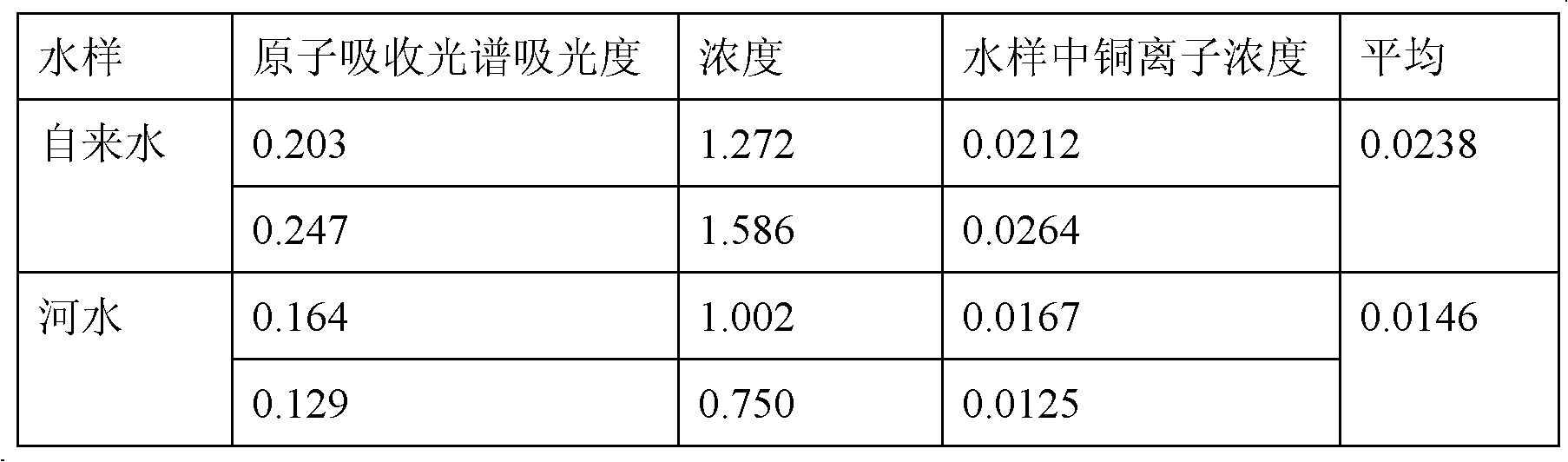
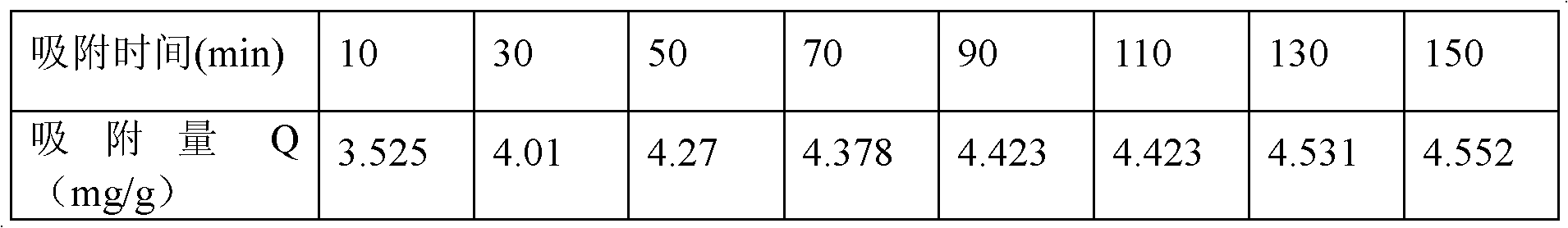Copper ion imprinted polymer and application thereof
A technology of imprinting polymers and copper ions, which can be applied to alkali metal compounds, other chemical processes, alkali metal oxides/hydroxides, etc. Effects of Reaction Steps
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0018] (1) Get 1,4-dihydroxyanthraquinone 1mmol, add acetonitrile 60ml, add copper nitrate 1mmol (solid or copper ion concentration is 1mol / L solution), fully react 3h on the magnetic stirrer, this reaction is at room temperature (10 ~30°C); add EGDMA (ethylene glycol dimethacrylate) 20mmol, azobisisobutyronitrile (AIBN) 0.6mmol, under nitrogen protection, heat in an oil bath, and react at 60°C for 24h;
[0019] (2) Take the solid, that is, the polymer generated by the reaction in step (1), and wash it with aqueous methanol (the volume ratio of methanol to water is 1:4) to remove unreacted substances; then wash with 0.1mol / L nitric acid oscillation Remove copper ions, wash 3 to 4 times, each shaking washing time is 30 to 60 minutes, the amount of nitric acid solution is 25 to 200ml / time; wash with water to pH 7, dry at 60°C to obtain copper ion imprinted polymer .
Embodiment 2
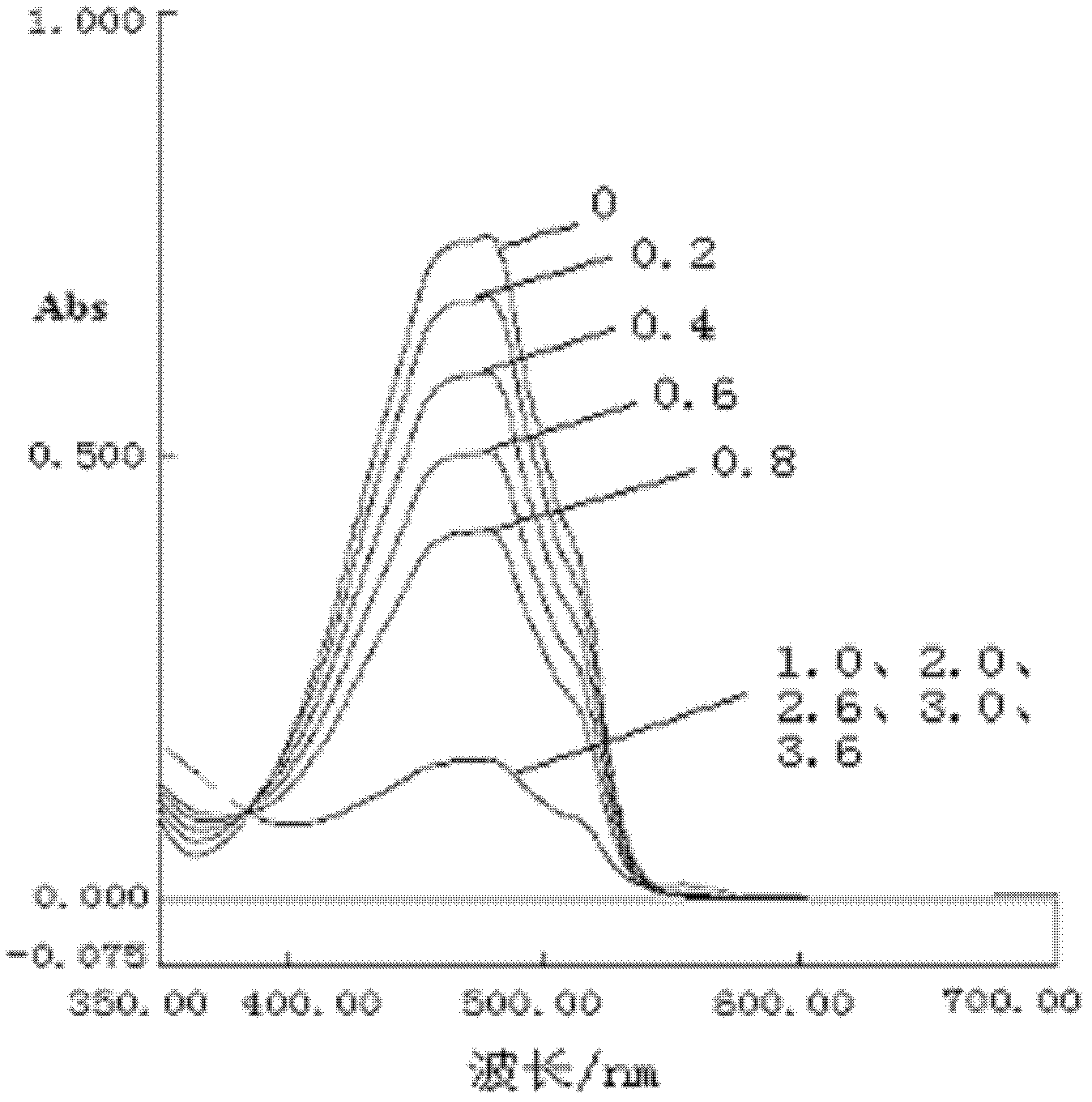
[0021] Dissolve 1,4-dihydroxyanthraquinone in acetonitrile, keep the amount of anthraquinone constant, change the amount of copper ion, make copper ion and 1,4-dihydroxyanthraquinone according to the ratio of substance respectively 0, 0.2, 0.4, 0.6, 0.8, 1.0, 2.0, 2.6, 3.0 and 3.6 combined, and the absorbance of the solution can be detected to judge the combination of the template and the functional monomer.
[0022] The combination of template copper ion and functional monomer 1,4-dihydroxyanthraquinone in acetonitrile solution is as follows: figure 1 shown. When the molar ratio of substances is 0, there is only anthraquinone in the system, and the absorbance is the largest. When copper ions are added, anthraquinone and copper ions are combined and coordinated, which means that the concentration of anthraquinone in the system decreases, which affects the absorbance of anthraquinone. Therefore, as the amount of copper ions added increases, the maximum absorbance decreases gra...
Embodiment 3
[0024] (1) Take 1 mmol of 1,4-dihydroxyanthraquinone, add 50 ml of acetonitrile, add 1 mmol of copper nitrate (solid or saturated solution), and fully react on a magnetic stirrer for 4 hours. The reaction is carried out at room temperature (10-30 ° C) ; Add EGDMA (ethylene glycol dimethacrylate) 18mmol, azobisisobutyronitrile (AIBN) 0.7mmol, under the protection of nitrogen, heat with an oil bath, and react at 65°C for 20h;
[0025] (2) Take the solid, that is, the polymer generated by the reaction in step (1), and wash it with methanol aqueous solution (the volume ratio of methanol to water is 1:4) to remove unreacted substances; then use 0.2mol / L nitric acid vibration washing Remove copper ions, wash 3 to 4 times, each shaking washing time is 30 to 60 minutes, and the amount of nitric acid solution is 25 to 150ml / time; wash with water until the pH is 7, and dry at 60°C.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com