Method for preparing 1,4-dihydroxy anthraquinone
A technology of dihydroxyanthraquinone and boric acid, applied in the field of 1,4-dihydroxyanthraquinone synthesis, can solve the problems of waste of raw materials, high cost, environmental pollution, etc. The effect of good catalytic effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
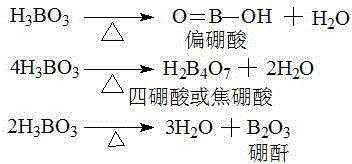
[0027] Boric acid was heated at 250°C for 10 hours to obtain boric acid dehydration compound; the boric anhydride in the boric acid dehydration compound was determined to be 99.3% and tetraboric acid 0.7% by elemental analysis;
[0028] In the condensation reactor, add oleum 350kg, phthalic anhydride 120kg, boric acid dehydration compound 40kg, p-chlorophenol 100kg (oleum: phthalic anhydride: metaboric acid: p-chlorophenol = 3.5: 1.2: 0.4: 1); oil bath Raise the temperature to 100°C for the material, precisely control the reaction temperature at 100~105°C, and keep it warm for 0.5 hours; press the material into a hydrolysis kettle with 300L of water, adjust the temperature to 50°C, keep it at 50°C for 0.1 hour, discharge the material, press filter, and wash with water To neutrality, blow dry to obtain 400.2kg of 1,4-hydroxyanthraquinone tide product, the moisture content is 54.2%, and the dry product is 183.3kg. Through liquid chromatography analysis, the content of 1,4-hydrox...
Embodiment 2
[0030] Boric acid was heated at 130°C for 2 hours to obtain boric acid dehydration compound; the content of metaboric acid in boric acid dehydration compound was 92.1% and tetraboric acid 7.9% according to elemental analysis.
[0031] In the condensation reaction kettle, add oleum 450kg, phthalic anhydride 150kg, boric acid dehydration compound 60kg, p-chlorophenol 100kg (oleum: phthalic anhydride: metaboric acid: p-chlorophenol = 4.5: 1.5: 0.6: 1); oil bath Raise the temperature to 250°C of the material, and keep it warm for 30 hours. Press the material into a hydrolysis kettle with 4500L of water, adjust the temperature to 105°C, keep it at 105~115°C for 4 hours, press filter, wash with water until neutral, and dry to obtain 1,4-hydroxyanthraquinone trendy product 507.1kg, tested moisture content 53.6%, converted to dry product 235.3kg, through liquid chromatography analysis, the content of 1,4-hydroxyanthraquinone reached 87.26%, dry product purity 81.43%, yield 89.5%.
Embodiment 3
[0033] Boric acid was heated at 220°C for 5 hours to obtain boric acid dehydration compound; the boric acid dehydration compound was determined to be 20.7% tetraboric acid and 79.3% boric anhydride in the boric acid dehydration compound.
[0034] In the condensation reactor, add oleum 400kg, phthalic anhydride 130kg, boric acid dehydration compound 50kg, p-chlorophenol 100kg (oleum: phthalic anhydride: boric acid dehydration compound: p-chlorophenol=4.0:1.3:0.5:1). Raise the temperature of the material in the oil bath to 190°C, and keep it warm for 15 hours; press the material into a hydrolysis kettle with 2000L of water, adjust the temperature to 100°C, keep it at 100-105°C for 4 hours, discharge the material, press filter, wash with water until neutral, Blow dry to obtain 413.6kg of 1,4-hydroxyanthraquinone tidal product, the detected moisture content is 55.3%, and the dry product is 184.9kg. Through liquid chromatography analysis, the content of 1,4-hydroxyanthraquinone rea...
PUM
| Property | Measurement | Unit |
|---|---|---|
| purity | aaaaa | aaaaa |
| purity | aaaaa | aaaaa |
| purity | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com