Patents
Literature
134 results about "Dihydroxyanthraquinone" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
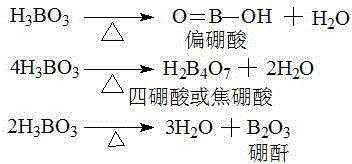
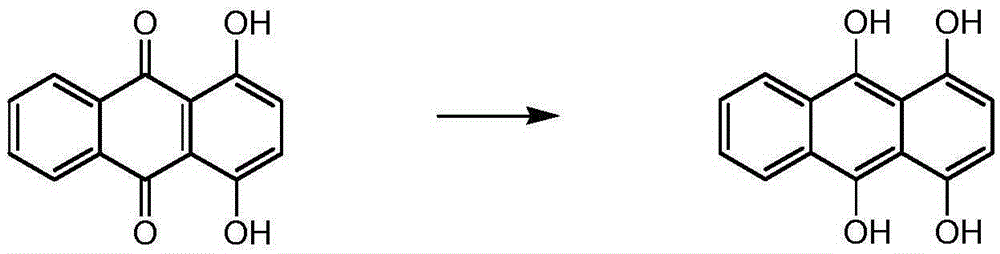
A dihydroxyanthraquinone is any of several isomeric organic compounds with formula C₁₄H₈O₄, formally derived from 9,10-anthraquinone by replacing two hydrogen atoms by hydroxyl groups. Dihyroxyantraquinones have been studied since the early 1900s, and include some compounds of historical and current importance. The isomers differ in the position of the hydroxyl groups, and of the carbonyl oxygens (=O) of the underlying anthraquinone.
Alizarin flow battery negative electrode electrolyte, and alizarin flow battery adopting electrolyte
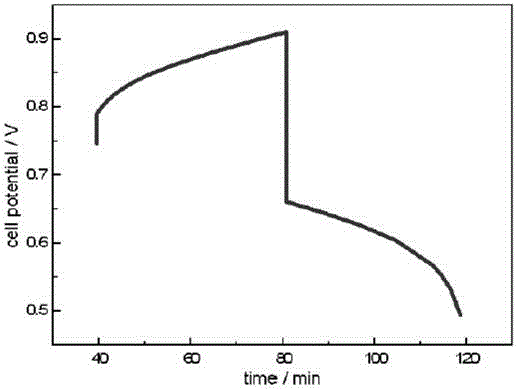
InactiveCN107171012ASolve the problem of electrolyte penetrationImprove cycle lifeRegenerative fuel cellsAlkaline electrolytesRare-earth elementPotassium hydroxide
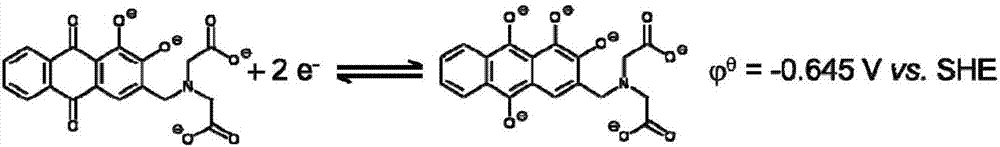
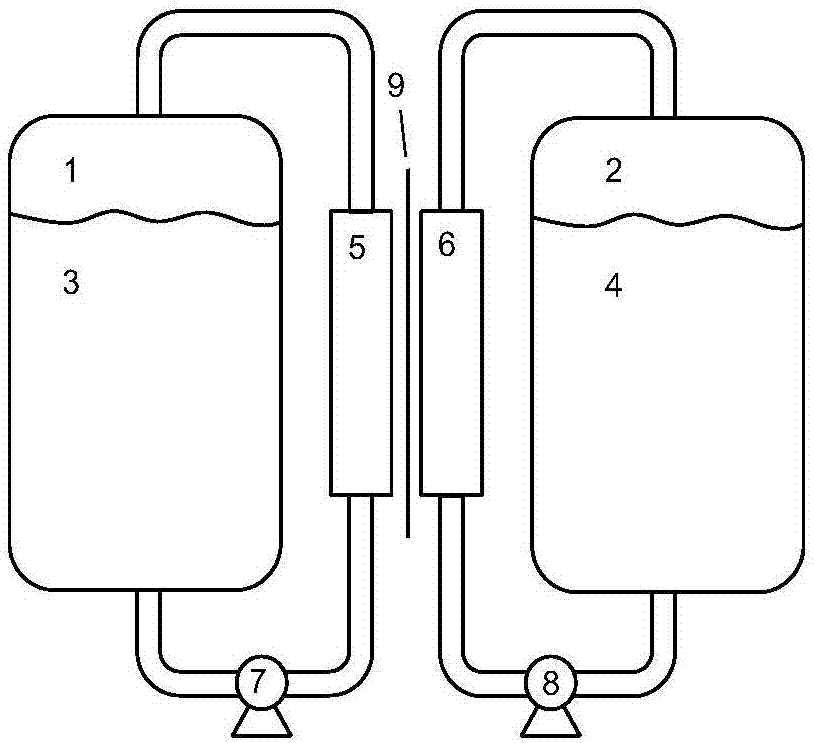
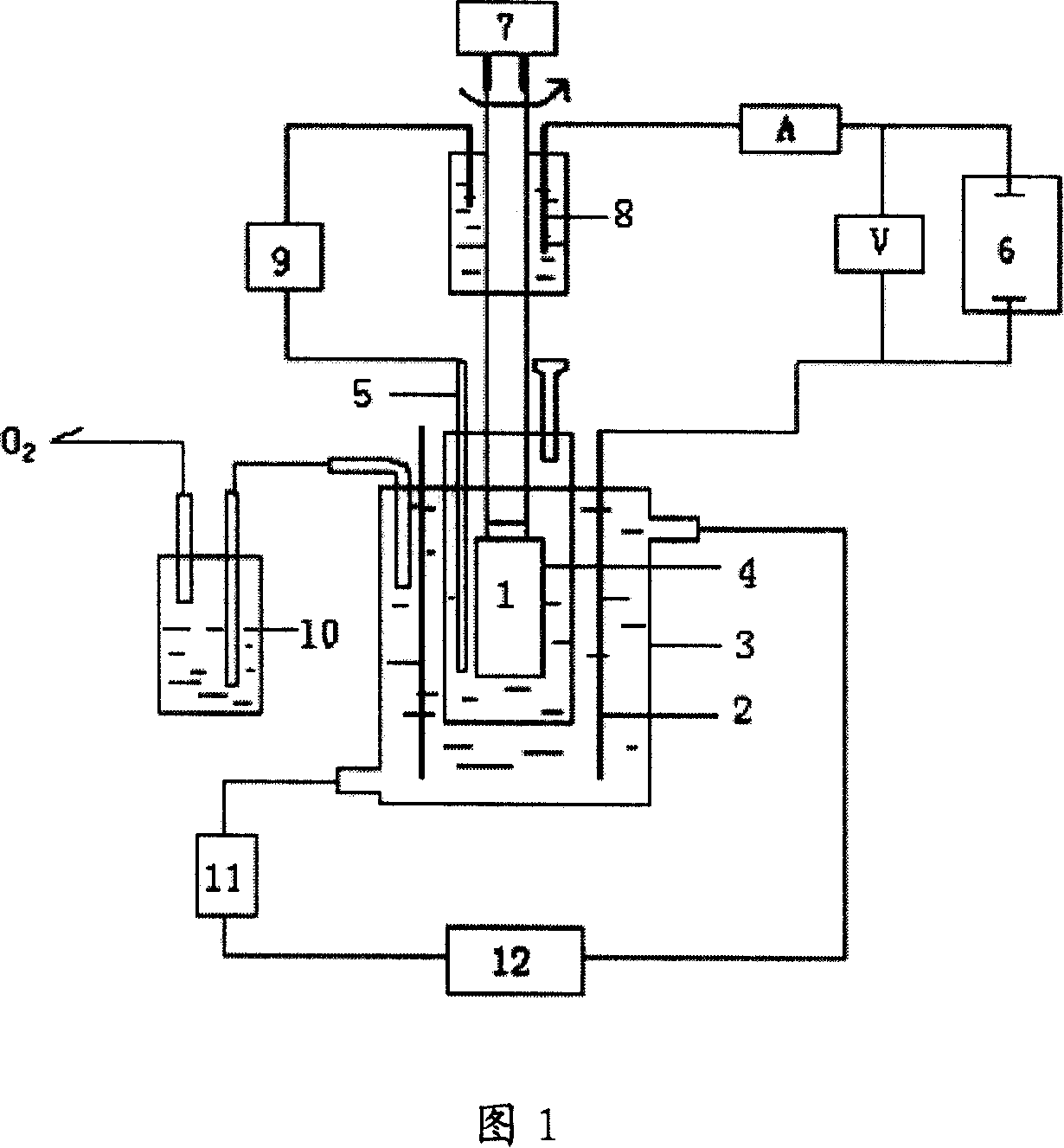
The invention provides an flow battery negative electrode electrolyte based on alizarin or derivatives thereof, and a flow battery adopting the electrolyte as a negative electrode. The electrolyte is an alkaline aqueous solution containing the alizarin or the derivatives thereof. The alizarin and the derivatives contain at least one of alizarin (1,2-dihydroxyanthraquinone), alizarin red (1,2-dihydroxyanthraquinone-3-sulfonic acid) or alizarin fluorin blue (3-alizarin methylamine-N,N-diacetic acid and mixtures thereof. An alkali used in the invention comprises at least one of sodium hydroxide, potassium hydroxide and a sodium hydroxide and potassium hydroxide mixture. The working temperature of the electrolyte is 10-50 DEG C; and the flow battery based on the electrolyte is a traditional structure, and comprises a positive electrode liquid storage tank 1, a positive electrode electrolyte 3, a negative electrode liquid storage tank, the negative electrode electrolyte 4, pumps 7 and 8, and a cation exchange membrane 9 for conducting cations. The active substance of the alizarin flow battery negative electrode electrolyte is bulk anions, and difficultly penetrates through a proton exchange membrane; and the electrolyte disclosed in the invention has the characteristics of no toxicity, no pollution, no rare earth elements, and obvious price advantage.
Owner:BEIHANG UNIV
Liquid crystal polycarbonates and methods of preparing same
ActiveUS20050192424A1Liquid crystal compositionsOrganic-compounds/hydrides/coordination-complexes catalystsHydroquinone CompoundDiol
Liquid crystal polycarbonates are made by forming a reaction mixture containing (a) an activated diaryl carbonate; (b) at least two species of aromatic diols selected from among resorcinol, 4,4′-biphenol, hydroquinone, methylhydroquinone, 4,4′-dihydroxyphenylether, dihydroxynaphthalene, including in particular the 2,6, 1,5, and 2,7 isomers, 4,4′-dihydroxybenzophenone and 2,6-dihydroxyanthraquinone (anthraflavic acid); and (c) optionally bisphenol A in a maximum amount of 10 mole %; and processing the reaction mixture in a melt transesterification reaction to form a liquid crystal polycarbonate. While the product composition has the same overall characteristics as compositions made using diphenyl carbonate as the donor moiety for the carbonate linkage, they are analytically distinguishable because of limited incorporation of intermediate or end-cap residues derived from the activated diaryl carbonate.
Owner:SHPP GLOBAL TECH BV +1
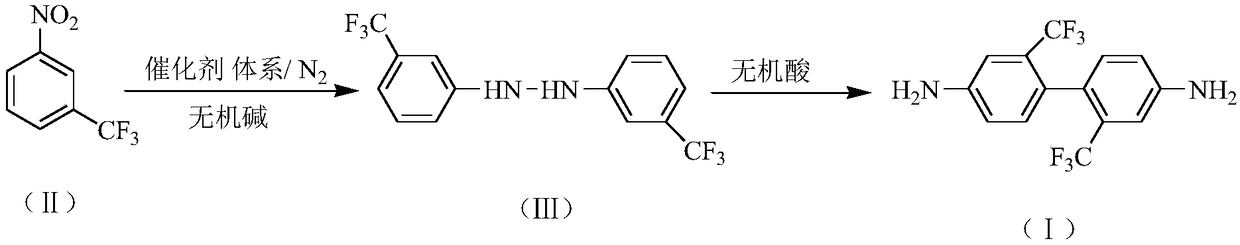
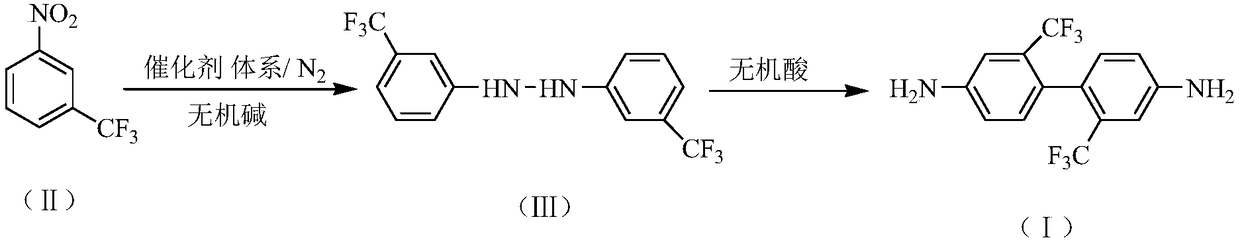
Preparation method of 2,2'-bis(trifluoromethyl)-4,4'-diaminodiphenyl
InactiveCN109232273AReduce generationHigh purityHydrazine preparationPreparation by rearrangement reactionsHydroxyanthraquinoneDodecylsulfonic acid
The invention relates to a preparation method of 2,2'-bis(trifluoromethyl)-4,4'-diaminodiphenyl. The preparation method comprises the following steps: synthesizing 3,3'-bis(trifluoromethyl)hydrazo-benzene in an inorganic alkaline aqueous solution by adopting nitrobenzotrifluoride as a raw material, adopting a phase transfer catalyst, a co-catalyst and Pd / C as a catalytic system and adopting aromatic hydrocarbon as a solvent, and performing the re-arrangement reaction on the 3,3'-bis(trifluoromethyl)hydrazo-benzene in an inorganic acid aqueous solution, thus obtaining 2,2'-bis(trifluoromethyl)-4,4'-diaminodiphenyl, wherein the phase transfer catalyst is one or a mixture of more of sodium dodecyl benzene sulfonate, sodium dodecyl sulfate and cetyl trimethyl ammonium bromide, and the co-catalyst is one or a mixture of more of 2,3-dichloro-1,4-naphthoquinone, 2-hydroxyanthraquinone and 2,6-dioxyanthraquinone. The preparation method has the advantages of mild reaction condition, simple process, high product quality and yield, low production cost, environmental friendliness, suitability for continuous production and the like.
Owner:烟台海川化学制品有限公司
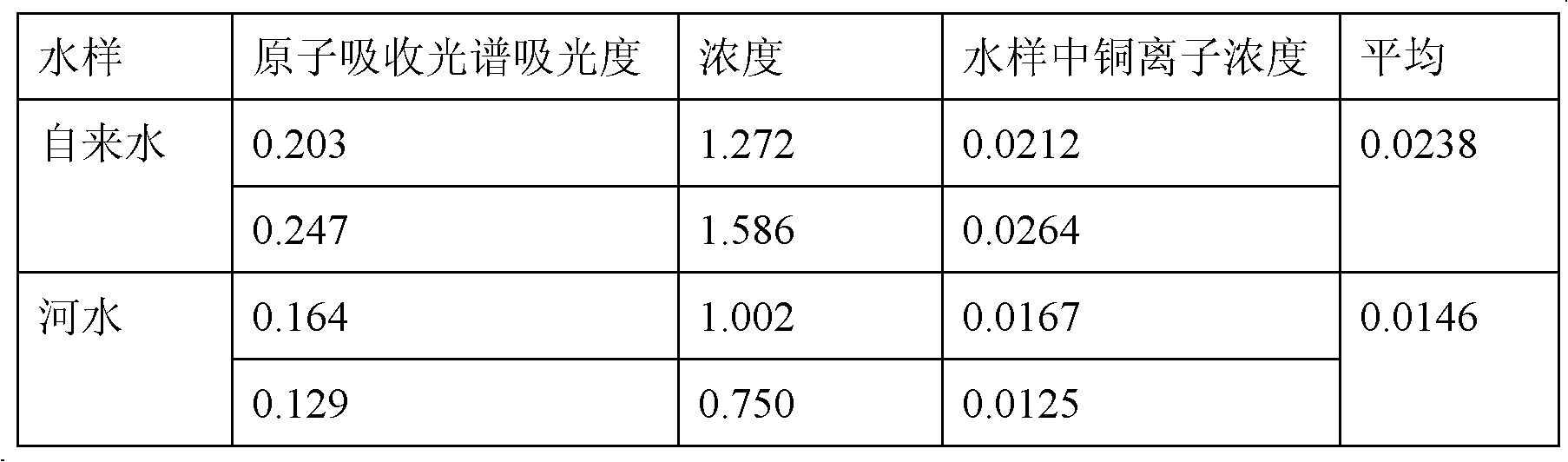
Ferroferric oxide nano-composite particle and preparation method and applications thereof
InactiveCN103241776AGood dispersionEfficient detectionFerroso-ferric oxidesNanotechnologySodium acetateN dimethylformamide
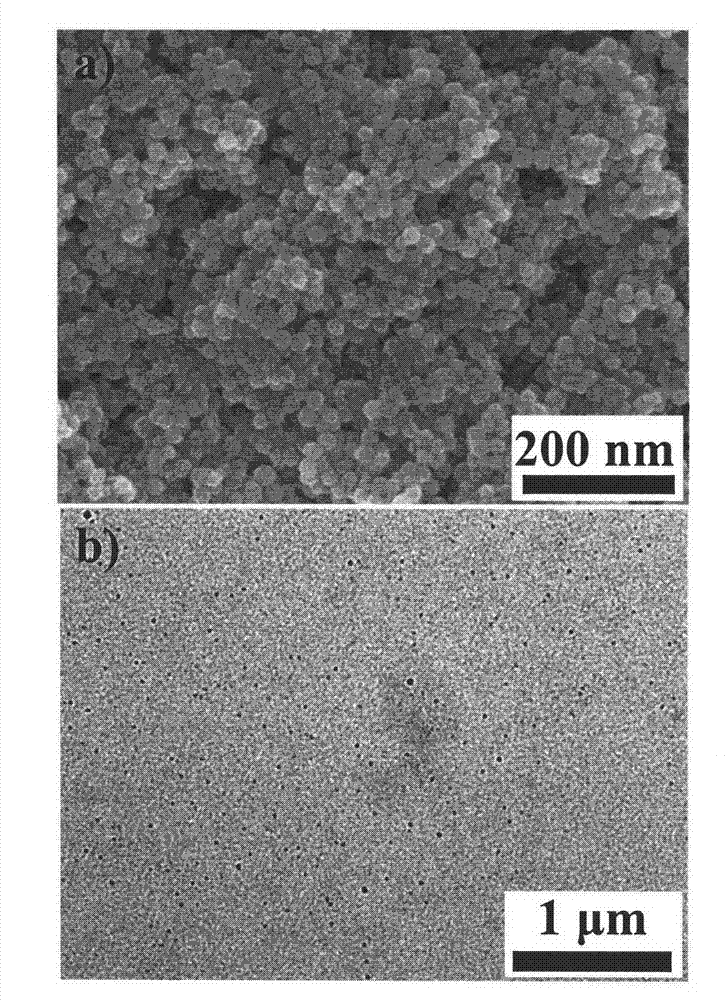
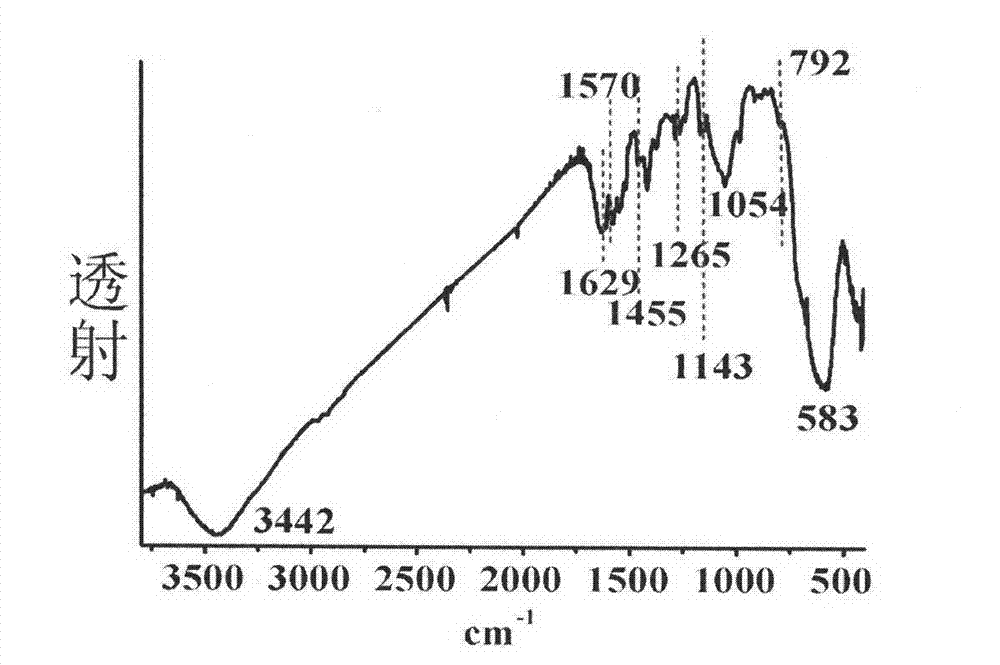
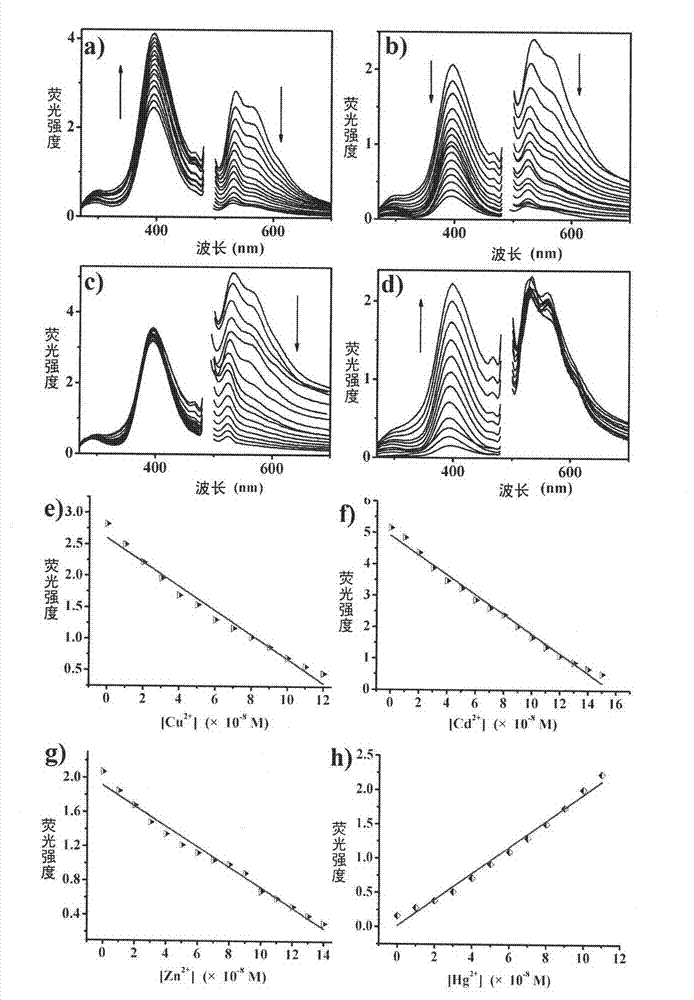
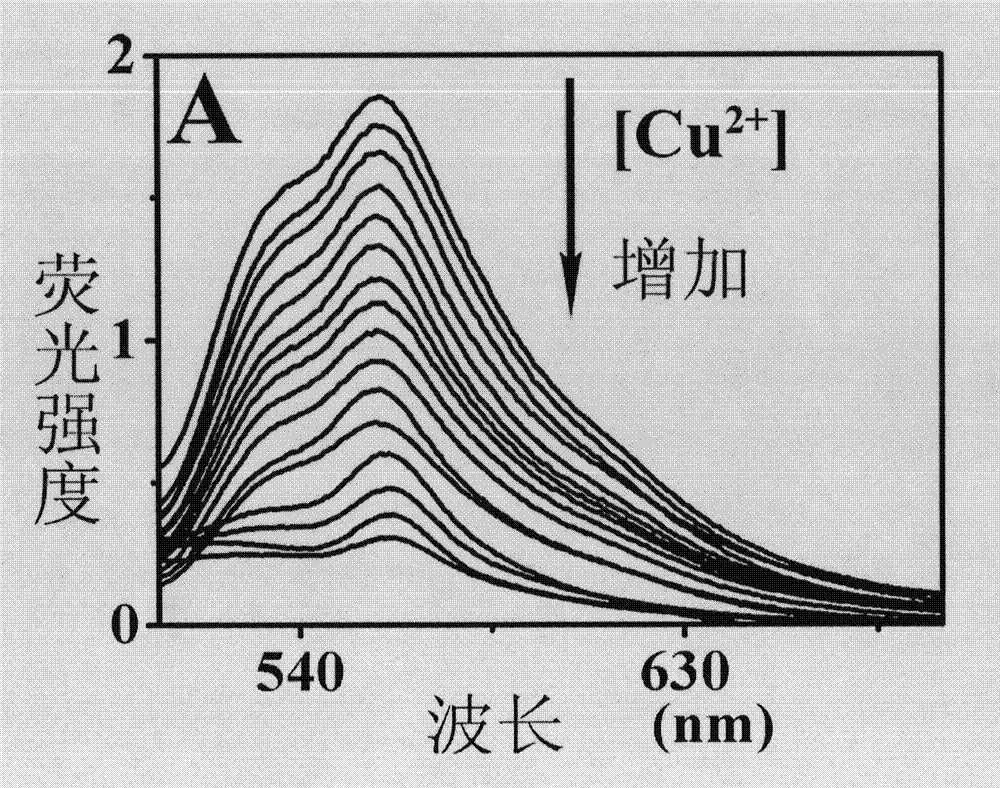
The invention discloses a ferroferric oxide nano-composite particle and a preparation method and applications thereof. The composite particle is a ferroferric oxide nano-composite particle with the particle size of 10-30nm, and 1,4-dihydroxy anthraquinone and fluorenylmethoxycarbonyl are modified on the surface of the ferroferric oxide nano-composite particle. The preparation method comprises the steps of: firstly, carrying out a hydrothermal method on ferric trichloride hexahydrate, sodium acetate, 1,6-hexanediamine and ethanediol to obtain the ferroferric oxide nano-composite particle, then producing 1,4-dihydroxy anthraquinone, chloroacetyl chloride, and N,N-dimethylformamide into dyes, then adding the ferroferric oxide nano-composite particle, sodium carbonate and the dyes into acetonitrile to carry out a reflux reaction, carrying out solid-liquid separation, washing and drying on the obtained reaction liquor to obtain an intermediate product, then dispersing the intermediate product into the N,N-dimethylformamide, adding dispersion liquid into the fluorenylmethoxycarbonyl, stirring for 2 hours, and carrying out solid-liquid separation, washing and drying to obtain a target product. The ferroferric oxide nano-composite particle can be used for fast detecting four heavy metal ions, namely copper, zinc, cadmium and mercury in an aqueous solution.
Owner:HEFEI INSTITUTES OF PHYSICAL SCIENCE - CHINESE ACAD OF SCI
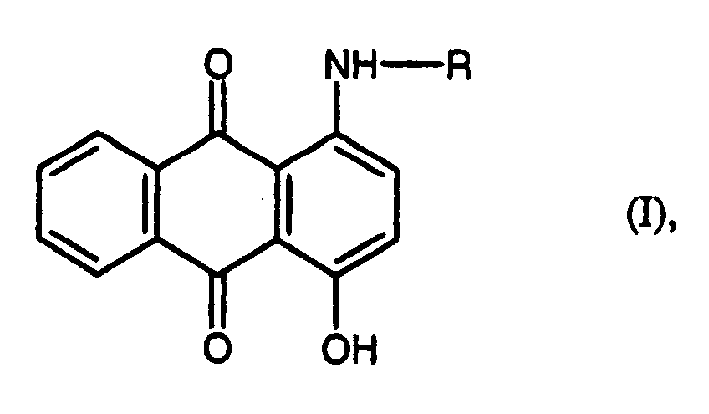
Preparation of 1-amino-4-hydroxy anthraquino
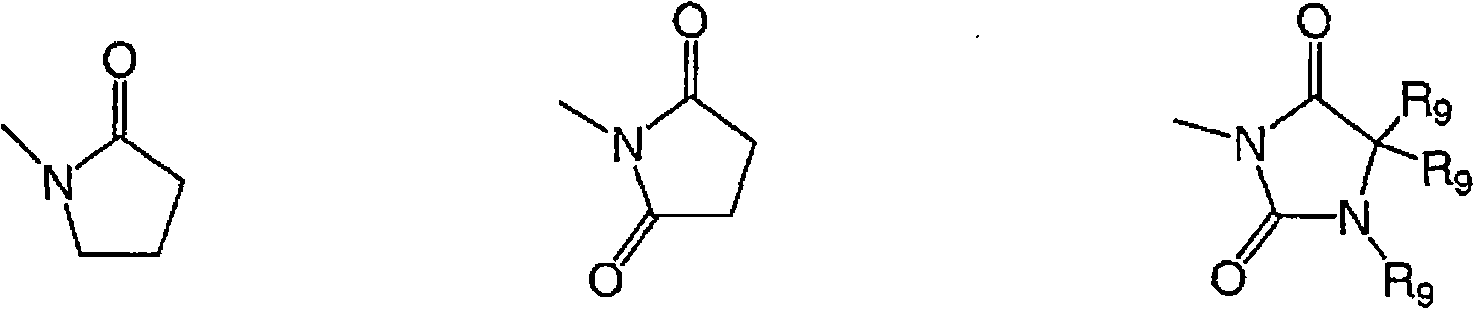
The present invention relates to a process for preparing 1-amino-4-hydroxyanthraquinones of the formula (I) wherein R is an aliphatic or aromatic radical, by reacting 1,4-dihydroxyanthraquinone, with aliphatic or aromatic amines in the presence of N-methyl-2-pyrrolidone.
Owner:BAYER AG
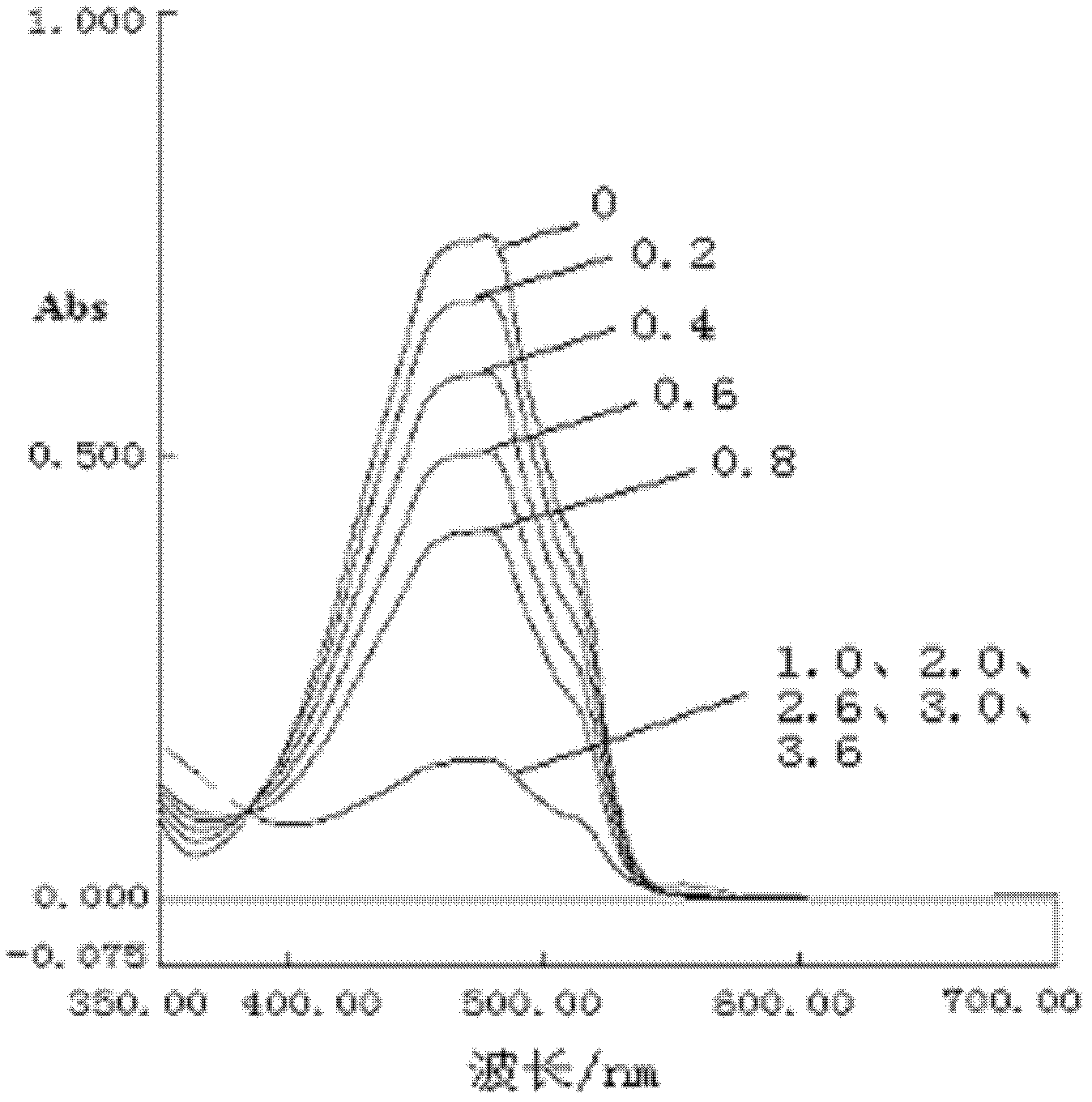
Copper ion imprinted polymer and application thereof
InactiveCN102659971AIncrease varietyExpand the scope ofOther chemical processesAlkali metal oxides/hydroxidesFunctional monomerPolymer science
The invention belongs to the field of analysis and detection of metal ions in water environment, and discloses a copper ion imprinted polymer, which comprises a polymer carrier and a functional monomer 1,4 - dihydroxy anthraquinone embedded in the polymer carrier, wherein the polymer carrier uses ethylene glycol dimethacrylate as a monomer. The preparation method comprises the following steps of: (1) mixing the 1,4 - dihydroxy anthraquinone with an organic solvent and a soluble copper salt, adding a crosslinking agent and an initiator agent, and reacting for 18 to 20hr at a temperature of 50 to 65 DEG C in the absence of oxygen; and (2) after washing, adding nitric acid to remove the copper ions. By a way that the functional monomer is embedded in a polymer network and a precipitation polymerization method, the imprinted polymer with selective adsorption for the copper ions is synthesized. The ion imprinted polymer has a specific recognition capability for the copper ions, and has better selectivity. Without connecting a double bond capable of being reacted with the crosslinking agent with the 1,4-dihydroxy anthraquinone in advance, the selection type and scope of the functional monomer is expanded, and the reaction steps are simplified.
Owner:SHANGHAI OCEAN UNIV
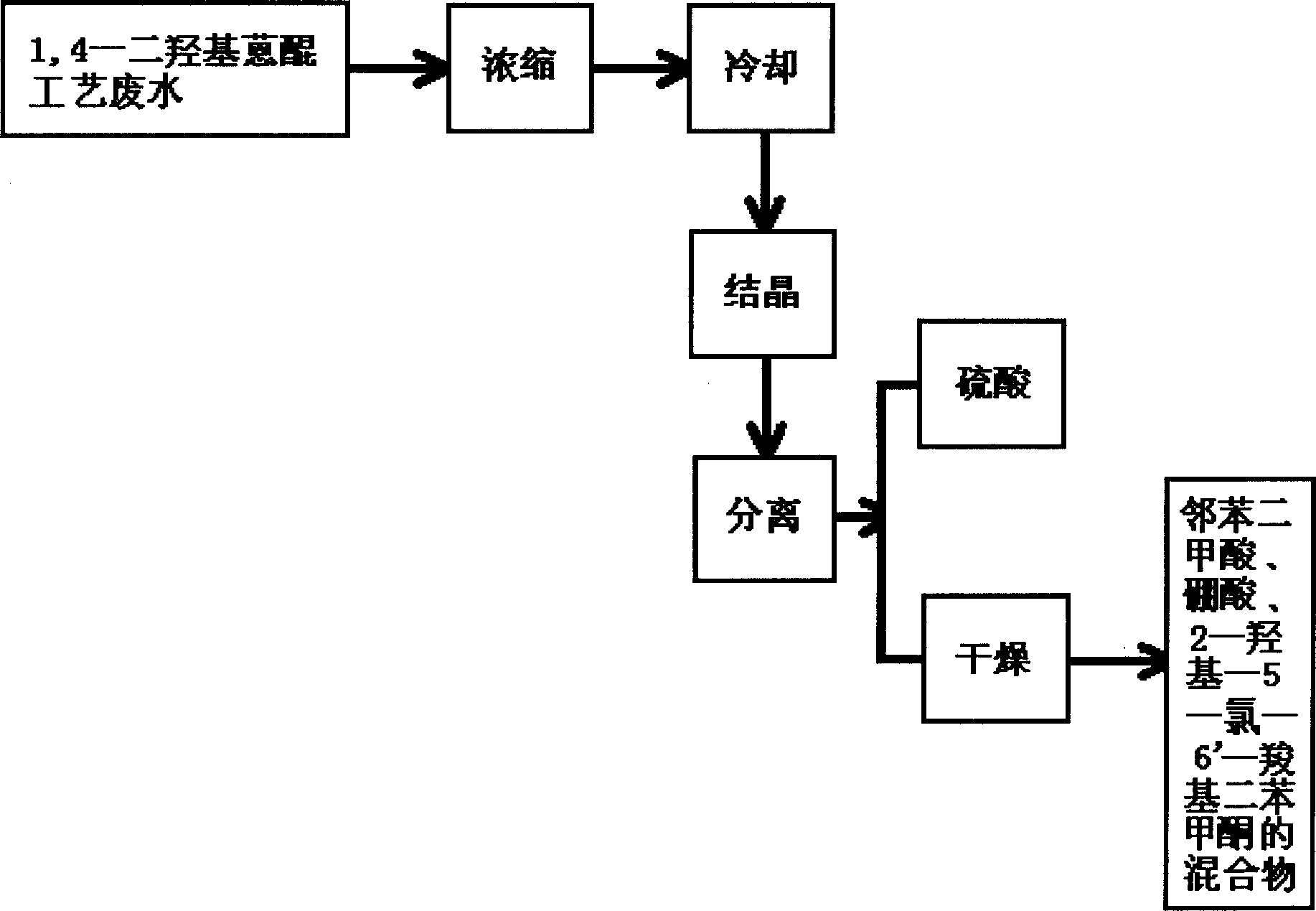
Recovery method for waste water of 1,4-dihydroxyanthraquinone production
The invention discloses a recovery method for waste water of 1,4-dihydroxyanthraquinone productionwhich consists of subjecting 1,4-dihydroxyanthraquinone processing waste water to condensation till sulfuric acid concentration is 20%-80%, cooling to 30-0 deg. C for crystallization, proceeding solid-liquid separation, the filtered body is 20-80% of sulfuric acid, finally drying the filter cakes.
Owner:湖北开元化工科技股份有限公司
Preparation method of light-resistant and waterproof water-based flame retardant polyurethane coating and adhesive
InactiveCN104194607ANon-macromolecular adhesive additivesOrganic-compounds/hydrides/coordination-complexes catalystsPolymer sciencePtru catalyst
The invention discloses a preparation method of light-resistant and waterproof water-based flame retardant polyurethane coating and adhesive. The preparation method of the light-resistant and waterproof water-based flame retardant polyurethane coating and adhesive comprises the following steps: mixing polytetrahydrofuran glycol with isophorone diisocyanate in the presence of a fullerene catalyst, reacting at the temperature of 60-85 DEG C for 1-2 hours, adding coumaphos and propantheline bromide, and reacting at the temperature of 70 DEG C for 3 hours, so that polyurethane prepolymer A is obtained; adding a chain extender, butanone and riboflavin sodium phosphate into the polyurethane prepolymer A, reacting at the temperature of 60-85 DEG C for 2.5-3.5 hours, adding a flame retardant, reacting at the temperature of 75-85 DEG C for 1-2 hours, then adding 1,4-dihydroxy anthraquinone, polyacrylamide and a tackifying agent, reacting at the temperature of 75 DEG C for 1-2 hours, then adding triethylamine and carrying out neutralization reaction for 30-50 minutes, adding water and emulsifying for 30 minutes, so that the light-resistant and waterproof water-based flame retardant polyurethane coating and adhesive are obtained. The prepared light-resistant and waterproof water-based flame retardant polyurethane coating has triple performance of flame retardance, light resistance and water resistance; meanwhile, polyurethane is environment-friendly and low in cost, so that the prepared light-resistant and waterproof water-based flame retardant polyurethane coating can be widely applied to surfaces of a wall body, furniture and metalware; the prepared light-resistant and waterproof water-based flame retardant polyurethane adhesive can be taken as a binder for plastic, glass, paper, textiles and leather.
Owner:慧融高科新型材料科技有限公司
Process for synthesizing anthraquinone compound
ActiveCN103319379AReduce dosageSave raw materialsOrganic compound preparationSulfonic acid preparationOrganic solventOrganic synthesis
The invention relates to the field of organic synthesis and in particular relates to a process for synthesizing an anthraquinone compound. The invention provides a synthesis process for condensing 1,4-dihydroxy anthraquinone and paratoluidine to obtain 1,4-diparatoluidine anthraquinone under the action of a catalyst and carrying out a SO3 sulfonation reaction on the 1,4-diparatoluidine anthraquinone to obtain 1,4-di(2-sulfo-4-methylanilino) anthraquinone under the action of an organic solvent. The conventional process is greatly improved, so that the raw materials are saved, the product quality is improved, the product cost is reduced, and more important, the content of wastewater and waste acid in industrial production is obviously reduced, and the wastes are easily treated and are environment-friendly.
Owner:江西开元生物医药科技有限公司
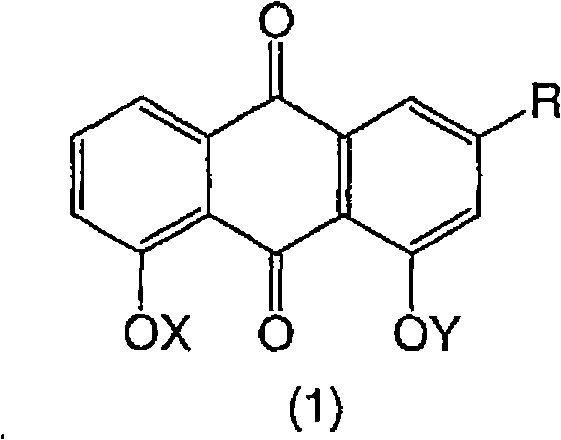
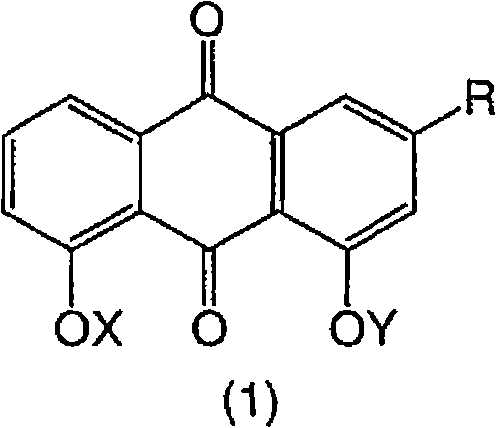
Dihydroxyanthraquinones and their use
A compound of formula (1), wherein X is H or -OCR1 and Y is H or -OCR2, provided that X and Y are not both H; and R is CH2OR9, CONR11R12, CN, tetrazole or COOR17; or a salt thereof, has therapeutic utility.
Owner:SOSEI R&D LTD
Electrolyte capable of improving hydroxy-anthraquinone solubility in anthraquinone redox flow battery and preparation method for electrolyte
InactiveCN107248585AImprove solubilityImprove charge and discharge performanceRegenerative fuel cellsChemical industrySolubility
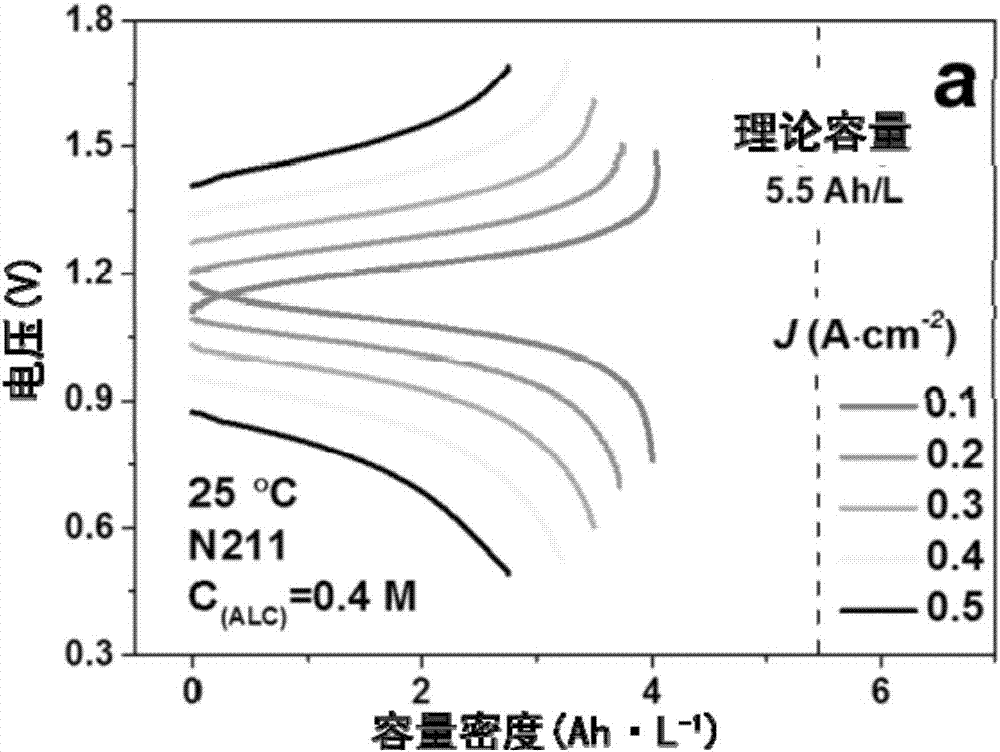
The invention discloses an electrolyte capable of improving hydroxy-anthraquinone solubility in an anthraquinone redox flow battery and a preparation method for the electrolyte, and belongs to the fields of energy and chemical industry. The electrolyte comprises deionized water, potassium hydroxide, hydroxy-anthraquinone and tetra alkyl ammonium halide, wherein the hydroxy-anthraquinone is selected from at least one kind of 1, 4-dyhydroxyl anthraquinone, 1, 8-dyhydroxyl anthraquinone, 2, 6-dyhydroxyl anthraquinone and 1, 4, 5, 8-tetrahydroxy anthraquinone; the tetra alkyl ammonium halide is preferably selected from tetraethyl ammonium chloride and tetraethyl ammonium bromide, wherein the adding mass percentage of the tetra alkyl ammonium halide is 0.1-20% of the total weight of the electrolyte. At the room temperature, hydroxy-anthraquinone and tetra alkyl ammonium halide are dissolved into potassium hydroxide at the mass ratio of 1-5 to 1, and steps of stirring and heating are performed to reach 60-90 DEG C to obtain the electrolyte. The hydroxy-anthraquinone is high in solubility in the electrolyte provided by the invention; and in addition, when the electrolyte is used for the anthraquinone redox flow battery, the battery obtains very excellent charging-discharging performance.
Owner:DALIAN UNIV OF TECH
Novel process for producing 1,4-dihydroxy anthraquinone
InactiveCN103664567AHigh yieldReduce manufacturing costOrganic compound preparationHydroxy-anthraquinone dyesSolventHydrolysis
The invention discloses a novel process for producing 1,4-dihydroxy anthraquinone, and relates to the technical field of chemical production. The technological process is as follows: firstly, co-heating and dehydrating fuming sulphuric acid and boric acid for 30-60 minutes, adding phthalic anhydride, heating up to 130 DEG C, adding parachlorophenol into the reaction system for multiple times in batches, heating up to 200-205 DEG C after adding the parachlorophenol, continuously reacting for 5-10 hours, slowly putting a reactant in 8 times by volume of water for hydrolysis after the reaction is finished, controlling the hydrolysis temperature at 100-130 DEG C, extracting the product to an organic phase by use of a solvent after hydrolysis, discharging the lower-layer waste acid, washing the upper-layer extracting solution by use of hot water, distilling the solvent for cycle use, and carrying out high-vacuum distillation and sublimation to obtain high-quality 1,4-dihydroxy anthraquinone. The novel process disclosed by the invention is used for improving the product yield and reducing the production cost by regulating process conditions such as raw material feeding ratio, feeding method, feeding temperature and the like on the premise of ensuring the product yield and the product quality.
Owner:PENGZE XINGDA CHEM
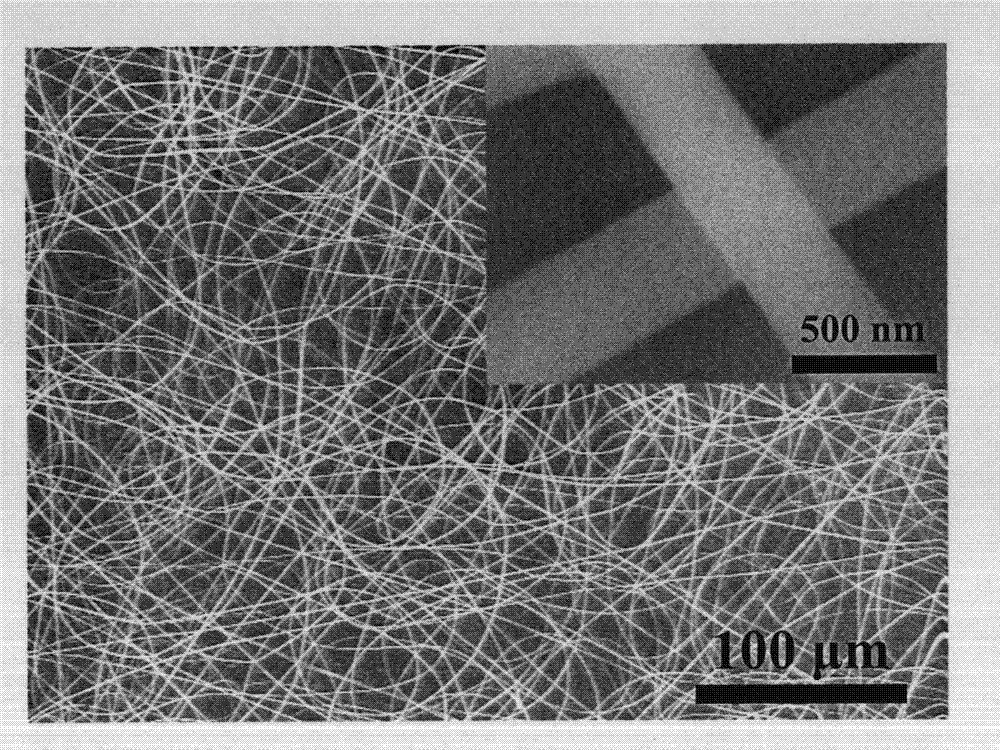
Porous membrane mixed by 1,4-dihydroxy anthraquinone and cellulose, preparation method and usage
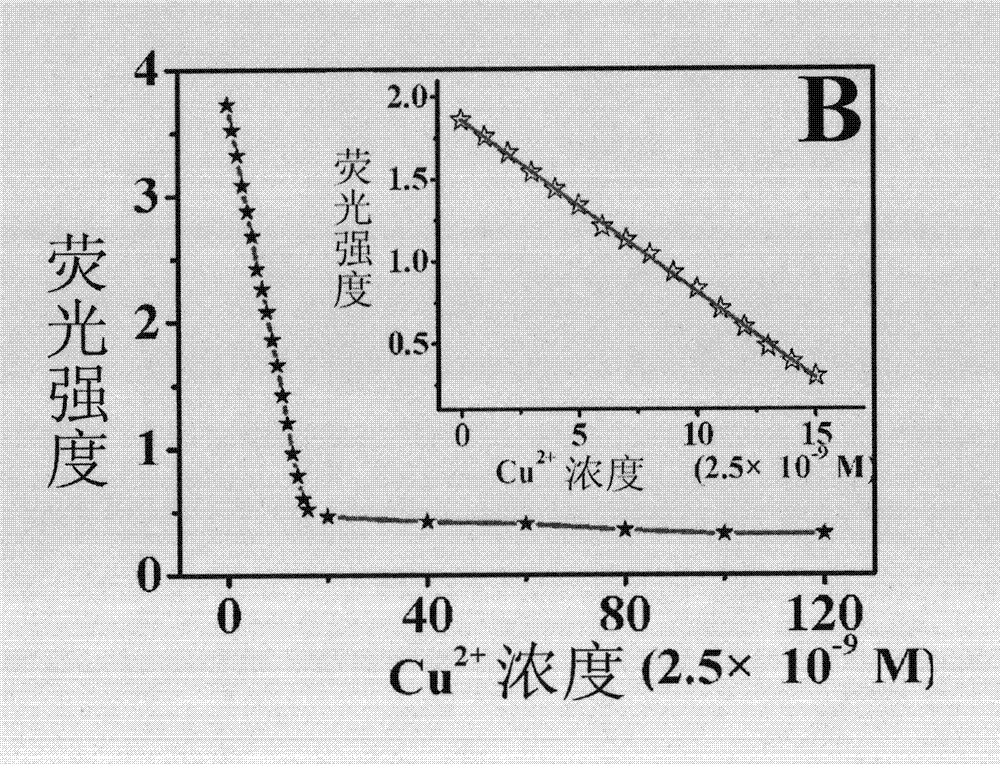
InactiveCN103088554AEfficient detectionGood choiceFibre treatmentFluorescence/phosphorescenceFiberFluorescence
The invention discloses a porous membrane mixed by 1,4-dihydroxy anthraquinone and cellulose, preparation method and usage. The porous membrane is in a porous mesh type structure composed of nanofibers, and the surfaces of the nanofibers are fully distributed with micropores, the diameter of the micropores are 300-500nm, and the nanofibers are composed of cellulose with a 75-85:1 mass ratio and the 1,4-dihydroxy anthraquinone. The method is firstly mixing cellulose acetate, the 1,4-dihydroxy anthraquinone and solvent, wherein the solvent is acetone and deionized water mixed solution; obtaining spinning solution; then placing the spinning solution on an electrostatic spinning machine to perform electrostatic spinning for at least 3min to obtain porous membrane composed of cellulose acetate nanofibers mixed with the 1,4-dihydroxy anthraquinone; following by placing the porous membrane composed of the cellulose acetate nanofibers mixed with the 1,4-dihydroxy anthraquinone into sodium hydroxide solution to soak for at least 24h; and obtaining the porous membrane composed of the cellulose acetate nanofibers mixed with the 1,4-dihydroxy anthraquinone. The porous membrane can be used as fluorescent sensing materials during detections which adopt a fluorescence analytical method to perform fast detection on trace bivalent copper ion.
Owner:HEFEI INSTITUTES OF PHYSICAL SCIENCE - CHINESE ACAD OF SCI
Method for preparing solvent blue 78
InactiveCN104725251AHigh yieldReduce moisture contentOrganic chemistryOrganic compound preparationWastewaterRoom temperature
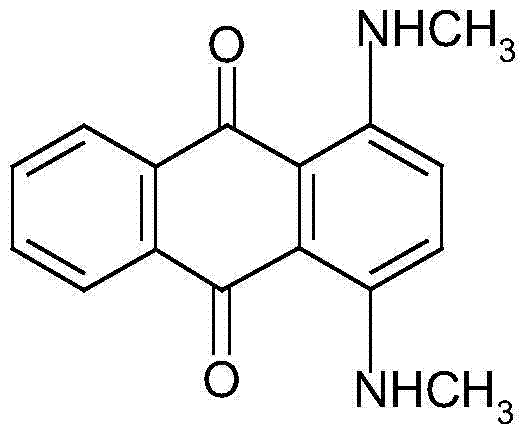
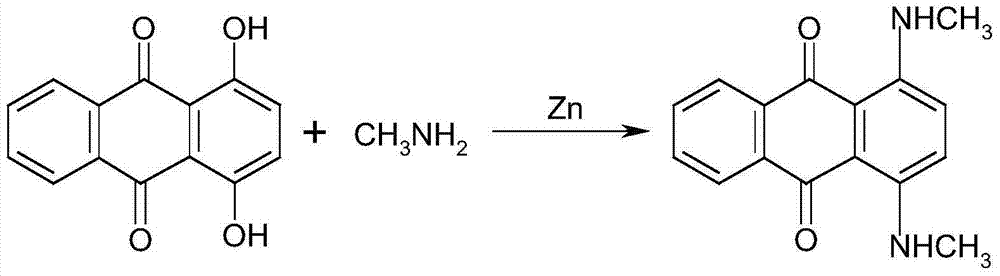
The invention discloses a method for preparing solvent blue 78. The method comprises the following steps: adding liquid caustic soda and 1,4-dihydroxyanthraquinone into a reactor, uniformly stirring, adding sodium hydrosulfite and monomethyl amine, sealing the reactor, heating, carrying out heat insulation for several hours, cooling to normal temperature after the reaction ends, filtering, washing, and drying to obtain a finished product. Compared with the prior art, the method has the following advantages: 1, the yield increases by near 20%; 2, the water content is small, easy drying is realized, and the drying time of 1t of a material is shortened to 12h from original 2d, so energy consumption is greatly reduced; and 3, environmental protection, energy saving and great reduction of wastewater are realized.
Owner:JIANGSU DAOBO CHEM
Method for preparing solvent blue 35
ActiveCN104725252AQuick responseHigh yieldOrganic chemistryOrganic compound preparationSolventN-Butylamine
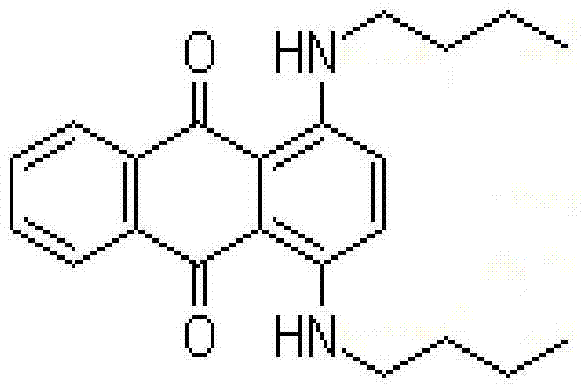
The invention discloses a method for preparing solvent blue 35. The method comprises the following steps: adding ethanol, anhydrous sodium sulfate, 1,4-dihydroxyanthraquinone, 1,4-dihydroxyanthraquinone leuco, acetic acid and n-butylamine into a reactor, closing the reactor, heating to a refluxing temperature, reacting under a pressure of 0.08Mpa or less for 1-4h, distilling off an ethanol and n-butylamine mixed solvent, adding alkaline water to realize separation, filtering, washing, drying, and discharging. In the invention, acetic acid is used as a catalyst, anhydrous sodium sulfate is used as a water absorbing agent, and 1,4-dihydroxyanthraquinone is mixed with the 1,4-dihydroxyanthraquinone leuco in reasonable proportion, so the reaction speed is improved, and the yield is improved; and ethanol is adopted as a solvent to reduce the generation of tar, and a recovered ethanol and n-butylamine mixed solution can be directly used after dehydration in order to reduce the generation of wastewater.
Owner:JIANGSU DAOBO CHEM
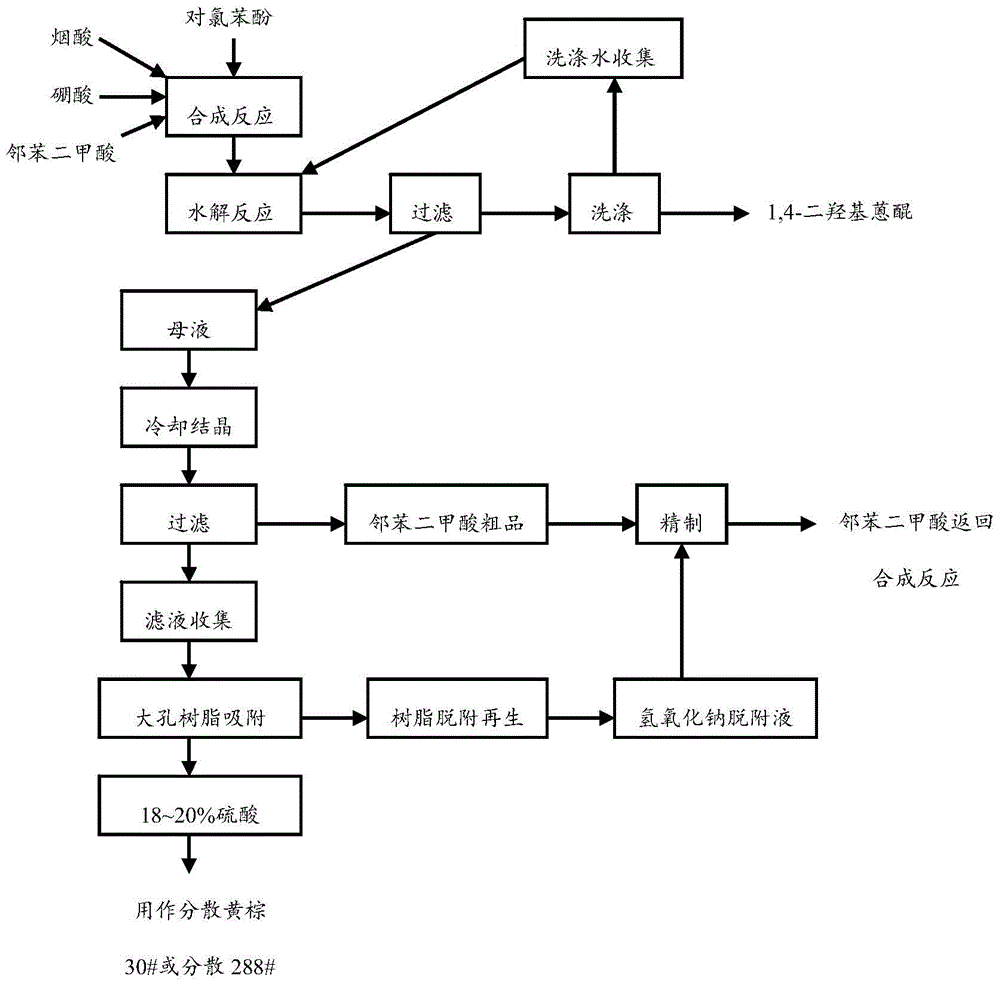
Clean production technique of 1,4-dihydroxy anthraquinone
InactiveCN104557504AAvoid pollutionSave resourcesOrganic compound preparationQuinone separation/purificationDesorptionHydrolysis
The invention discloses a clean production technique of 1,4-dihydroxy anthraquinone, which comprises the following steps: phthalic acid is used as a raw material, synthesis technique washing water is directly used as diluting hydrolysis bottom water for the next batch, a mother solution wastewater is cooled, crystallized, separated and subjected to resin adsorption to recover sulfuric acid, the separated phthalic acid crude product and the resin desorption solution are together refined to recover the phthalic acid, and low-temperature drying is performed to directly reuse the phthalic acid into the product synthesis process. The technique can effectively treat wastewater generated in the 1,4-dihydroxy anthraquinone production process and recover the organic chemical raw materials phthalic acid and sulfuric acid in the wastewater, thereby implementing unification of the wastewater treatment and resource recovery and implementing clean production.
Owner:ZHEJIANG RUNTU INST
Electrolytic synthesis method for 1,5-diamino-4,8-dihydroxyanthraquinone by one-step method
InactiveCN101054681AShort process routeHigh selectivityElectrolysis componentsElectrolytic organic productionElectrolysisSynthesis methods
The present invention relates to a one-step method for electrolytic synthesis of 1,5-diamino-4,8-dihydroxyanthraquinone. Principally, according to the method, by adopting a cathode rotation cellular-type electrolytic tank, adopting sulfuric acid of 7.5-14.2 mol / L as electrolysing solution, adopting 1,5-dinitroanthraquinone as raw material, adopting a phase transfer catalyst, controlling the temperature at 100-160 DEG C, controlling the cathode electric potential of -0.1--0.4 V, controlling the electric current density at 200-2000A, an electroanalysis is performed to prepare said 1,5-diamino-4,8-dihydroxyanthraquinone final product, with one one step. Said phase transfer catalyst is SnCl2, BiCl3, cetyl trimethyl ammonium bromide or octadecyl trimethyl ammonium bromide. Said electrolytic synthesis method in accordance with the present invention is characterized by its short process route, high selectivity, low cost of manufacture, few environment pollution, high total outcome yield, and the like. Especially, the method is capable of one-step preparing 1,5-diamino-4,8-dihydroxyanthraquinone product. At the same time, said method can be extensively used for electrolytic synthesis of other aromatics aminophenol compounds.
Owner:ZHEJIANG UNIV OF TECH
Quinone compound modified activated carbon particle electrode and preparation method and application thereof
ActiveCN106396094AImprove valence conversion efficiencyAccelerated denitrificationTreatment by combined electrochemical biological processesTreatment with anaerobic digestion processesActivated carbonAdhesive
The invention discloses a quinone compound modified activated carbon particle electrode and a preparation method and application thereof and belongs to the technical field of environment engineering. The technical scheme includes that the quinone compound modified activated carbon particle electrode comprises, by weight, 20-30 parts of activated carbon powder and 1-3 parts of 1, 4-dihydroxyanthraquinone loaded on the surface of activated carbon. The preparation method includes: 1, weighing the 20-30 parts of activated carbon powder and the 1-3 parts of 1, 4-dihydroxyanthraquinone powder, and mixing well to obtain mixed powder; 2, adding an adhesive and a curing agent into the mixed powder, and stirring well to obtain mixed paste; 3, granulating and drying the mixed paste to obtain the quinone compound modified activated carbon particle electrode. The quinone compound modified activated carbon particle electrode and the preparation method and the application thereof have the advantages that a quinone compound does not shed, so that secondary pollution of treated water is avoided; the particle electrode has good catalytic effect on electrode biomembrane denitrification; the particle electrode is suitable for industrial production and popularization and application.
Owner:ZHEJIANG UNIVERSITY OF SCIENCE AND TECHNOLOGY
Porous membrane composed of cellulose doped with 1,4-dihydroxy anthraquinone and bivalent copper ion and preparation method and application thereof
InactiveCN103084073AGood choiceEfficient detectionSemi-permeable membranesFluorescence/phosphorescenceFiberCellulose acetate
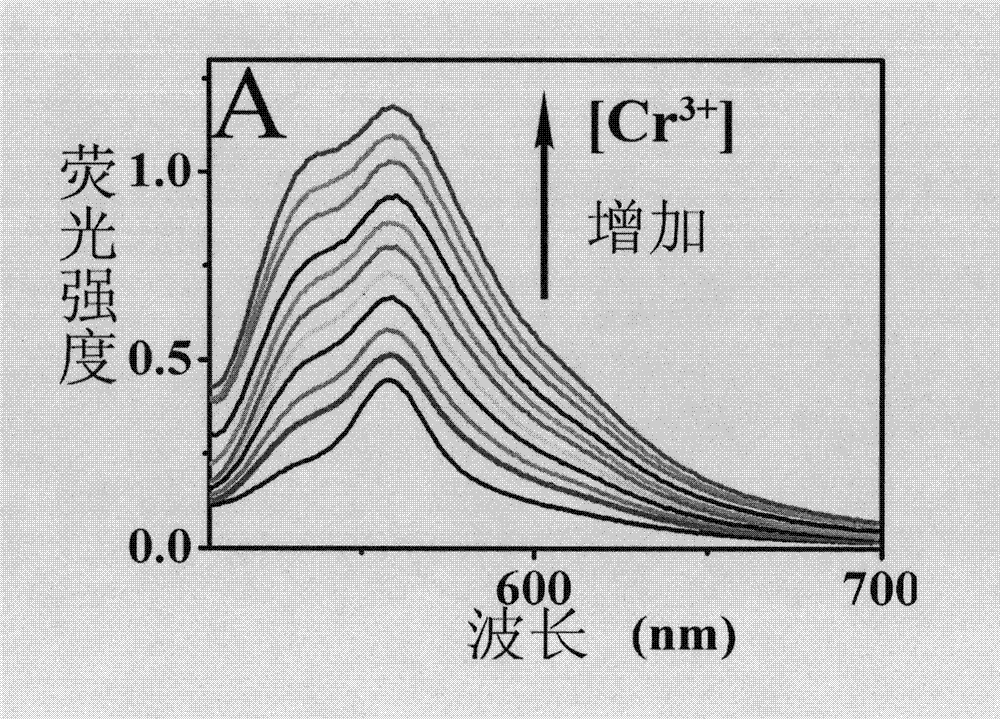
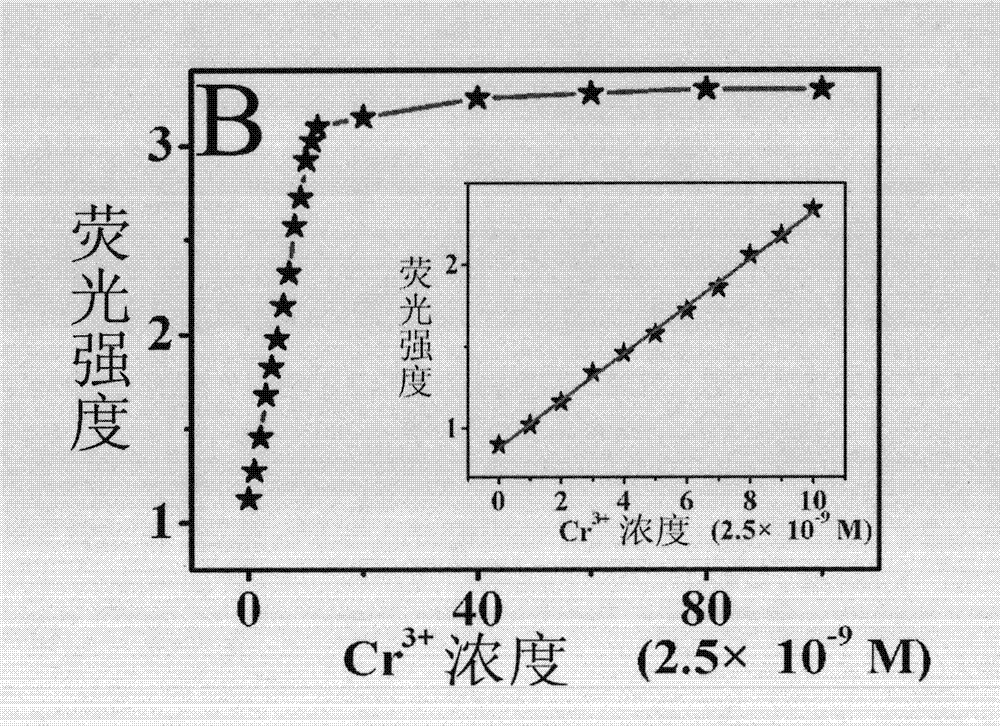
The present invention discloses porous membrane composed of cellulose doped with 1,4-dihydroxy anthraquinone and bivalent copper ions and a preparation method and application thereof. The porous membrane has a porous net structure composed of nano-fibers with a diameter of 300-500 nm and with surface covered with micropores. The nanofiber is composed of cellulose, 1,4-dihydroxy anthraquinone and divalent copper ions with a mass ratio of 75-85:1:1.8-2.2. The preparation method includes: mixing cellulose acetate, 1,4-dihydroxy anthraquinone, copper nitrate hydrate, and a solvent to obtain a spinning solution, placing the spinning solution on an electrostatic spinning machine for electrostatic spinning to obtain the porous membrane composed of cellulose doped with 1,4-dihydroxy anthraquinone and bivalent copper ions, soaking the porous membrane composed of cellulose doped with 1,4-dihydroxy anthraquinone and bivalent copper ions in a sodium hydroxide solution to get the target product. The porous membrane is used as a fluorescence sensing material for trace rapid detection of trivalent chromium ions, when the fluorescence analysis method is used for detection.
Owner:HEFEI INSTITUTES OF PHYSICAL SCIENCE - CHINESE ACAD OF SCI
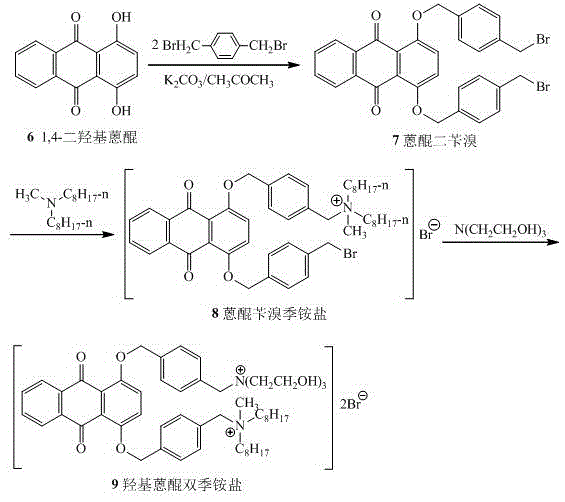
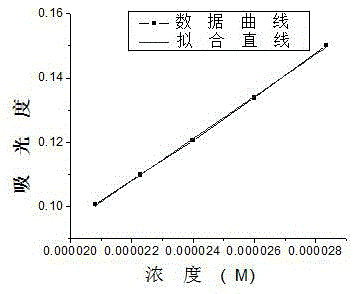
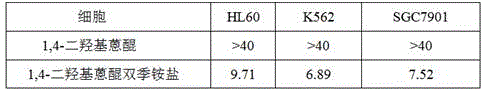
1,4-dihydroxy anthraquinone bisbenzyl quaternary ammonium salt having water solubility and anticancer activity
InactiveCN105523948AGood inhibitory effectEasy to transportOrganic compound preparationQuinone preparationSolubilityBenzoyl bromide
The invention discloses 1,4-dihydroxy anthraquinone bisbenzyl quaternary ammonium salt having water solubility and anticancer activity and preparation thereof. Particularly, the preparation comprises the steps: 1,4-dihydroxy anthraquinone is subjected to a reaction with excessive p-dibenzyl bromide to obtain anthraquinone dibenzyl bromide; the anthraquinone dibenzyl bromide is subjected to a reaction with N-methyldi-n-octylamine to obtain anthraquinone benzyl bromide quaternary ammonium salt; then the anthraquinone benzyl bromide quaternary ammonium salt is subjected to a reaction with triethanolamine to obtain the corresponding hydroxy anthraquinone bisquaternary ammonium salt. The anthraquinone bisquaternary ammonium salt has relatively good inhibition ability to a variety of cancer cells while maintaining water solubility. The compound which has both water solubility and anticancer activity is expected to be developed as an anticancer drug and has good application prospects.
Owner:FUZHOU UNIV
Method for preparing 1,4-dihydroxy anthraquinone
InactiveCN104926636AImprove catalytic performanceReduce dosageOrganic compound preparationQuinone preparationHydroxyanthraquinonePtru catalyst
The invention discloses a method for preparing 1,4-dihydroxy anthraquinone by taking a boric acid dehydration compound as a catalyst. The method comprises the following steps: (1) heating boric acid at 100-250 DEG C to prepare the boric acid dehydration compound; (2) adding fuming sulphuric acid, phthalic anhydride, the boric acid dehydration compound and parachlorophenol into a condensation kettle in sequence, and carrying out condensation reaction at 100-250 DEG C; (3) adding a proper amount of water and the materials in the condensation kettle into a hydrolysis kettle, carrying out hydrolysis reaction at 50-115 DEG C, discharging a reactant, filtering the reactant by pressing, washing and drying the reactant to prepare 1,4-dihydroxy anthraquinone. According to the method, the boric acid dehydration compound is used as the catalyst, so that the addition amount of the raw materials including fuming sulphuric acid and phthalic anhydride is reduced; the resource consumption can be reduced; the cost is reduced; the waste emission is reduced; the method is relatively environmentally-friendly and economical.
Owner:JIANGSU YABANG DYE
Synthesis method of solvent green 3
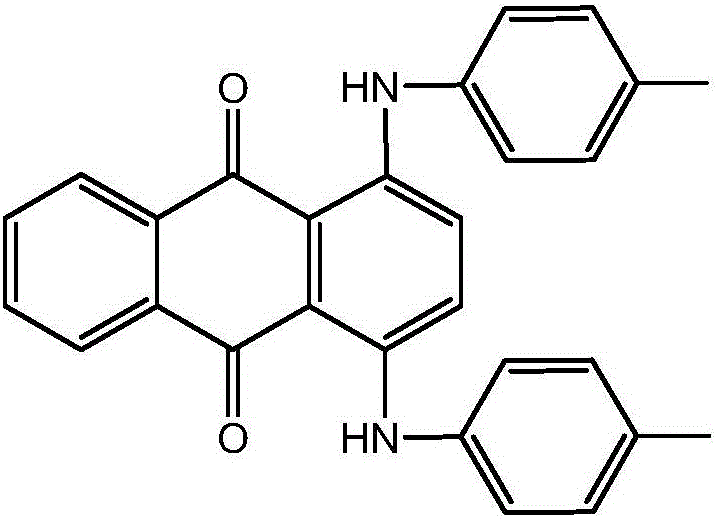
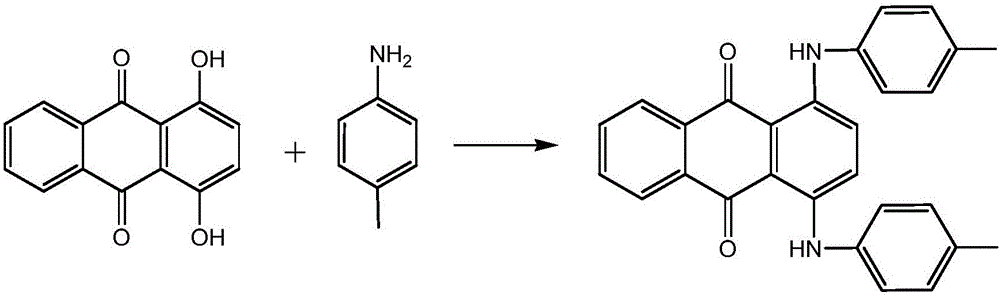
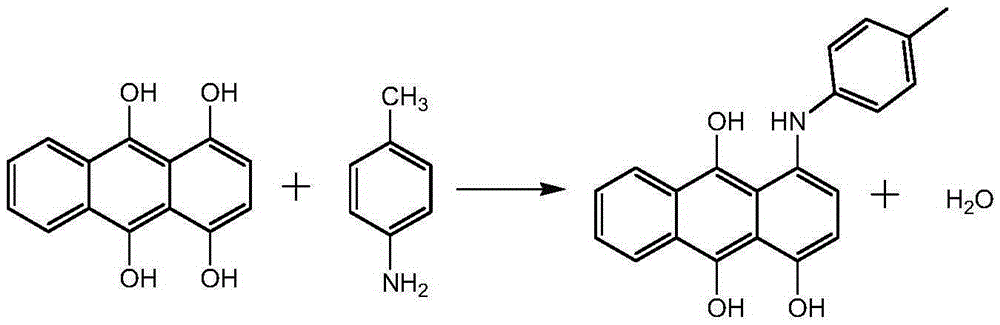
ActiveCN106675080AReduction of recycling energy consumptionHigh yieldAnthracene dyesSynthesis methodsAntioxidant
The invention discloses a synthesis method of a solvent green 3. According to the synthesis method, a target product is obtained by taking 1,4-dihydroxy anthraquinone and p-toluidine as raw materials, ethanol as a solvent, boric acid as a catalyst, a 1,4-dihydroxy anthraquinone leuco body as an initiator, sodium hydrogensulfite as an antioxidant and benzene as an azeotrope agent of a system through condensation reaction. According to the synthesis method, the ethanol with a low boiling point is used as the solvent and the solvent green 3 is hardly dissolved in the ethanol, so that the yield of a product is improved and the recycling energy consumption of the solvent is also reduced; the 1,4-dihydroxy anthraquinone leuco body is used as the initiator, has the effect of a reaction initiator and also has a catalytic action in a reaction process; the activity of the 1,4-dihydroxy anthraquinone leuco body can be guaranteed in the presence of the antioxidant, namely the sodium hydrogensulfite, and the initiation and catalysis effects are improved.
Owner:JIANGSU DAOBO CHEM
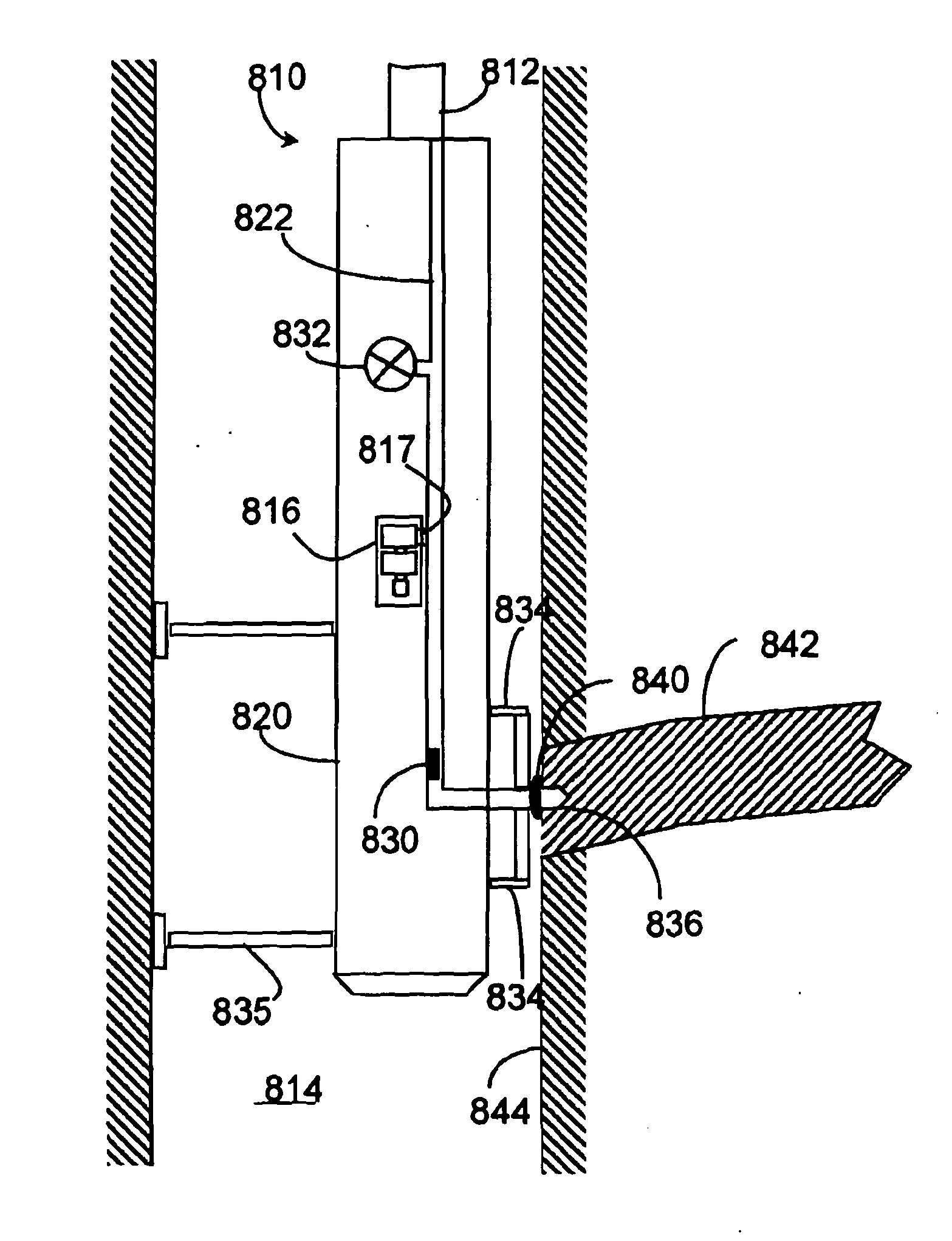
Electrochemical sensor
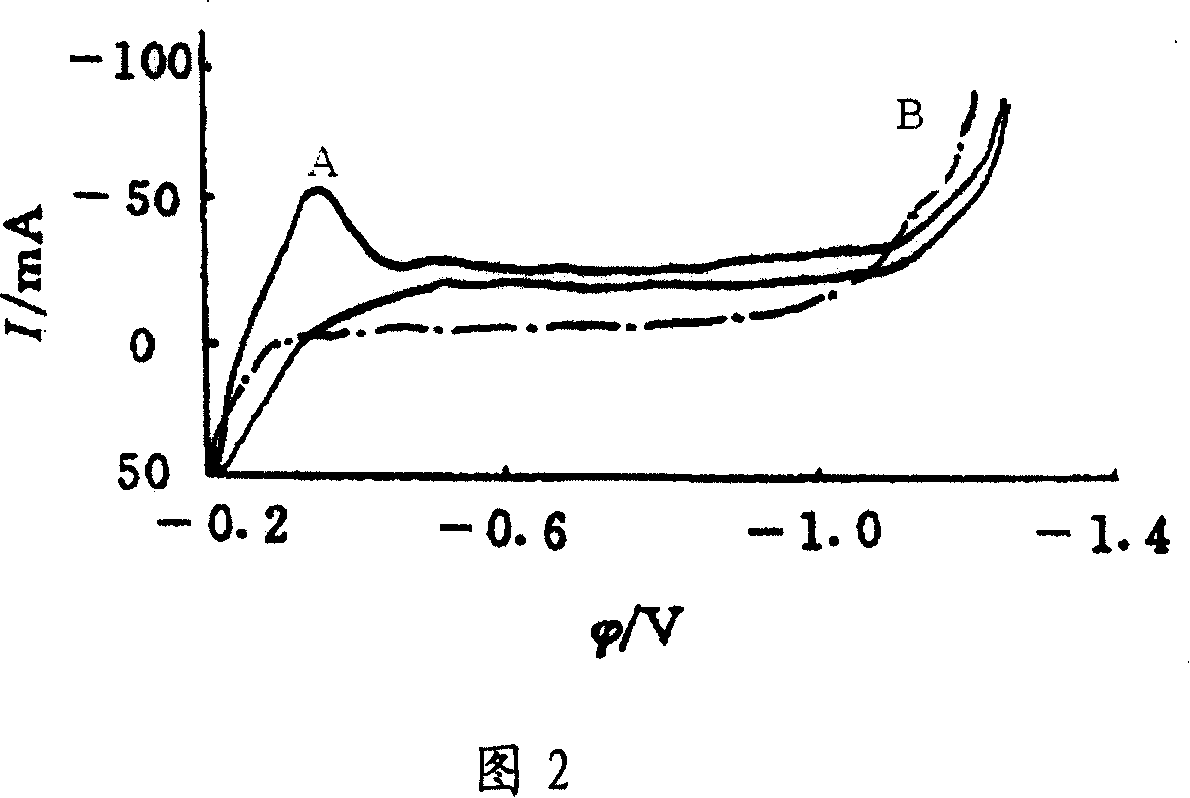
InactiveUS20130256133A1Improve reaction speedLower activation energyWeather/light/corrosion resistanceVolume/mass flow measurementElectrochemical gas sensorReaction rate
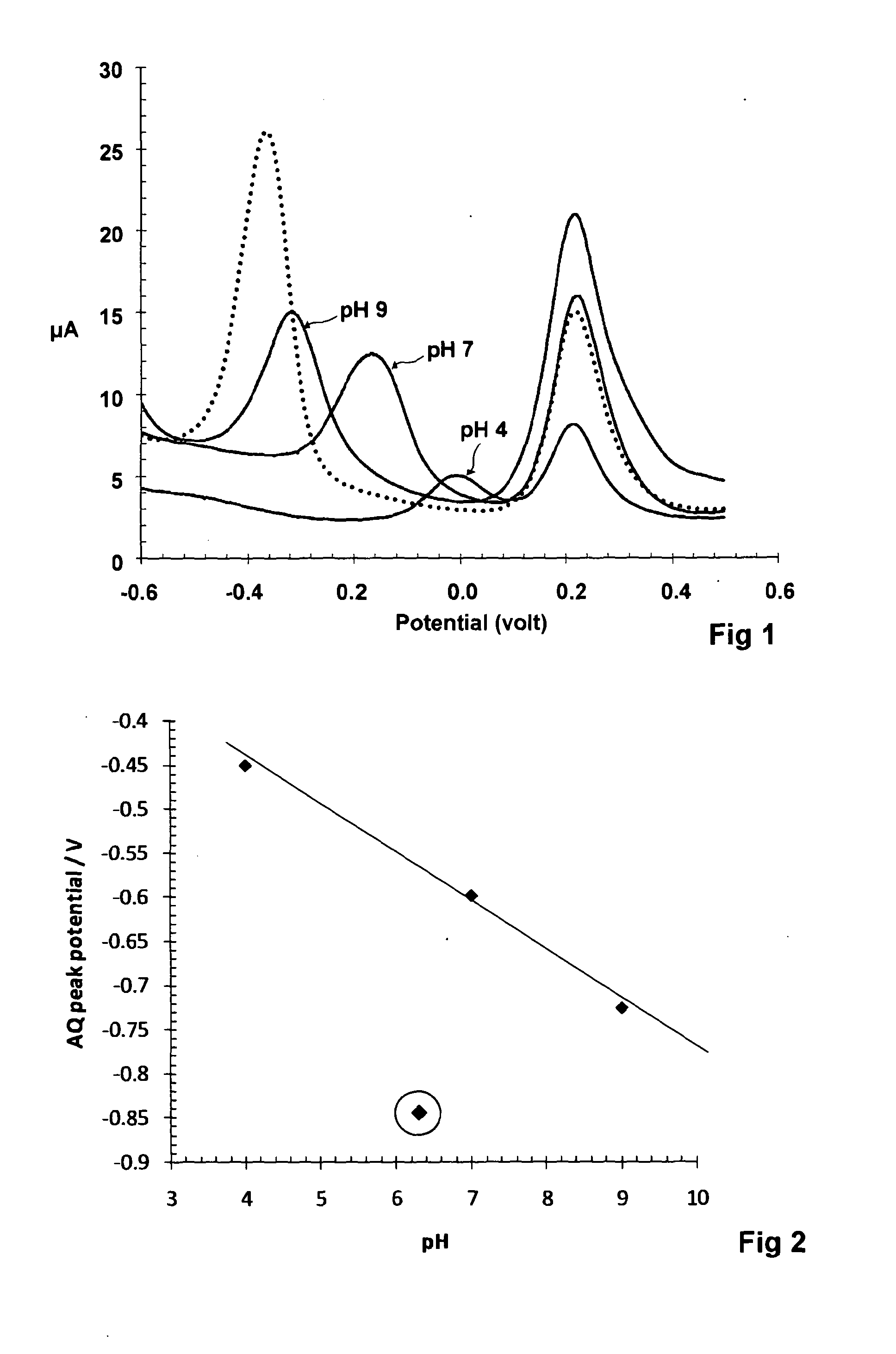
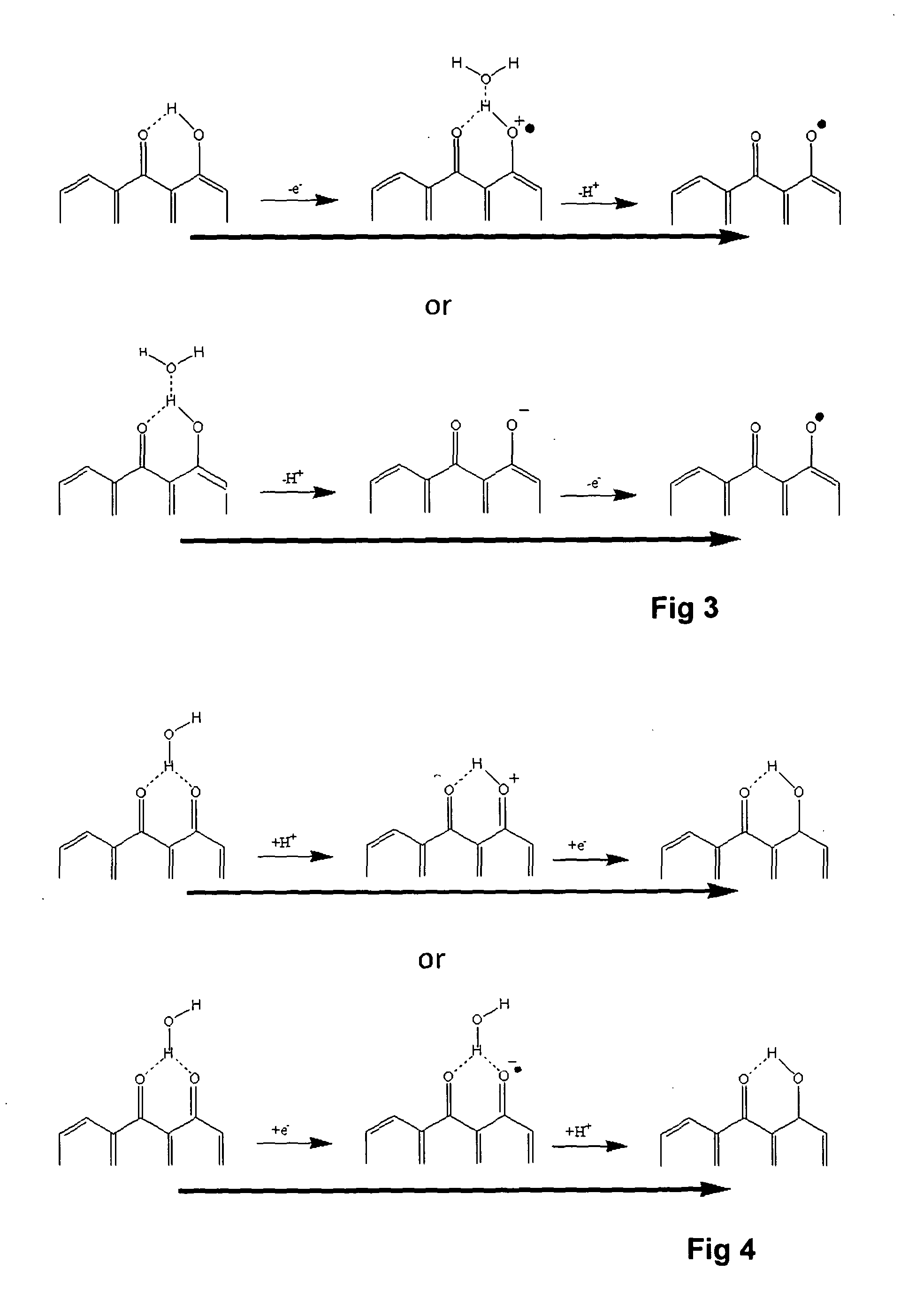
A voltammetric pH sensor, especially for characterising wellbore fluids, comprises a plurality of electrodes with a redox active organic compound attached to an electrode and having at least one functional group convertible electrochemically between reduced and oxidized forms with transfer of at least one proton between the compound and surrounding aqueous phase, wherein the compound has at least one substituent group which promotes hydrogen bonding at a said functional group and thereby increases the reaction rate of proton transfer. The substituent group may form an internal hydrogen bond with a redox-convertible group or may enhance polarity to promote electrostatic interaction with water molecules and reduce activation energy. Typical examples include alizarin or 1,2-dihydroxy-anthraquinone (RH=72-48-0), quinizarin or 1,4-dihydroxy-anthraquinone (RN=81-64-1), 2-acetoxy-benzoquinone (RN=1125-55-9), chloranil or 2,3,4,5-tetrachloro-benzoquinone (RN=118-75-2) and 1,4-diamino-2,3-dichloro-anthraquinone (RN=81-42-5) deposited on a glassy carbon electrode. In this way, anomalous measurements at low ionic strength and low concentrations of pH buffering species can be overcome.
Owner:SCHLUMBERGER TECH CORP
Low-cost flow battery anode electrolyte and preparation method thereof
InactiveCN104835962ALow costEliminate Energy Storage BarriersElectrode manufacturing processesSupporting electrolyteUninterruptible power supply
The invention belongs to the field of flow batteries and discloses low-cost anode electrolyte and a preparation method thereof. The low-cost flow battery anode electrolyte is based on a sulfuric acid solution of small organic molecule 1,2-dihydroxy anthraquinone-3-sodium sulfonate (ARS). The preparation method of the low-cost flow battery anode electrolyte comprises the following steps: adding electrolyte raw material 1,2-dihydroxy anthraquinone-3-sodium sulfonate or derivatives thereof or a mixture of the 1,2-dihydroxy anthraquinone-3-sodium sulfonate and derivatives thereof in water, dissolving and evenly mixing the added materials, using ion exchange resin to remove positive ions, then adding supporting electrolyte sulfuric acid, and diluting the obtained solution to a preset volume to obtain the low-cost flow battery anode electrolyte. The flow battery is simple in structure, low in cost, high in energy density and watt density. Compared with a vanadium flow battery, the flow battery using the anode electrolyte provided by the invention is 50% lower in cost. The flow battery can be used as a large-scale electric energy storage device in a wind energy and solar power system, and can be used for regulating peak load, supplying power for remote areas and providing uninterruptible power supply.
Owner:BEIJING INSTITUTE OF TECHNOLOGYGY
Synthetic method for solvent violet 13
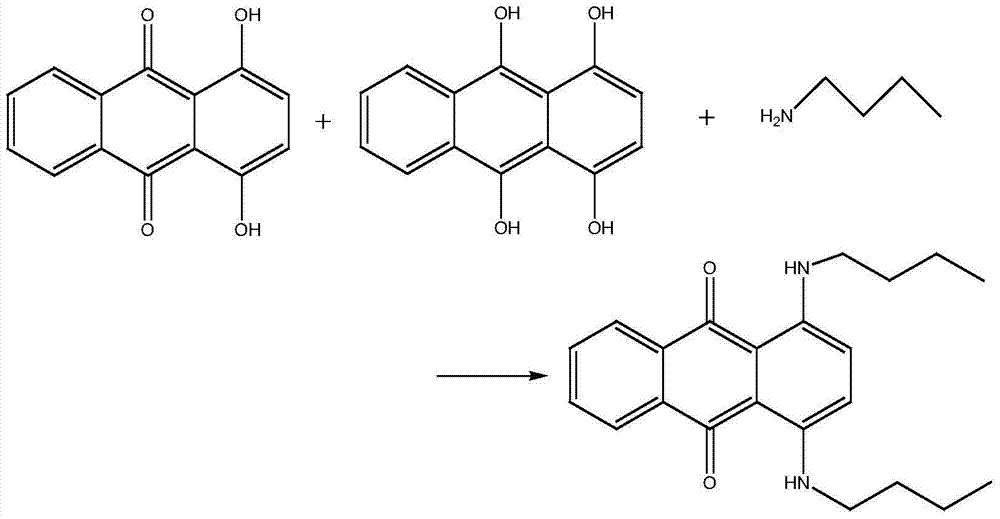
ActiveCN105237417AReduced post-processingSave manpower and material resourcesOrganic chemistryOrganic compound preparationToluidineMaterial resources
The invention discloses a synthetic method for solvent violet 13, and develops a new route for synthesizing violet 13 by employing a one-pot process. The method comprises successively adding methanol, 1,4-dihydroxyanthraquinone, 4-nitrotoluene, iron powder and boric acid into a pressure container, sealing the container and performing nitrogen displacement, then heating with stirring, introducing hydrogen for hydrogenation reduction, after the reaction system stops absorbing hydrogen, performing warm-keeping reaction, heating after the reaction is finished, performing condensation reaction, cooling after the reaction is finished, releasing the pressure, introducing air and performing oxidation reaction, and finally adding hydrochloric acid for beating, filtering, washing and drying, so as to obtain violet 13. According to the method, the initial raw material p-toluidine is replaced by 4-nitrotoluene, direct usage of the highly-toxic raw material p-toluidine is avoided, and the synthetic technology is low in toxicity and friendly to environment. By pouring all of the raw materials into the container, the postprocessing process of the intermediate step is saved, and a large amount of manpower and material resource is saved.
Owner:JIANGSU DAOBO CHEM
Low-cost flow battery negative electrode electrolyte and preparation method therefor
InactiveCN106532096ALow costImprove performanceRegenerative fuel cellsOrganic electrolytesHigh energyPotassium hydroxide
The invention belongs to the field of a flow battery, and discloses a low-cost negative electrode electrolyte and a preparation method therefor. The low-cost flow battery negative electrode electrolyte is a negative electrode electrolyte based on a potassium hydroxide solution of small organic molecule 1, 8-dihydroxy anthraquinone (DHAQ). The preparation method for the low-cost flow battery negative electrode electrolyte comprises following steps of preparing 1M of KOH solution, adding an electrolyte raw material 1, 8-dihydroxy anthraquinone or derivative thereof or a mixture into the 1M KOH solution, dissolving and mixing uniformly, and performing constant volume by the 1M KOH solution to obtain the low-cost flow battery negative electrode electrolyte. The flow battery has the advantages of simple structure, low material cost, high energy density and high power density, and the like; and compared with an all-vanadium redox flow battery, the cost of the low-cost flow battery is reduced by 40%, so that the low-cost flow battery is quite suitable for being used as large-scale electric energy storage equipment for regulating peak and filling valley for a power grid.
Owner:BEIJING INSTITUTE OF TECHNOLOGYGY
1,8-dihydroxy anthraquinone copper compound and preparation method and application thereof
ActiveCN103641703AIncrease burn rateGood catalyticOrganic compound preparationOrganic-compounds/hydrides/coordination-complexes catalystsCombustionCopper
The invention discloses a 1,8-dihydroxy anthraquinone copper compound as shown in a structural formula (I). The 1,8-dihydroxy anthraquinone copper compound disclosed by the invention has excellent catalysis effect on a modified biradical solid propellant as a combustion catalyst and can outstandingly increase the combustion speed of the modified biradical solid propellant and reduce the pressure index.
Owner:XIAN MODERN CHEM RES INST
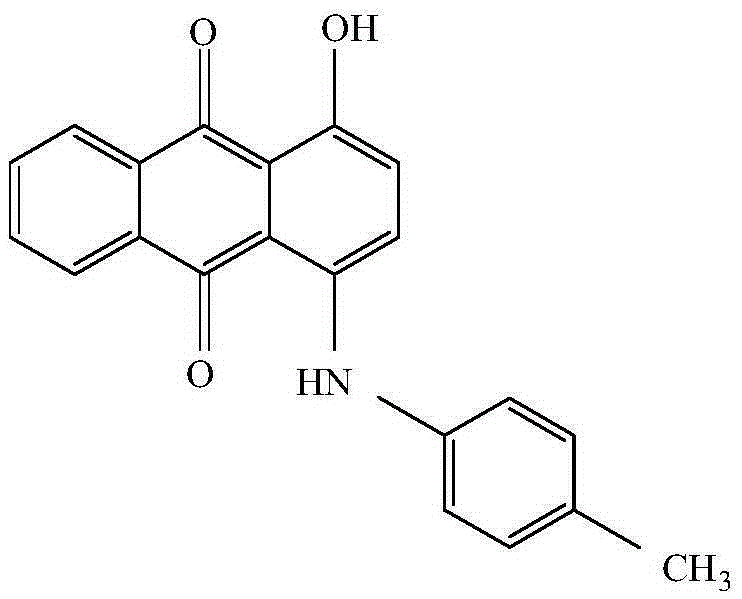
Preparation method of 1-hydroxy-4-(p-toluidino)anthraquinone
InactiveCN107501987ASmooth responseHigh yieldOrganic-compounds/hydrides/coordination-complexes catalystsCatalytic reactionsEtherEther solvent
The invention provides a preparation method of 1-hydroxy-4-(p-toluidino)anthraquinone. 1,4-dihydroxy anthraquinone, p-toluidine, ether solvents and a condensation catalyst are added to a reaction kettle, a system is gradually heated for a reaction, a product is filtered after the reaction ends, a filter cake is washed, dried and treated with a refining process, and a finished product, namely, 1-hydroxy-4-(p-toluidino)anthraquinone is obtained.
Owner:宁波德欣科技有限公司
Anthraquinone type solvent dye and preparation and application thereof
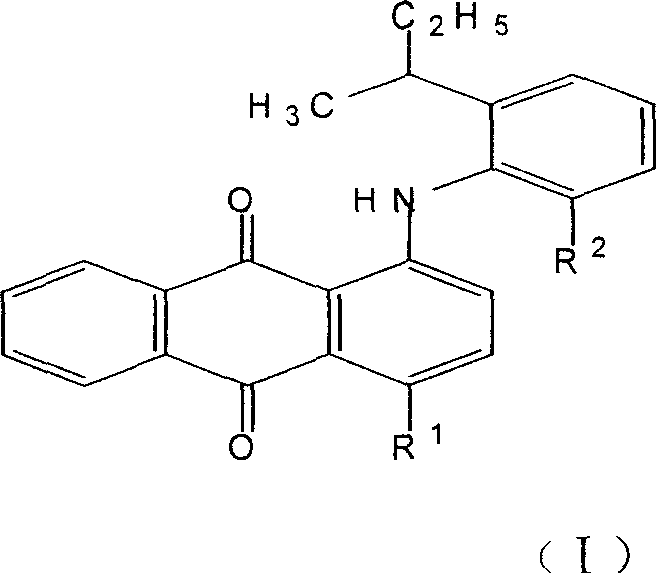
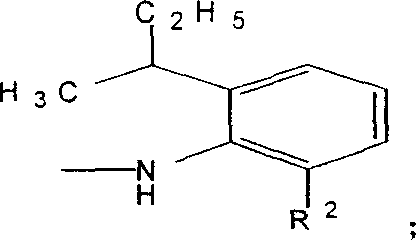
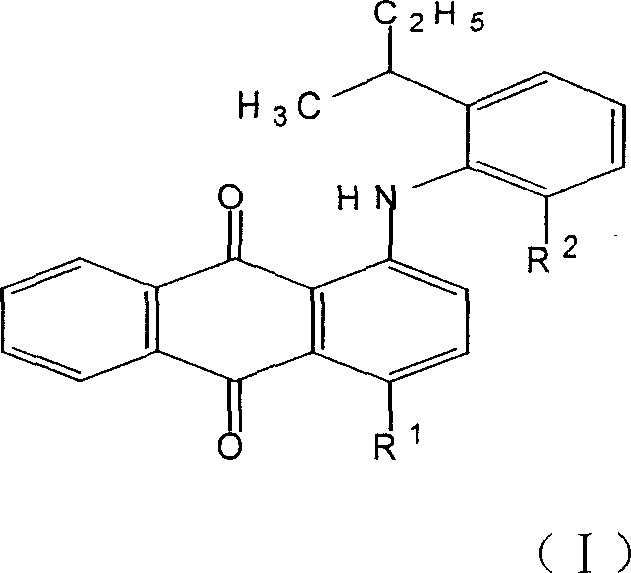
The invention discloses an anthraquinone dissolvent dye and preparation and application in dye technical domain, which is characterized by the following: setting the composite as general formula (I); choosing R1 from hydroxyl group or II; choosing R2 from metyl group or ethyl group; adopting vacuum rectifying craft; extracting 2-ethyl group-6-secondary butyl aniline or 2-metyl group-6-secondary butyl aniline; proceeding condensation reaction with 1, 4-alizarin; preparing the general formula (I) component; decreasing the cost of dye. This dye can be used to color oil, hard plastic rubber and resin, which can get brilliance red light blue and bright violet.
Owner:SHENYANG RES INST OF CHEM IND +1
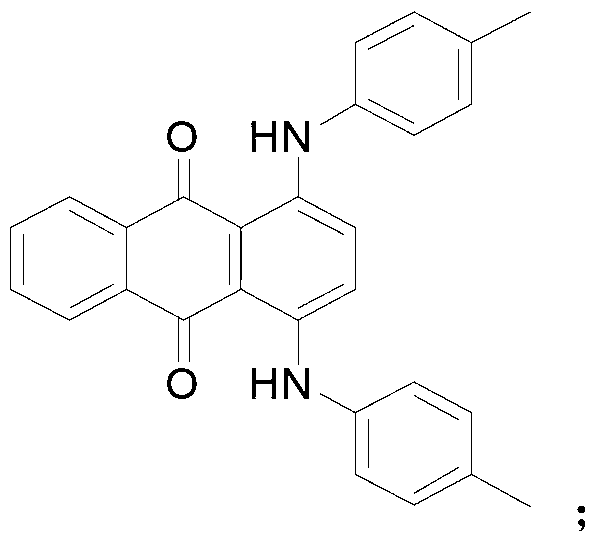
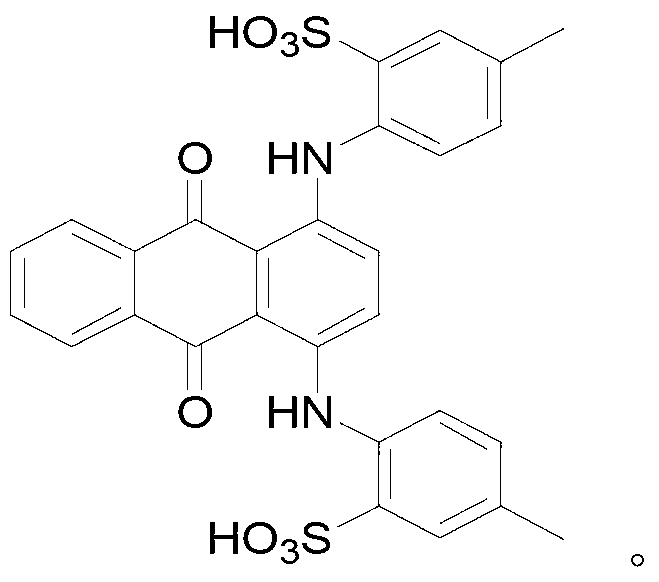
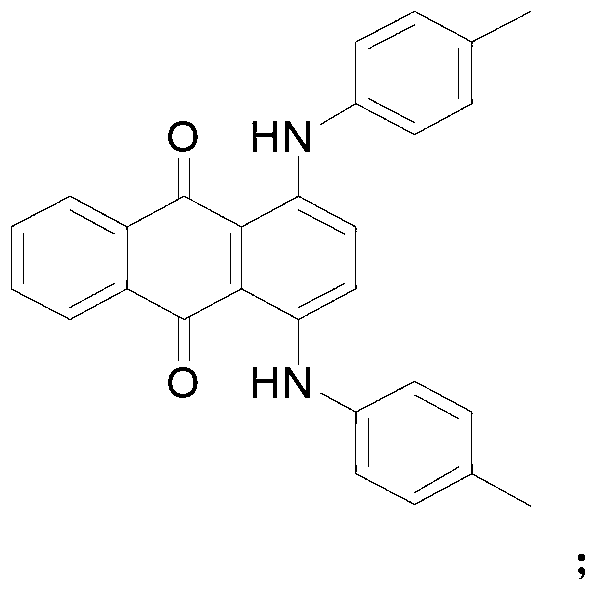
Method for synthesizing N-[4-[(4-hydroxyanthraquinone-1-yl) amino] benzyl] acetamide
ActiveCN102050757AIncrease contentReduce contentOrganic compound preparationCarboxylic acid amides preparationPolyesterHydroxyanthraquinone
The invention relates to a method for synthesizing N-[4-[(4-hydroxy anthraquinone-1-yl) amino] benzyl] acetamide. The method comprises the steps of: with hydrochloric acid as a catalyst, carrying out condensation reaction on 1,4-dihydroxy anthraquinone, a 1,4-dihydroxy anthraquinone leuco body and p-phenylenediamine for 10-16h at 95-105 DEG C in a butanol-water solution medium; after the condensation reaction is completed, sequentially carrying out water adding and distilling, filtering, heating treatment with dilute alkali liquor, water washing till reaching a neutral state and drying to obtain a condensation material; and carrying out acylation reaction on the condensation material and acetic anhydride for 2h at 110-112 DEG C in a acetic acid medium, cooling to normal temperature, filtering, washing and drying to obtain a product of N-[4-[(4-hydroxy anthraquinone-1-yl) amino] benzyl] acetamide. The N-[4-[(4-hydroxy anthraquinone-1-yl) amino] benzyl] acetamide product synthesized with the method has high purity, controllable content, fewer 1,4-dihydroxy anthraquinone residues, and low byproduct content and product ash content, and is suitable for polyester spinning and dying.
Owner:宁波龙欣精细化工有限公司
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com
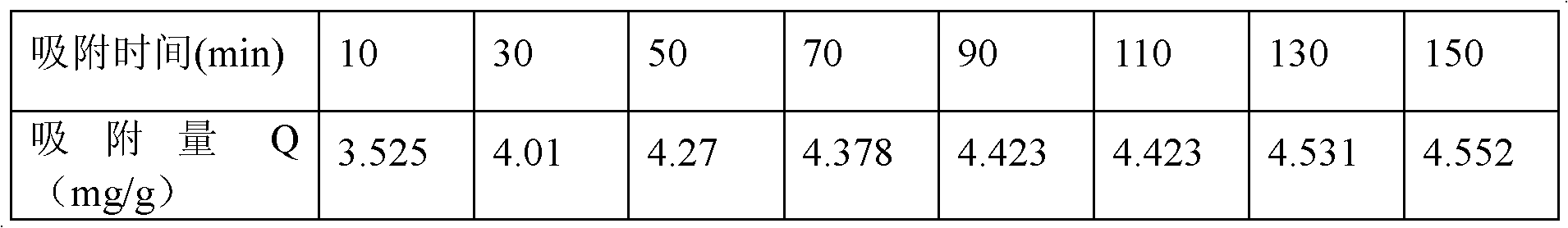


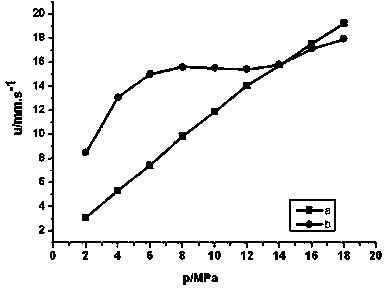
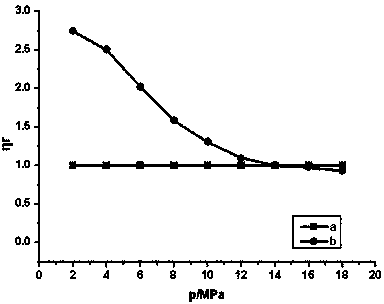
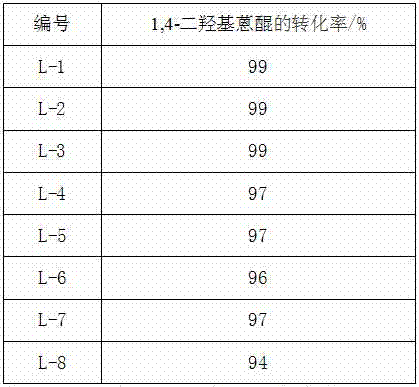
![Method for synthesizing N-[4-[(4-hydroxyanthraquinone-1-yl) amino] benzyl] acetamide Method for synthesizing N-[4-[(4-hydroxyanthraquinone-1-yl) amino] benzyl] acetamide](https://images-eureka.patsnap.com/patent_img/6ed0e043-8bde-4019-80ff-0988495ad228/BSA00000370286200021.PNG)
![Method for synthesizing N-[4-[(4-hydroxyanthraquinone-1-yl) amino] benzyl] acetamide Method for synthesizing N-[4-[(4-hydroxyanthraquinone-1-yl) amino] benzyl] acetamide](https://images-eureka.patsnap.com/patent_img/6ed0e043-8bde-4019-80ff-0988495ad228/BSA00000370286200022.PNG)