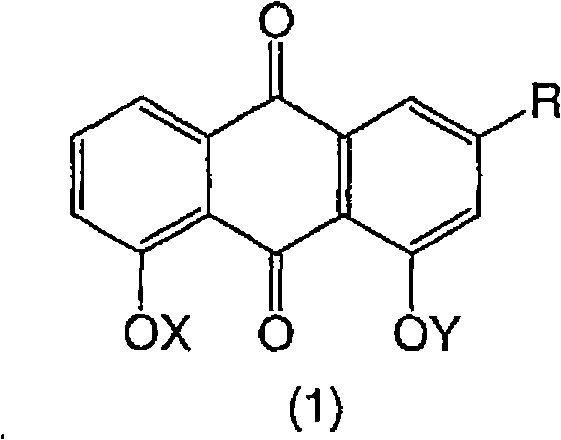
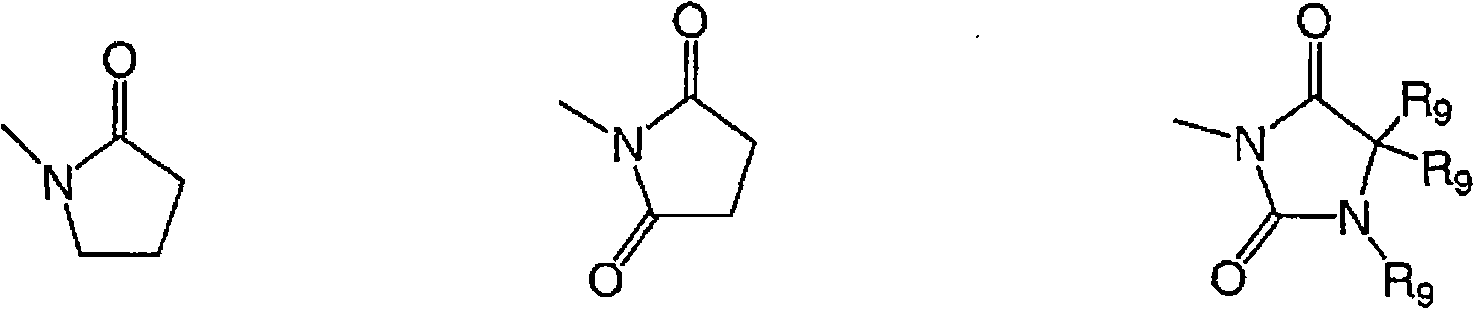
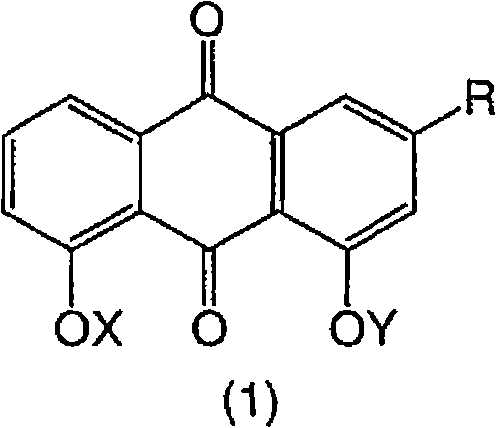
Dihydroxyanthraquinones and their use
An alkyl and cyclic group technology, applied in the field of dihydroxyanthraquinones and their uses, can solve problems such as poor physical and chemical properties
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0065] 1,8-bis(tetrahydropyran-4-carbonyloxy)-3-(morpholine-4-carbonyl)-9,10-dioxo-9,10-dihydroanthracene
[0066]
[0067] In a 100ml flask equipped with a magnetic stirrer, 4,5-ditetrahydropyranyloxy-9,10-dioxo-9,10-dihydroanthracene-2-carboxylic acid (2.00g, 3.9×10 -3 mol) was stirred in DCM (50 mL) under nitrogen. Add EDCI (0.90g, 4.7×10 -3 mol) and HOBt (0.65g, 4.7×10 -3 mol) and the solution was stirred for 15 minutes. The solid suspension was dissolved in DCM solvent. Then add morpholine (0.54mL, 5.9×10 -3 mol) and the solution was stirred for 30 minutes.
[0068] The reaction mixture was diluted in 300 mL DCM. Sat. NaHCO with 200 mL 3 solution, and 200 mL of saturated NaCl solution to wash the organic layer. The organic layer was separated and evaporated to dryness under reduced pressure. The resulting residue was then triturated in water, filtered and dried under high vacuum to afford the desired product as a yellow solid (1.95 g, 87%).
[0069] 1 H NMR...
Embodiment 2
[0071] 1,8-bis(tetrahydropyran-4-carbonyloxy)-3-ethylcarbamoyl-9,10-dioxo-9,10-dihydroanthracene
[0072]
[0073] In a 100ml flask equipped with a magnetic stirrer, 4,5-ditetrahydropyranyloxy-9,10-dioxo-9,10-dihydroanthracene-2-carboxylic acid (2.00g, 3.9×10 -3 mol) in DCM (50 mL) was stirred under nitrogen. Add EDCI (0.90g, 4.7×10 -3 mol) and HOBt (0.65g, 4.7×10 -3 mol) and the solution was stirred for 15 minutes. The solid suspension was dissolved in DCM solvent. Then add ethylamine (3.00mL, 5.9×10 -3 mol) and the solution was stirred for 15 minutes.
[0074] The reaction mixture was then diluted in 300 mL DCM. Sat. NaHCO with 200 mL 3 solution, and 200 mL of saturated NaCl solution to wash the organic layer. The organic layer was separated and evaporated to dryness under reduced pressure. The resulting residue was then triturated in water, filtered and dried under high vacuum to afford the desired product as a yellow solid (1.56 g, 75%).
[0075] 1 H NMR (50...
Embodiment 3
[0078] 4,5-bis(tetrahydropyran-4-carbonyloxy)-9,10-dioxo-9,10-dihydroanthracene-2-carboxylic acid 2-methoxyethyl ester
[0079]
[0080] Under nitrogen, to a 250 mL flask equipped with a magnetic stirrer, Dean-Stark apparatus, was added 2-methoxyethanol (300 mL) and 4,5-dihydroxy-9,10-dioxoanthracene-2-carboxylic acid (3.00g, 10.6×10 -3 mol) and 0.1 mL of sulfuric acid. The mixture was refluxed for 24 hours (external oil bath temperature 145°C). The reaction mixture was then cooled to room temperature.
[0081] The resulting solid was filtered and washed with 10 mL of ice-cold diethyl ether. 2-Methoxyethyl 4,5-dihydroxy-9,10-dioxanthracene-2-carboxylate was isolated as a yellow solid (2.9 g, 81%).
[0082] 1 H NMR analysis was consistent with the desired product, (500MHz, DMSO)
[0083] δ=8.1(s, 1H, Ar), 7.8(t, 1H, Ar), 7.7(s, 1H, Ar), 7.7(d, 1H, Ar), 7.4(d, 1H, Ar), 4.4(t , 2H, COOCH 2 ), 3.7(t, 2H, CH 2 OCH 3 ), 3.3(s, 3H, CH 2 OCH 3 ).
[0084] Under nitroge...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com