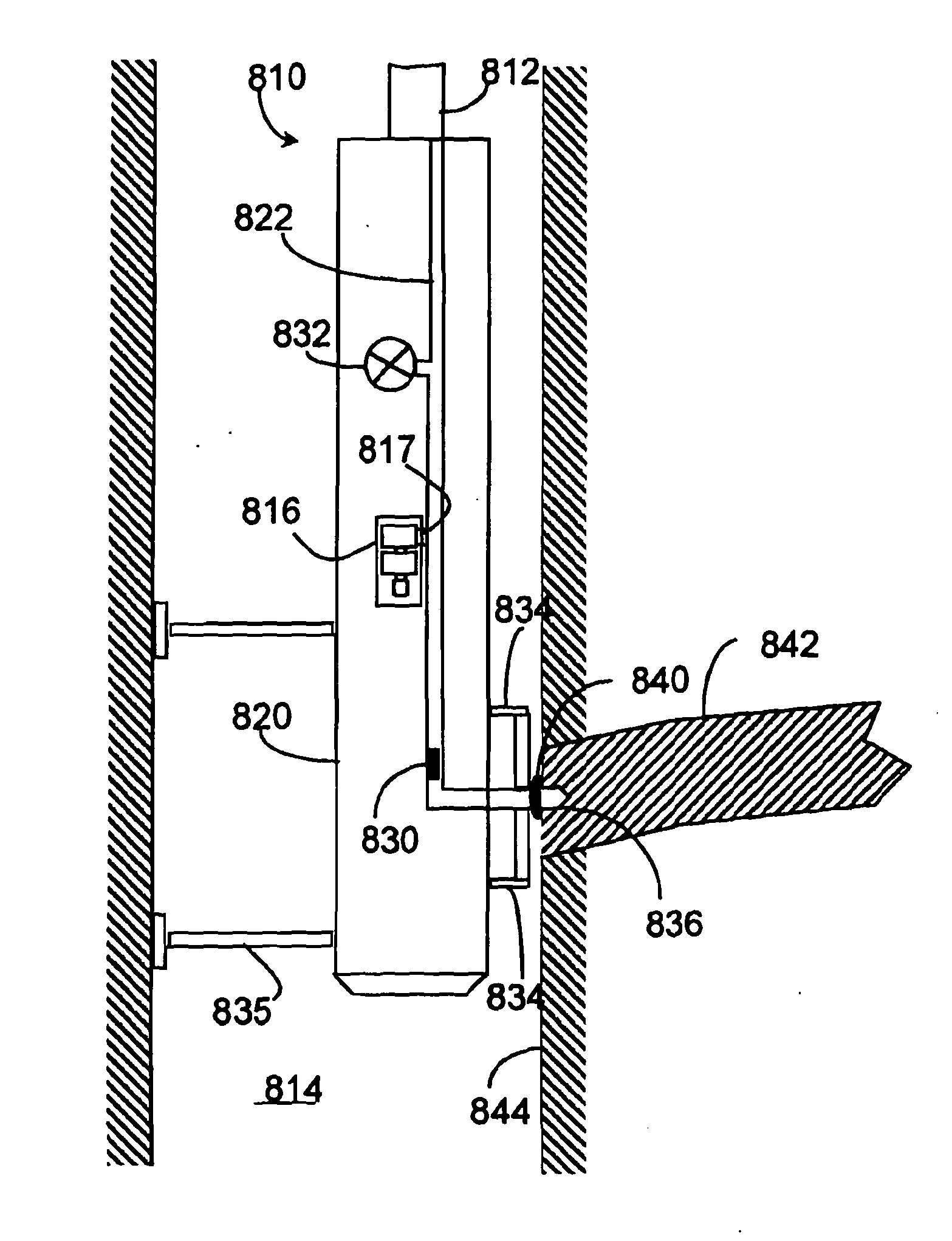
Electrochemical sensor
a technology of electrochemical sensors and sensors, applied in the field of electrochemical sensors, can solve the problems of difficult ph measurement, low ionic strength media, and high labor intensity, and achieve the effect of facilitating proton transfer
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
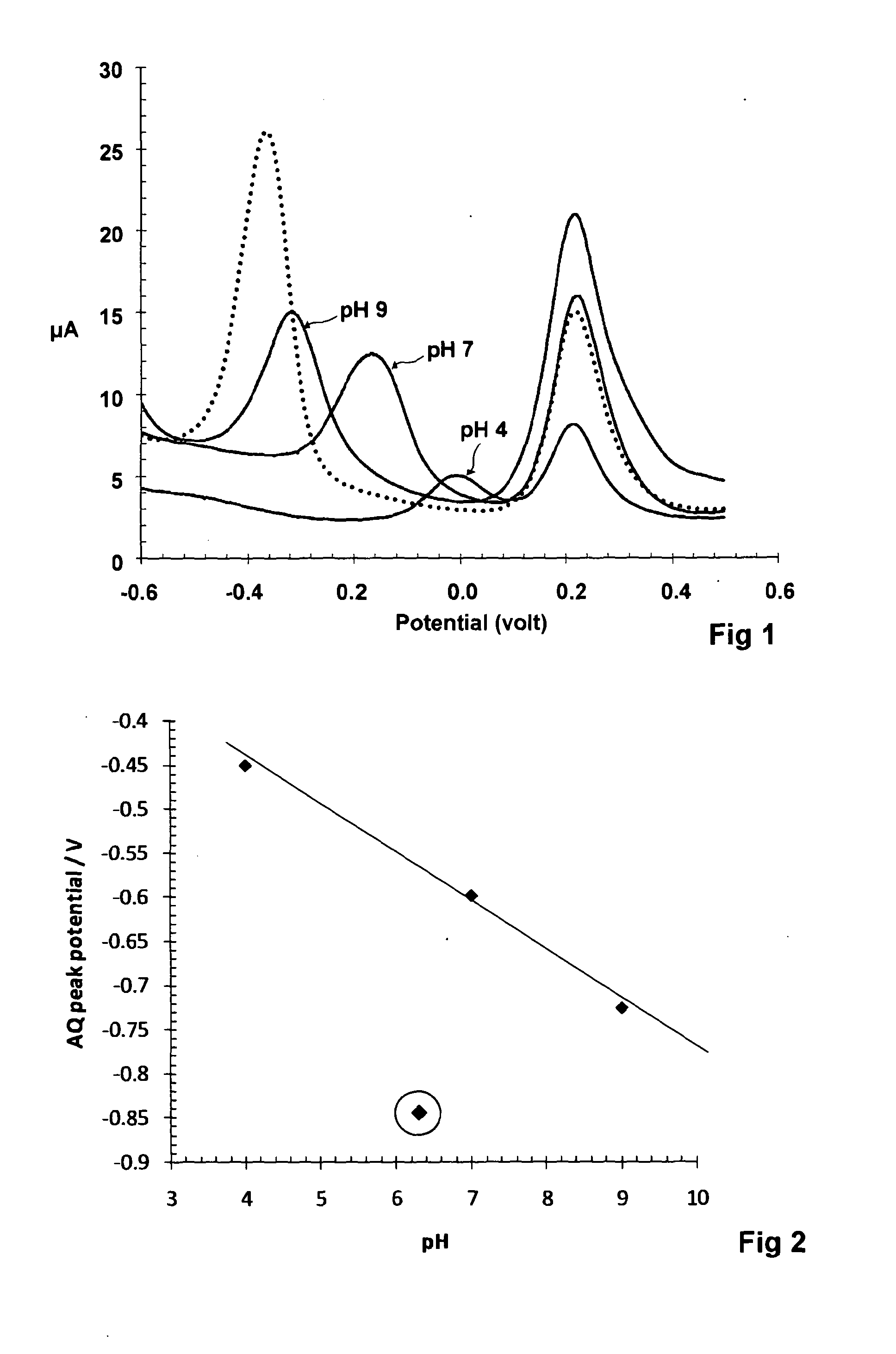
[0051]The procedure above was used to test four water-insoluble redox compounds with structures and redox reactions as follows:
[0052]When the electrodes with the compounds thereon were used in the above procedure using standard buffers, straight calibration lines were obtained for each of them. However, when used to measure pH of unbuffered water samples having pH of 8.06 and 7.92 as determined by a glass electrode, the measured oxidative peak voltage corresponded to the following pH values:
RedoxDetermined pH for waterDetermined pH for watercompoundsample A (actual pH 8.06)sample B (actual pH 7.92)AQ9.739.32PAQ9.909.211,4-DHAQ8.137.861,2-DHAQ7.907.84
[0053]It can be seen that the hydroxy substituted compounds give a measurement of pH which is very close to the value obtained with the glass electrode even though the water samples are not buffered. Without wishing to be bound as to theory, we attribute this to the hydroxy substituents facilitating inter and intra molecular hydrogen bon...
example 2
[0057]The procedure of Example 1 was repeated using unbuffered 0.1 molar potassium chloride solutions as electrolyte. This was done for the two dihydroxyanthraquinones used in Example 1 and also for 1,4-dihydroxynaphthoquinone whose redox conversion to and from a fully oxidised form is
[0058]The pH values of the solutions obtained from voltammetry as above and also as measured with a glass electrode are given in the following table:
pH determined bypH measured withCompoundvoltammetryglass electrode1,4-dihydroxyanthraquinone6.526.351,2-dihydroxyanthraquinone6.486.531,4-dihydroxynapthoquinone6.246.35
example 3
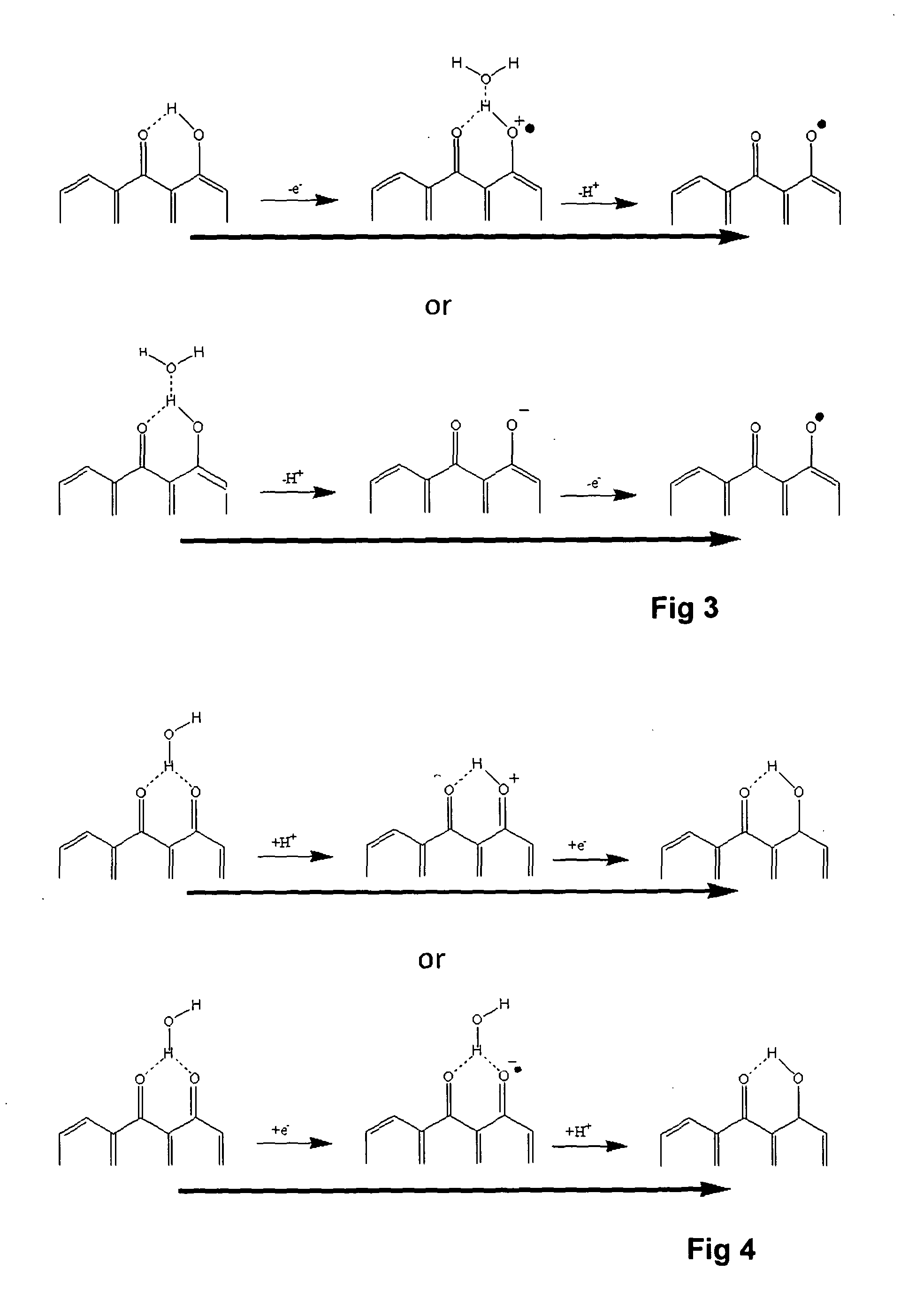
[0059]Further evidence that hydrogen bonding takes place and brings about proton transfer was obtained using the following compound, 2,3-dihydro-9,10-dihydroxy-1,4-anthracenedione which undergoes two electron two proton redox reaction as shown
[0060]This compound has two keto groups in an aliphatic cyclohexene ring (the left hand ring in the formulae above) which is fused with one ring of a dihydroxy naphthalene structure. This dihydroxy naphthalene portion of the molecule undergoes a two electron two proton redox process. Voltammetry using buffer solutions showed that voltage at peak current was linearly dependent on pH but measurement in unbuffered salt solution showed an anomalous pH value. The pH value determined by voltammetry was 8.13 whereas the true value measured with a glass electrode was 7.31.
[0061]This difference in behaviour compared to 1,4-DHAQ was attributed to the aliphatic ring positioning its keto oxygen atoms out of the plane of the naphthalene rings, so that inter...
PUM
| Property | Measurement | Unit |
|---|---|---|
| pH | aaaaa | aaaaa |
| concentration | aaaaa | aaaaa |
| pH | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com