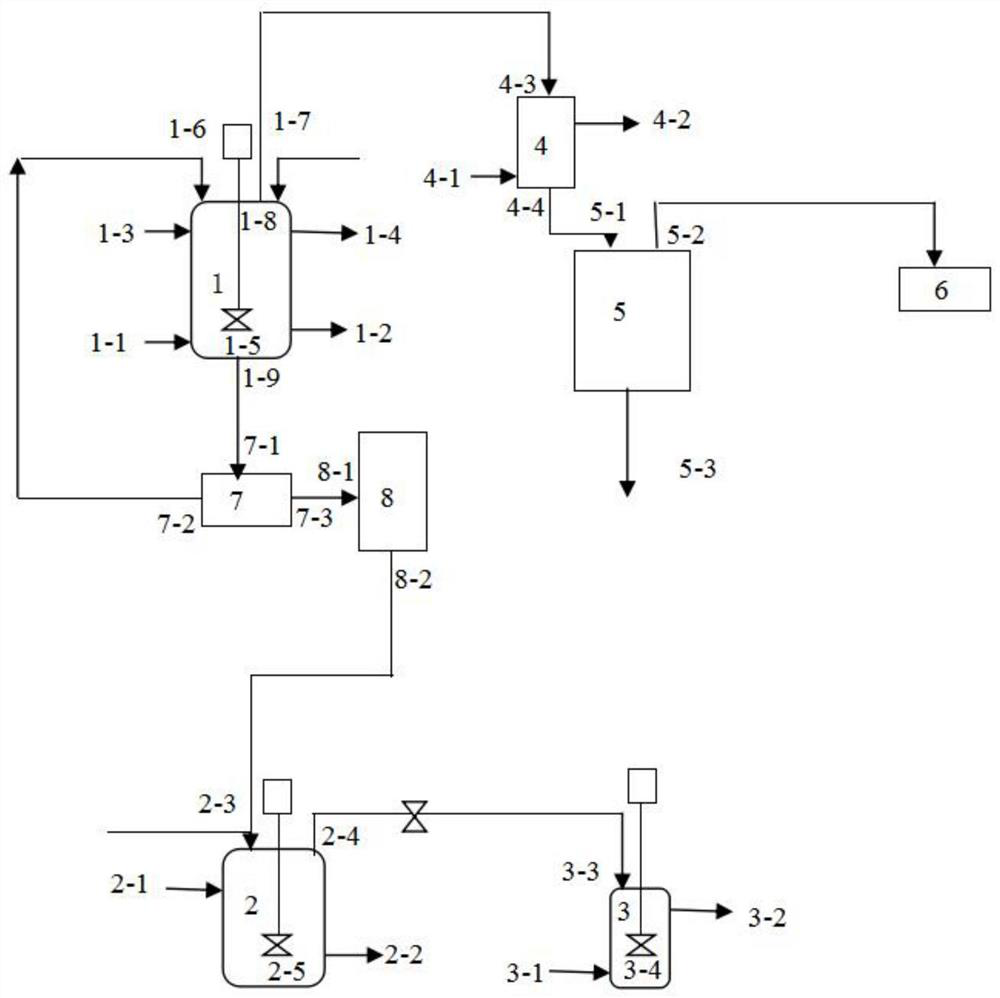
[0071] 100kg of waste water containing boron trifluoride (waste water produced in the process of preparing cefazolin) and 8.2kg of sodium hydroxide are dropped into the neutralization reaction tank 1 through the second inlet 1-7, and the agitator 1-5 is opened to carry out the acid reaction. Alkali neutralization reaction for 30min;
[0072] Inlet steam into the heating pipe inlet 1-3 provided on the outer surface of the neutralization reaction tank 1 to make the temperature of the feed liquid in the neutralization reaction tank 1 reach 80°C, close the first inlet 1-6, the second inlet 1-7 and The second outlet 1-9 feeds cooling circulating water into the cooling pipe inlet 4-1 on the outer surface of the condenser 4, opens the vacuum pump 6, and makes the vacuum degree in the neutralization reaction tank be-0.09MPa; carry out vacuum distillation, Stop the vacuum distillation when the quality of water removed from the feed liquid in the neutralization reaction tank 1 accounts ...
Embodiment 2
[0078] Recover boron trifluoride containing boron trifluoride waste water according to the method of embodiment 1, difference is, the mass ratio of boron trifluoride gas and acetonitrile is 1:6.8; The temperature of cooling crystallization is 25 ℃; Replacement reaction The temperature is 140°C.
Embodiment 3
[0080] Recover boron trifluoride containing boron trifluoride waste water according to the method of embodiment 1, the difference is that the mass ratio of boron trifluoride gas and acetonitrile is 1:6.2; the temperature of cooling crystallization is 20 ℃; replacement reaction The temperature is 160°C.
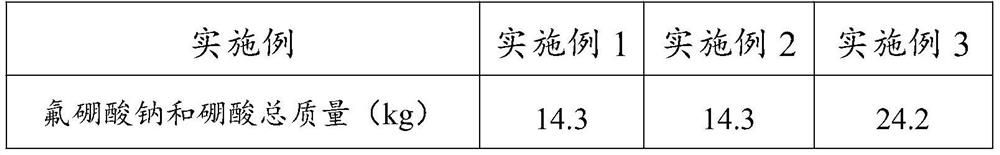
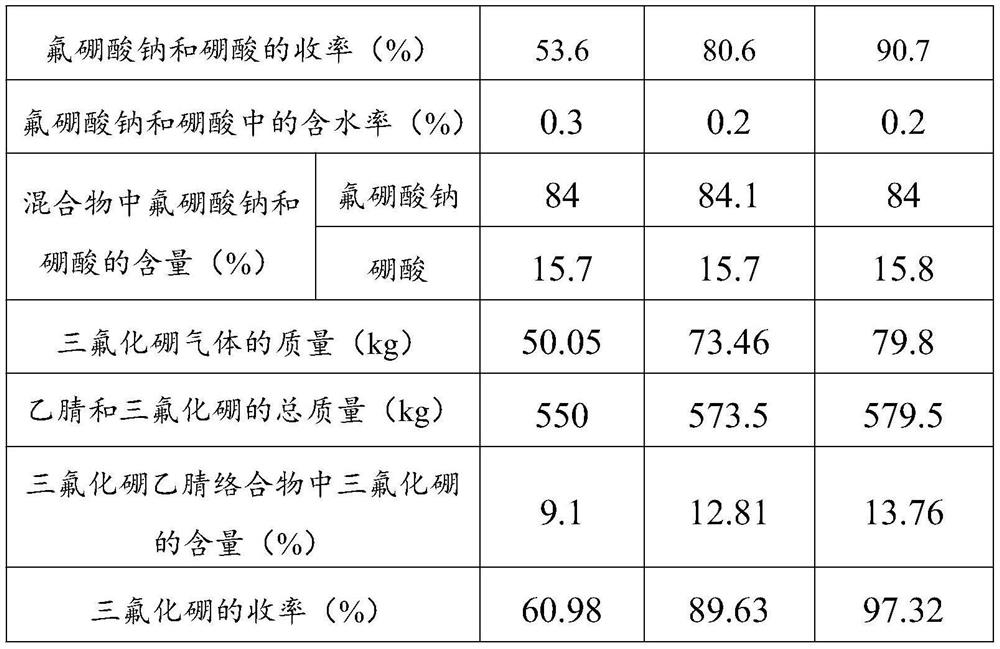
[0081] Weigh the sodium fluoroborate and boric acid total mass that embodiment 1~3 prepares, and its result is listed in table 1; Calculation embodiment 1~3 obtains the yield of sodium fluoroborate and boric acid, and its result is listed in table 1 Detect the water content of sodium fluoroborate and boric acid that embodiment 1~3 obtains according to Karl Fischer moisture detection method, its result is listed in table 1; According to GB / T22667-2008 and GB / T538-2006 detect embodiment 1~ 3 The mass percentages of sodium fluoroborate and boric acid in sodium fluoroborate and boric acid obtained, the results are listed in Table 1.
[0082] Weigh the quality of the boron trifluo...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com