Process for synthesizing anthraquinone compound
A synthesis process and compound technology, which is applied in the field of synthesis process of anthraquinone compounds, can solve the problems of wastewater, waste acid treatment difficulties, serious secondary pollution, environmental pollution, etc., and achieve the reduction of refining and purification process, less three wastes, and lower production cost effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
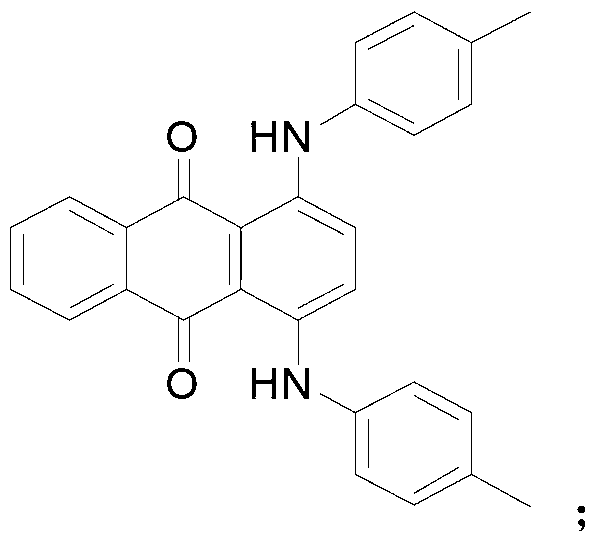
[0021] Synthesis of 1,4-di-p-toluidinoanthraquinone
[0022] 100mmol of 1,4-dihydroxyanthraquinone and 200mmol of p-toluidine were added to a round bottom flask filled with 400ml of water, followed by the addition of 210mmol of boric acid. The reaction was then stirred rapidly at 95°C for 10 hours. The completion of the reaction was followed by TLC spotting. After the reaction, the reaction liquid was filtered hot, and the obtained solid was washed twice with 100 ml of water and dried to obtain 36.1 g of a blue-green solid. The melting point is 220-222°C, and the yield is 86.4%.
Embodiment 2
[0024] Synthesis of 1,4-di-p-toluidinoanthraquinone
[0025] 100mmol of 1,4-dihydroxyanthraquinone and 200mmol of p-toluidine were added to a round bottom flask filled with 400ml of water, followed by the addition of 210mmol of boric acid. The reaction was then stirred rapidly at 100°C for 10 hours. The completion of the reaction was followed by TLC spotting. After the reaction, the reaction solution was cooled to 25° C. and filtered. The obtained solid was washed twice with 100 ml of water and dried to obtain 38 g of a blue-green solid. The melting point is 219-221°C, and the yield is 91.8%.
Embodiment 3
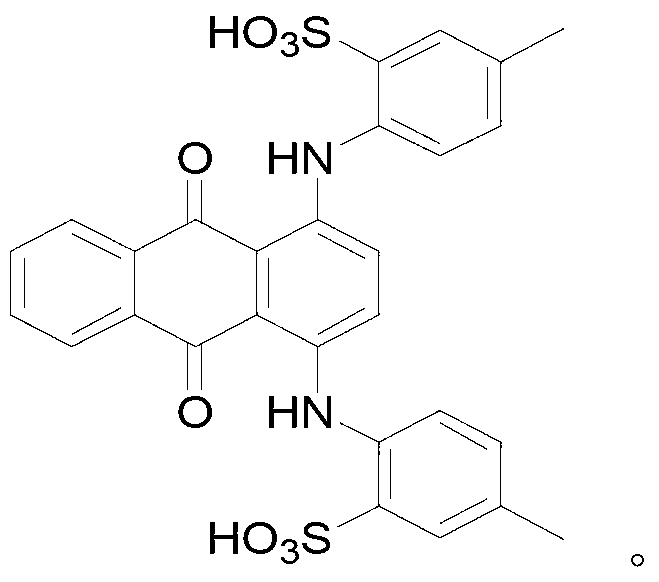
[0027] Synthesis of 1,4-bis(2-sulfo-4-methylanilino)anthraquinone
[0028] Dissolve 100mmol of 1,4-di-p-toluidinoanthraquinone in a three-necked flask filled with 150ml of methanol, and pass through SO while stirring at room temperature 3 gas. The completion of the reaction was followed by TLC spotting. After the reaction, the reaction liquid was cooled to 10° C. and filtered. The obtained solid was washed with 30 ml of methanol and dried to obtain 54 g of a green solid with a yield of 86%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com