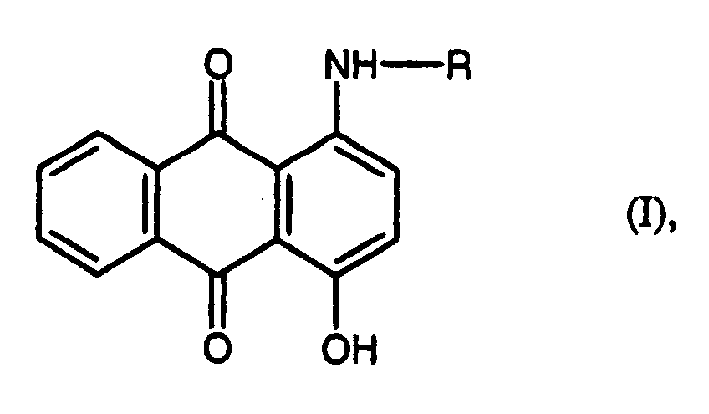
Preparation of 1-amino-4-hydroxy anthraquino
A technology of hydroxyanthraquinone and amino, which is applied in the field of preparing 1-amino-4-hydroxyanthraquinone, can solve the problems of low space-time yield and dye not very bright, and achieve improved product quality and excellent space-time yield. The effect of high efficiency and excellent thermal stability
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 11
[0046] Embodiment 11-p-toluidino-4-hydroxyl anthraquinone:
[0047] In a 1000ml four-necked bottle, in 413 parts of N-methylpyrrolidone, add 271 parts (1.13mol) of quinizarin, 17 parts (0.07mol) of dihydroquinizarin, 157 parts (1.47mol) under vigorous stirring p-Phenylenediamine, 4 parts (0.065 mol) of boric acid, 4.5 parts of water and 35.5 parts (0.355 mol) of 90% lactic acid. The mixture was heated to 90°C and maintained at this temperature for 12 hours, then cooled and filtered. The resulting product was washed with 500 parts of hot methanol at 60°C and dried. 370 parts (94% yield) of a dark purple product were obtained which, when used to dye polystyrene, give a very bright purple color.
Embodiment 2
[0048] Example 21-anilino-4-hydroxyanthraquinone:
[0049] Example 1 was repeated except that 157 parts of p-toluidine were replaced by 136 parts (1.46 mol) of aniline. 312 parts (83% yield) of a dark purple product were obtained which, when used to dye polystyrene, provided a purple shade of similar color strength to the dye described in Example 1, but significantly redder Some.
Embodiment 31
[0050] Example 31-P-acetamidoanilino-4-hydroxyanthraquinone:
[0051] Example 1 was repeated again, except that 220 parts (1.47 mol) of p-amino-acetanilide was used instead of 157 parts of p-toluidine, and 600 parts of NMP was used instead of 400 parts. 312 parts of a deep purple product were obtained which when used to dye polystyrene give a very bright purple. This shade has similar color strength to the dye described in Example 1, but is slightly bluer.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com