Sodium fluoborate compound, sodium fluoborate birefringent crystal as well as preparation method and application
A birefringent crystal, sodium fluoroborate technology, applied in chemical instruments and methods, boron halide compounds, crystal growth, etc., can solve the problems of small size, unreachable wave band, high impurity content, etc., and achieve good mechanical properties and growth speed Fast, high product purity effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
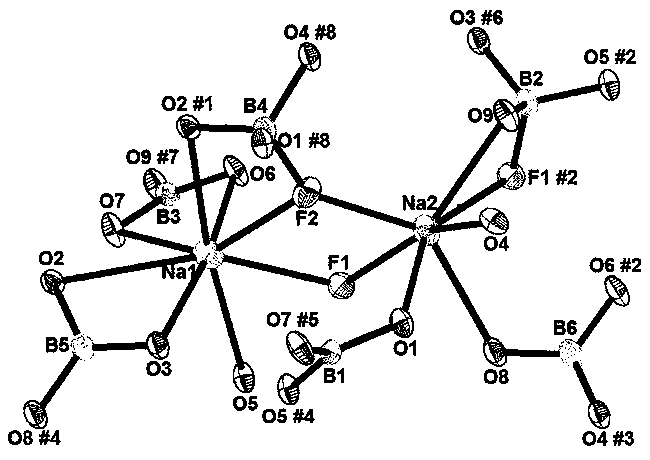
[0030] By chemical formula: NaBF 4 +NaBO 2 4H 2 O+4H 3 BO 3 =Na 2 B 6 o 9 f 2 +6H 2 O↑+2HF↑, sodium fluoroborate (Na 2 B 6 o 9 f 2 ) compounds:
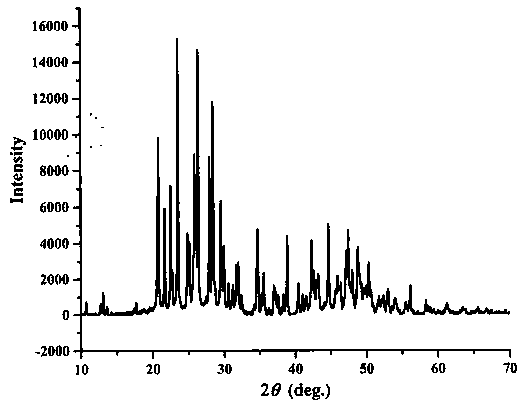
[0031] NaBF 4 :NaBO 2 4H 2 O:H 3 BO 3 Weigh it into a mortar with a molar ratio of 1:1:4, mix and grind it carefully, put it into a corundum crucible, put it into a muffle furnace, slowly raise the temperature to 200°C, and keep the temperature constant for 12 hours. After cooling, take out the crucible, grind the sample evenly, then place it in the crucible, raise the temperature of the muffle furnace to 400°C, keep the temperature for 48 hours, take out the sample, put it in a mortar, crush and grind it, and the compound sodium fluoroborate single-phase Polycrystalline powder, then carry out X-ray analysis to this polycrystalline powder, gained X-ray spectrogram and finished product Na 2 B 6 o 9 f 2 The X-ray spectra of single crystals ground into powders are consistent;
[0032] The synthesized compound Na ...
Embodiment 2
[0037] According to the reaction formula NaBF 4 +NaBO 2 4H 2 O+2B 2 o 3 =Na 2 B 6 o 9 f 2 +2HF↑synthesis of Na 2 B 6 o 9 f 2 Compound, specific operation steps are carried out according to embodiment 1;
[0038] The synthesized compound Na 2 B 6 o 9 f 2 Weigh it with co-solvent PbO according to the stoichiometric ratio of 1:3, mix evenly, put it into a Φ90mm×90mm open platinum crucible, put the crucible into a crystal growth furnace, raise the temperature to 460°C, keep the temperature for 10 hours, then cool down to 440°C ℃ to obtain a sodium fluoroborate solution;
[0039] Slowly cool down to room temperature at a rate of 0.5°C / h, and crystallize to obtain seed crystals;
[0040] Growing crystals on the surface of the melt or in the melt: fix the obtained seed crystal on the seed crystal rod, lower the seed crystal from the top of the crystal growth furnace, make the seed crystal and extend into the sodium fluoroborate melt, cool down to 410, Constant tempe...
Embodiment 3
[0043] The reaction formula NaF+6H 3 BO 3 =Na 2 B 6 o 9 f 2 +9H 2 O ↑ Synthesis of Na 2 B 6 o 9 f 2 Compound, specific operation steps are carried out according to embodiment 1;
[0044] The synthesized compound Na 2 B 6 o 9 f 2 with co-solvent PbF 2 Weigh it according to the stoichiometric ratio of 1:4, mix it evenly, put it into an open platinum crucible of Φ80mm×80mm, put the crucible into a crystal growth furnace, raise the temperature to 500°C, keep the temperature for 12 hours, and then cool it down to 430°C to obtain fluorine Sodium borate compound solution;
[0045] Slowly cool down to room temperature at a rate of 5°C / h, and use the platinum wire suspension method to obtain small crystals as seed crystals during the cooling;
[0046] Crystal growth on the surface of the melt or in the melt: fix the obtained seed crystal on the seed rod, lower the seed crystal from the top of the crystal growth furnace, make the seed crystal contact with the surface of ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com