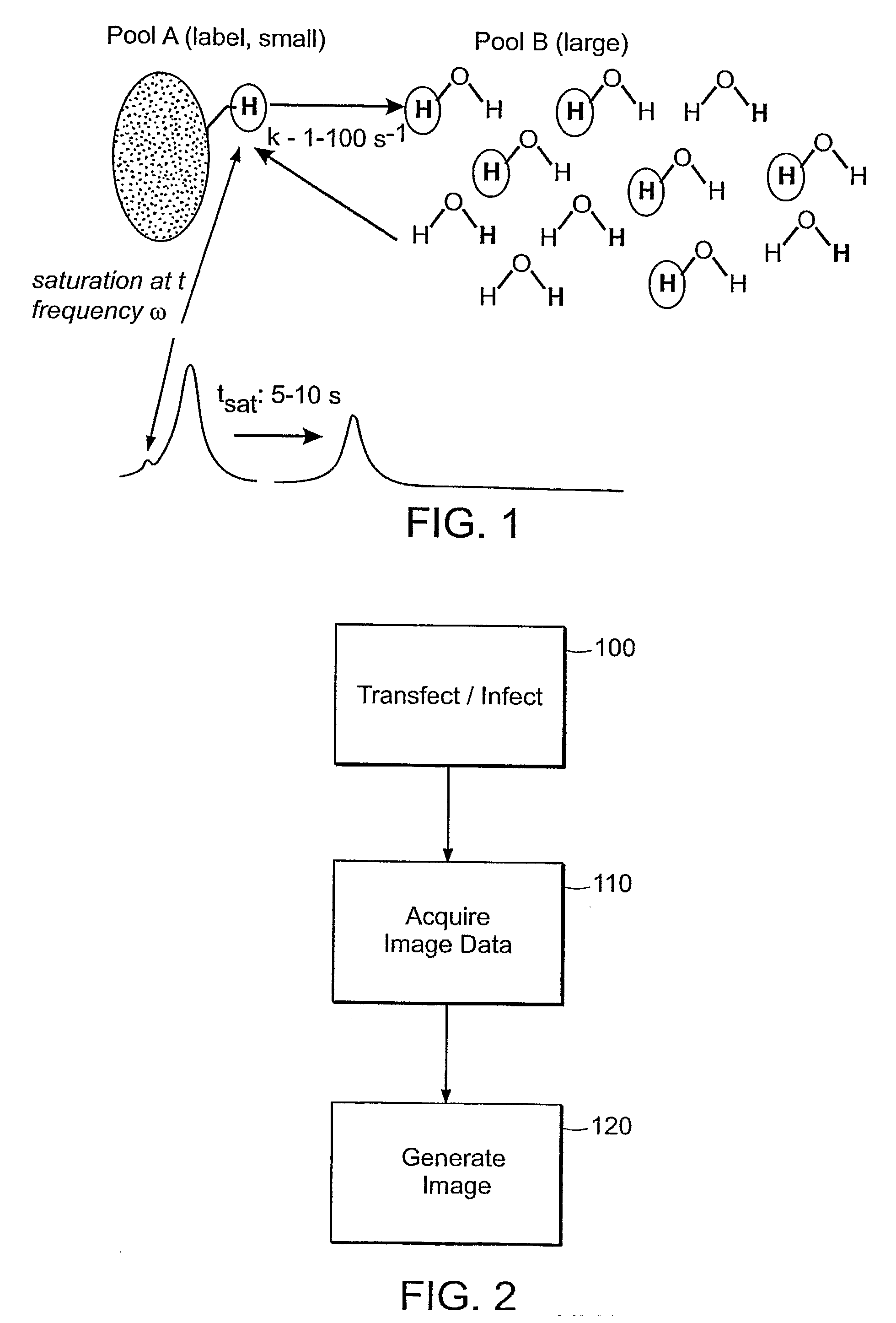
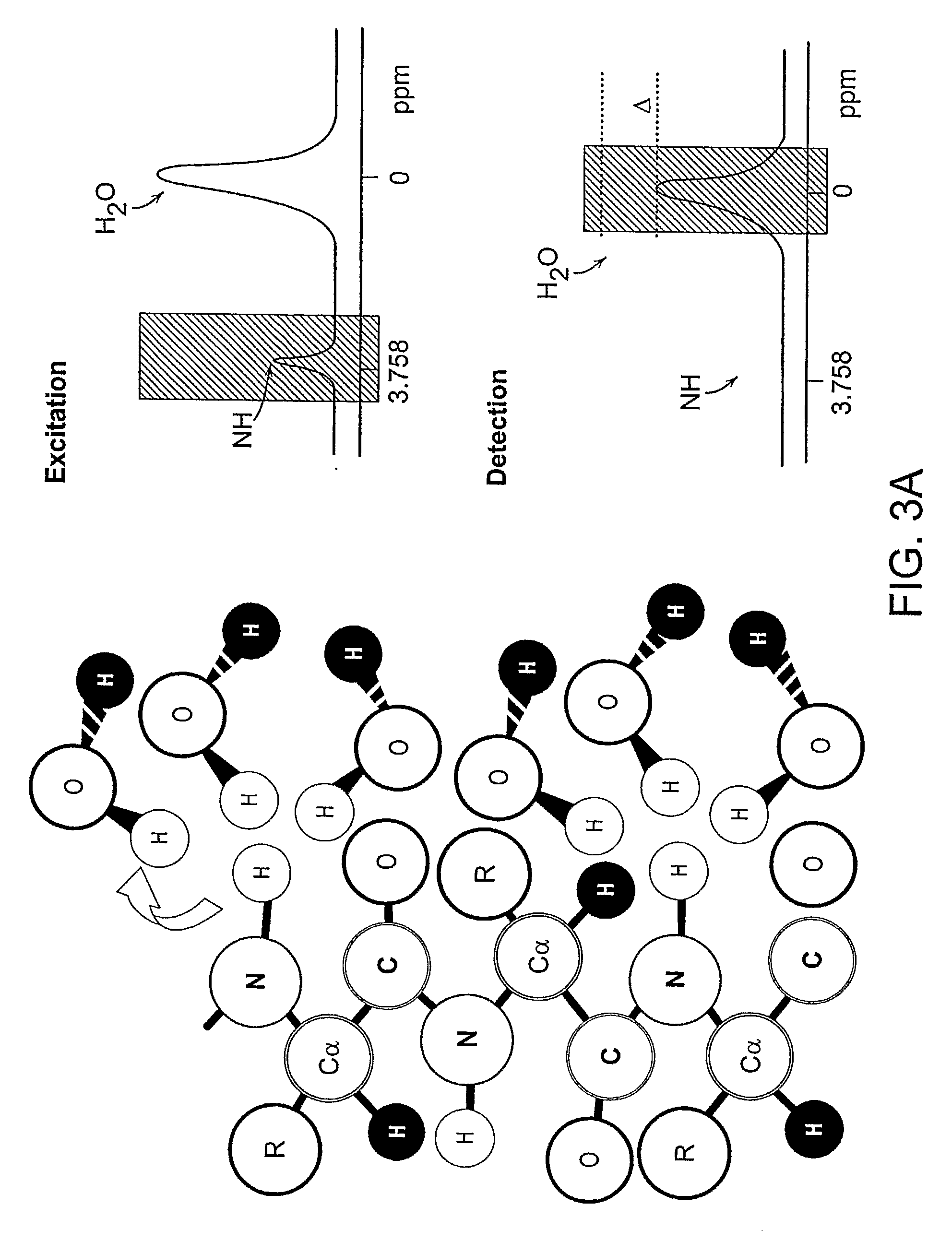
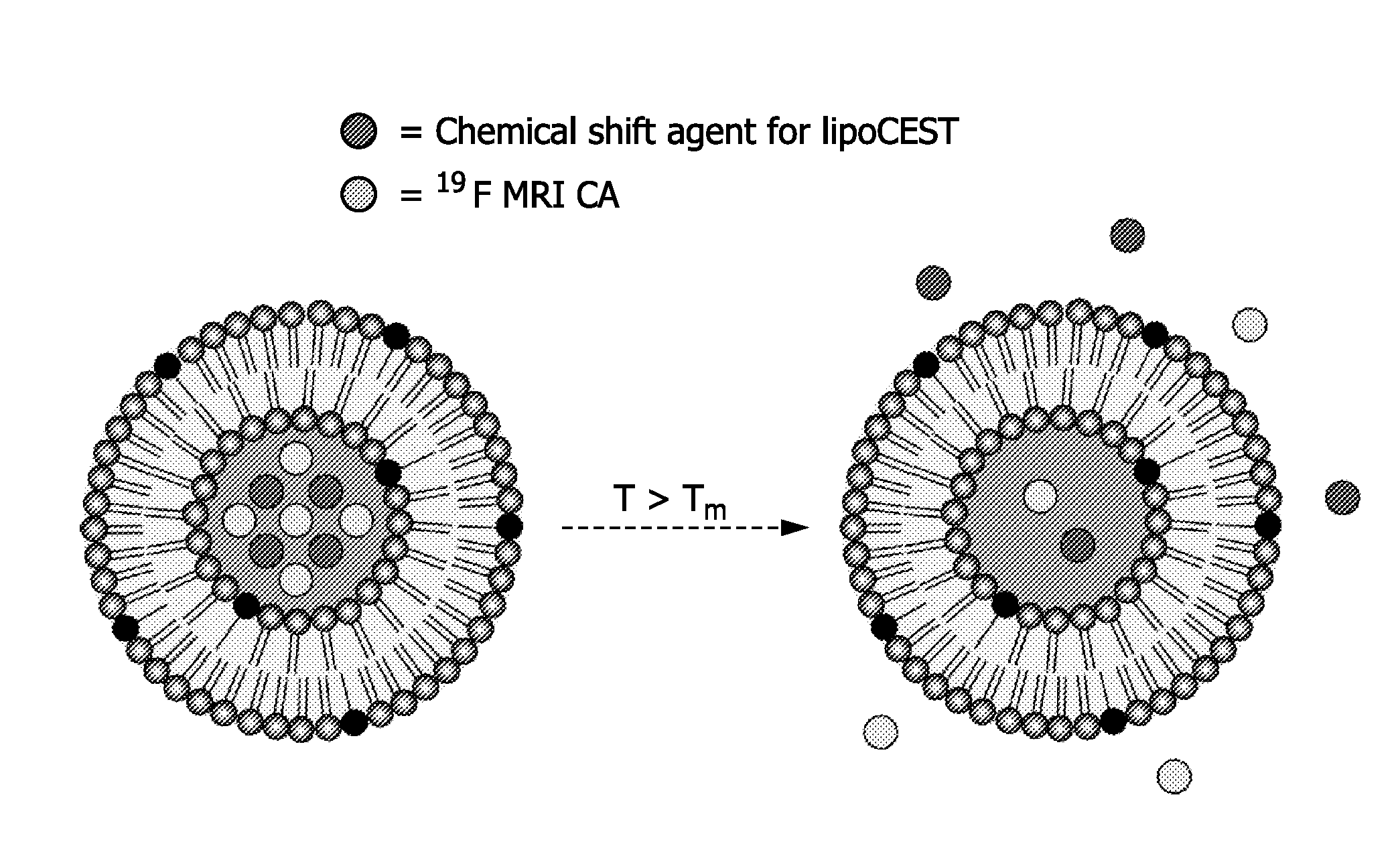
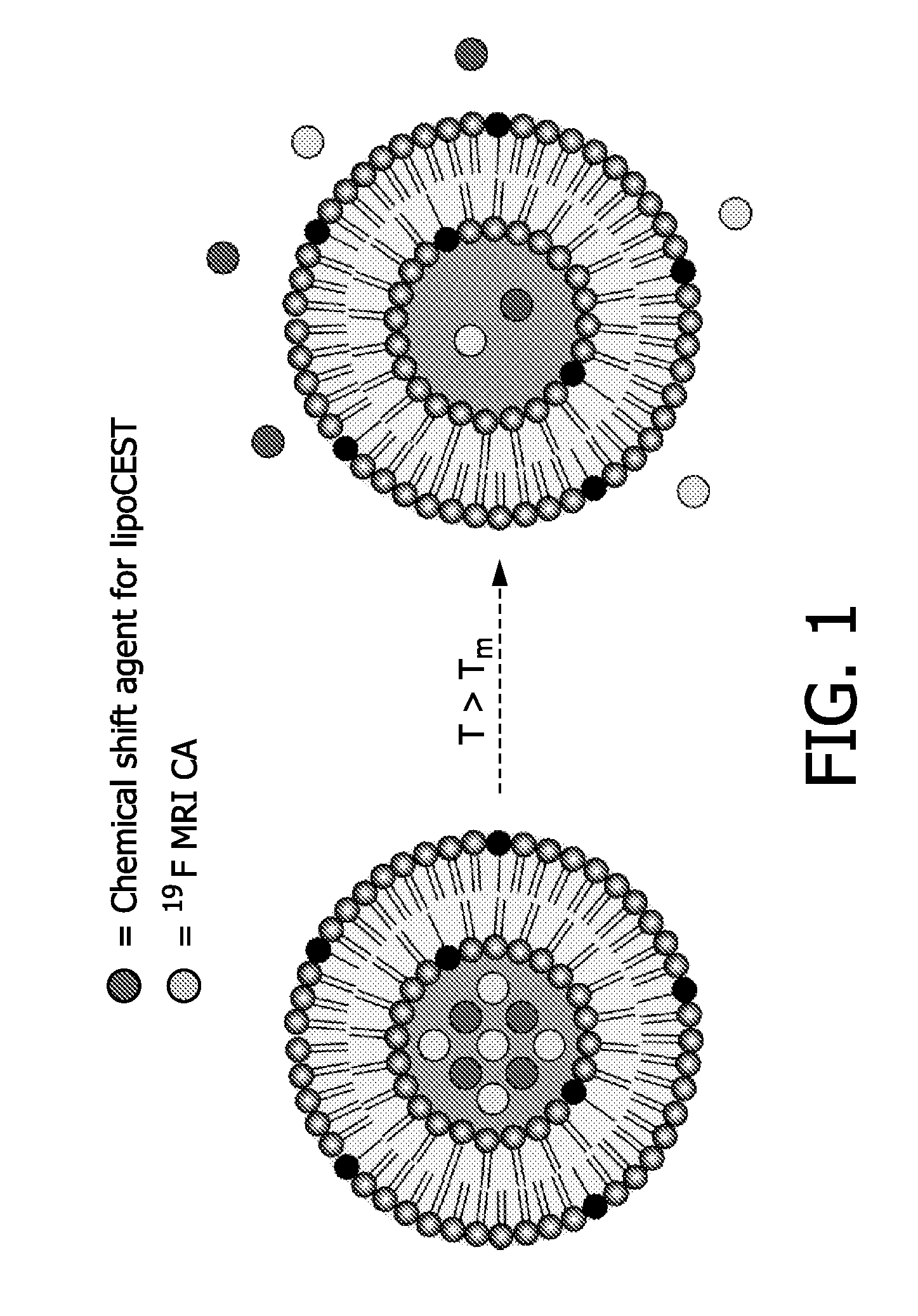
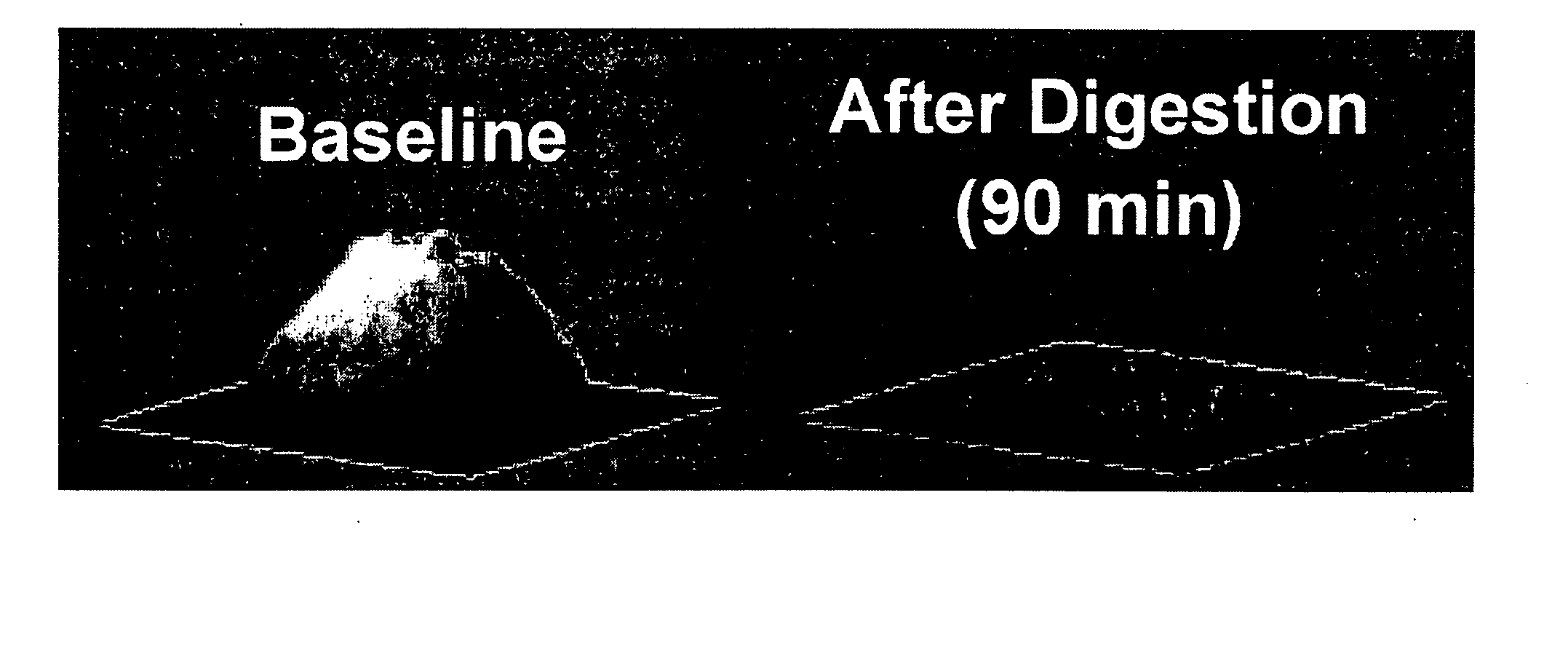
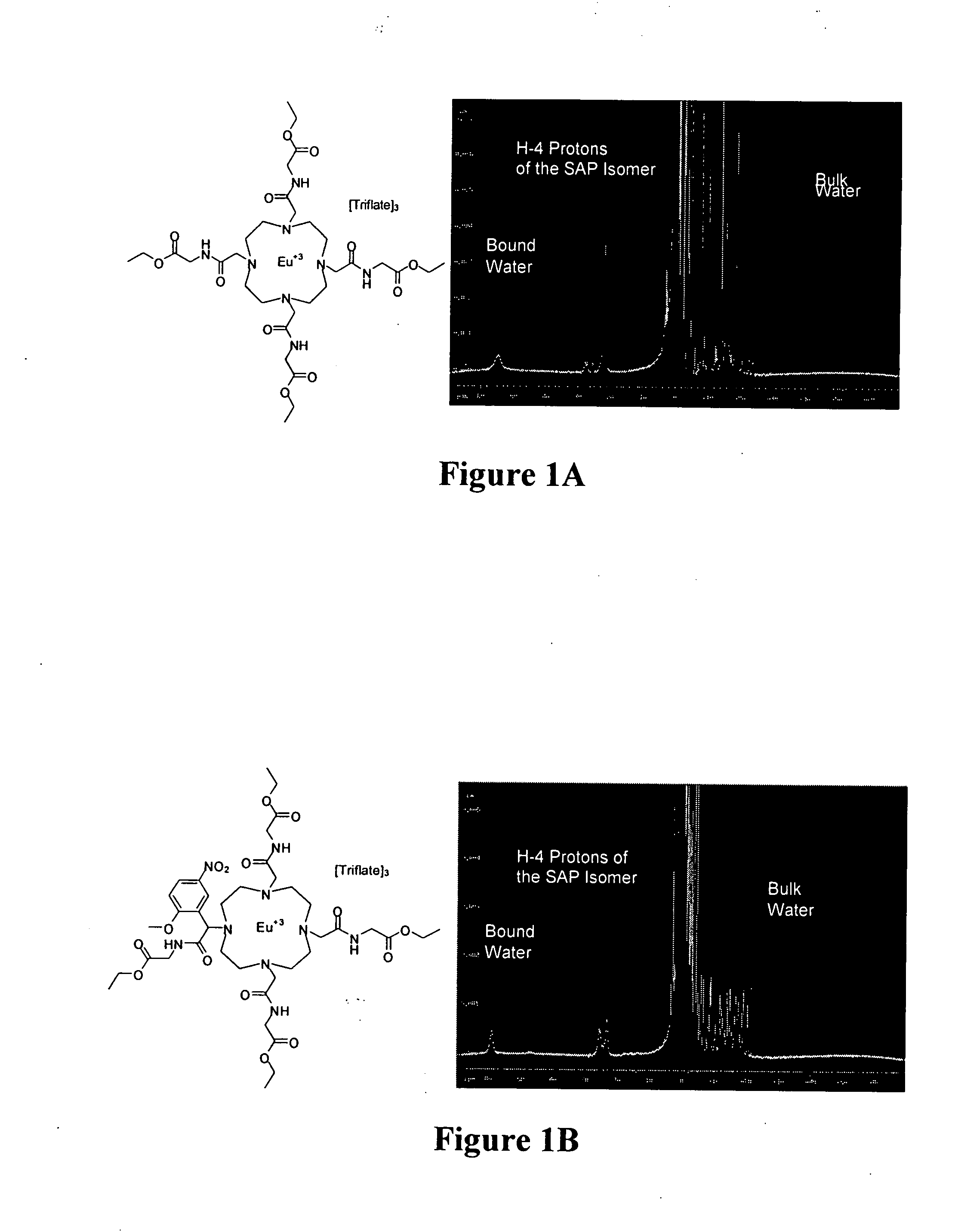
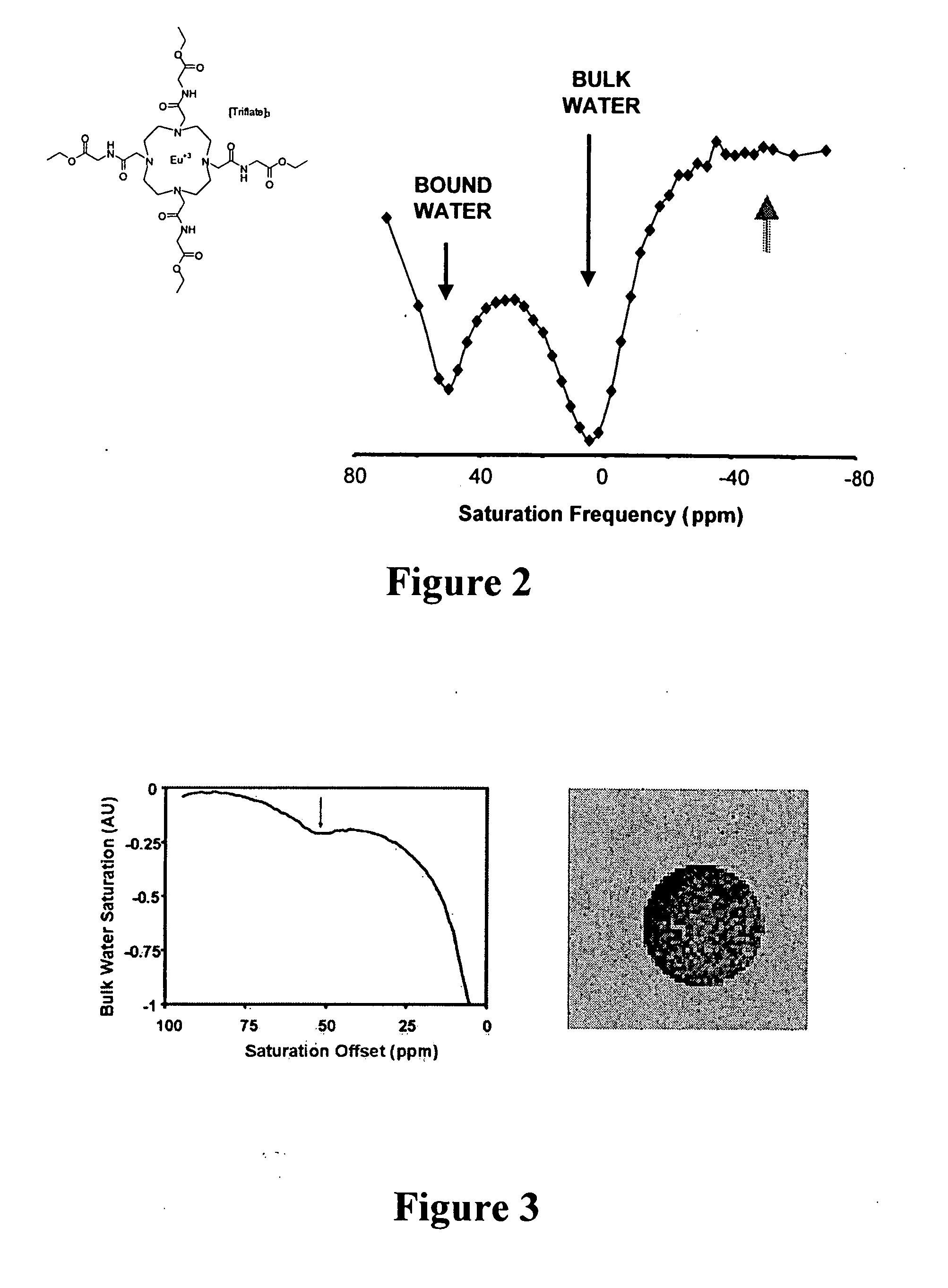
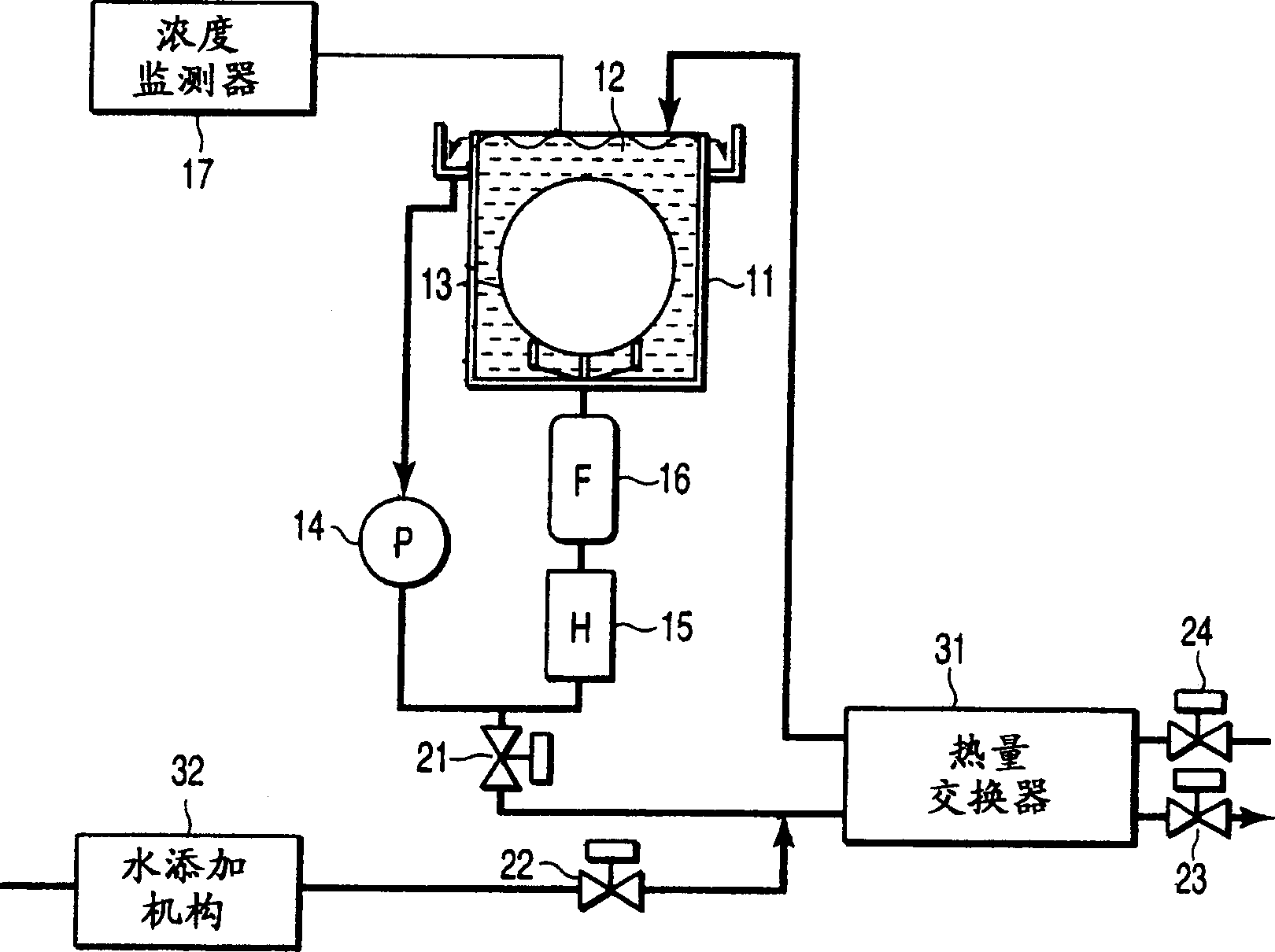
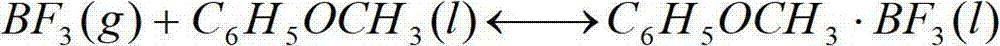Patents
Literature
146 results about "Chemical exchange" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
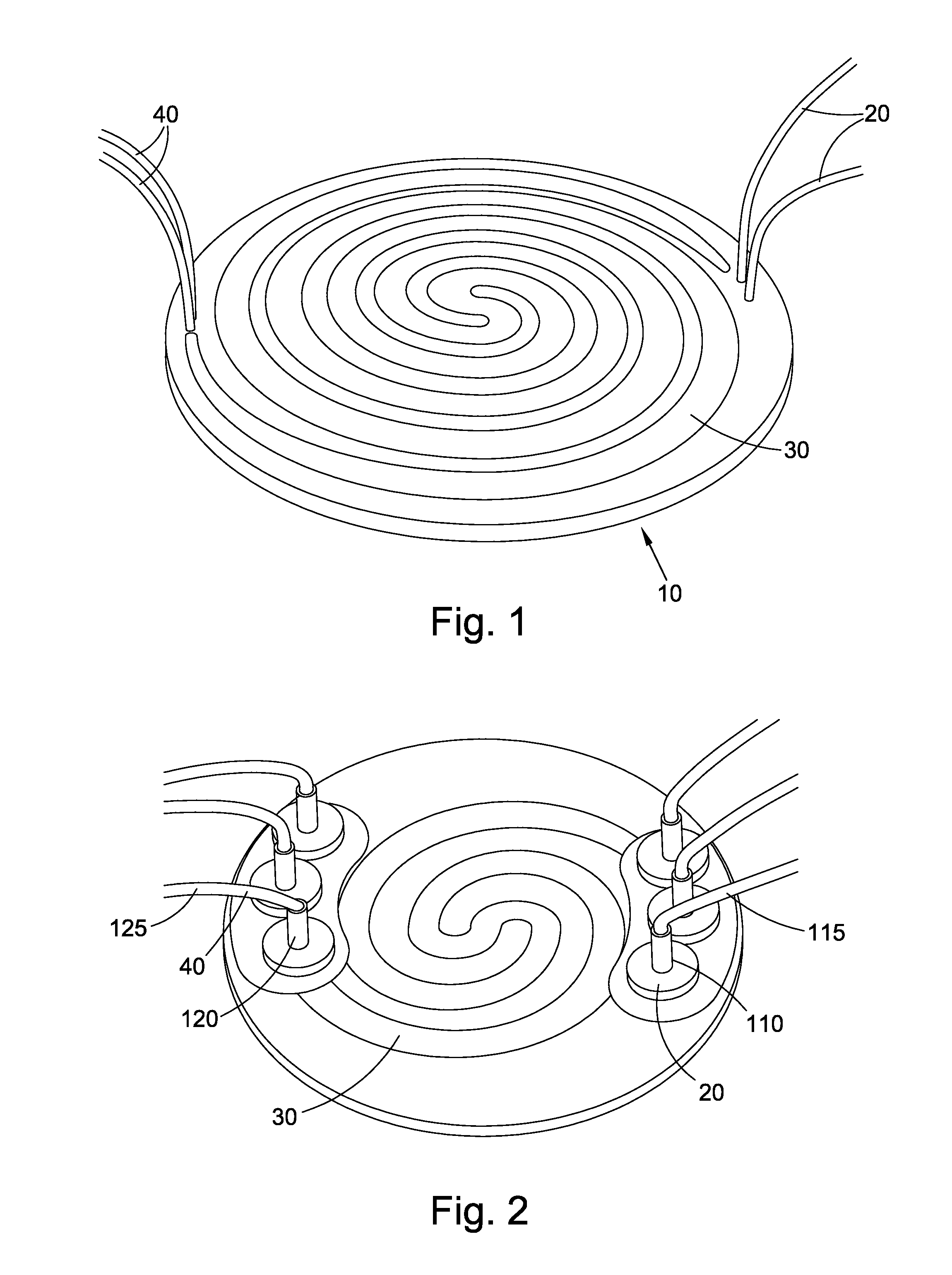
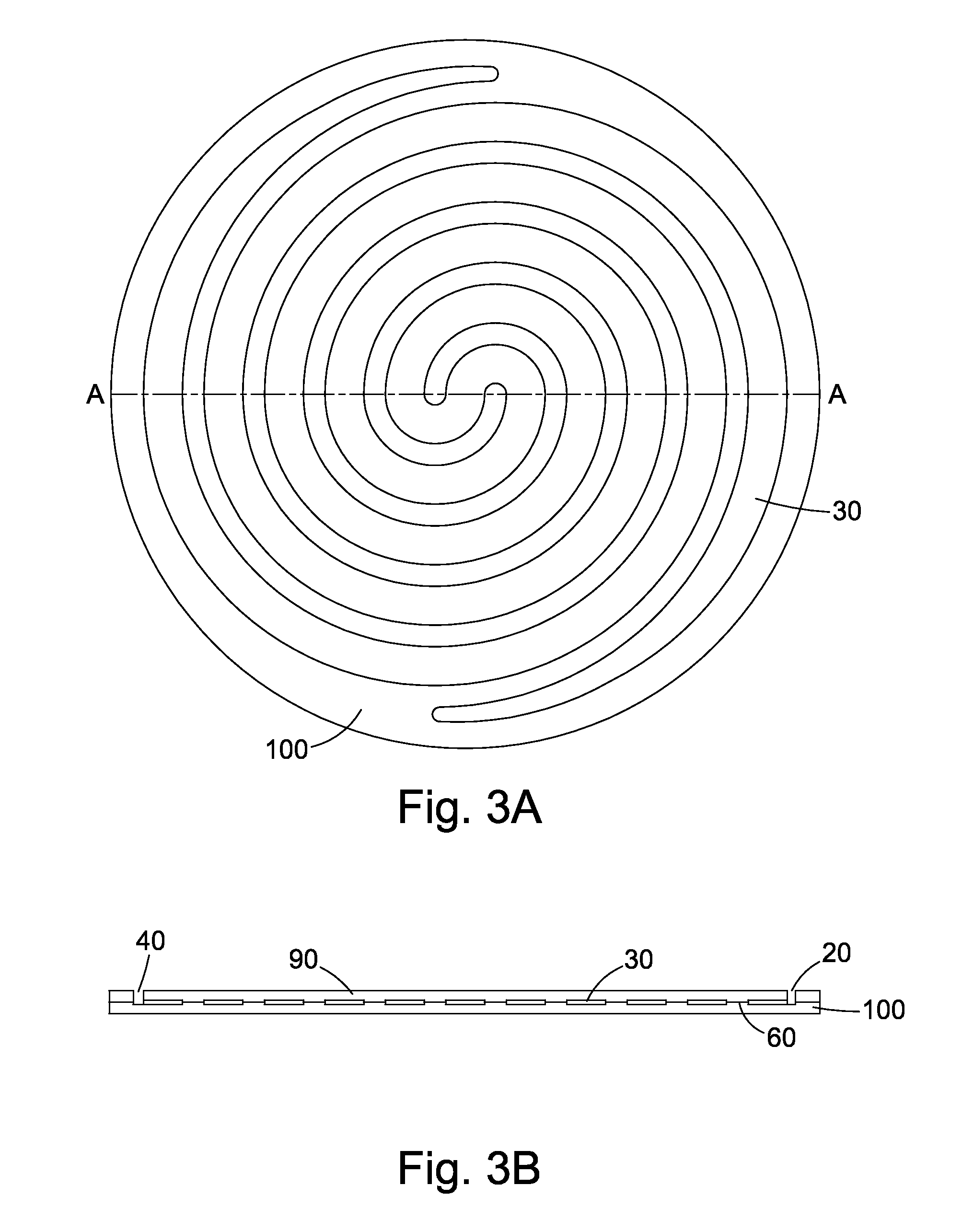
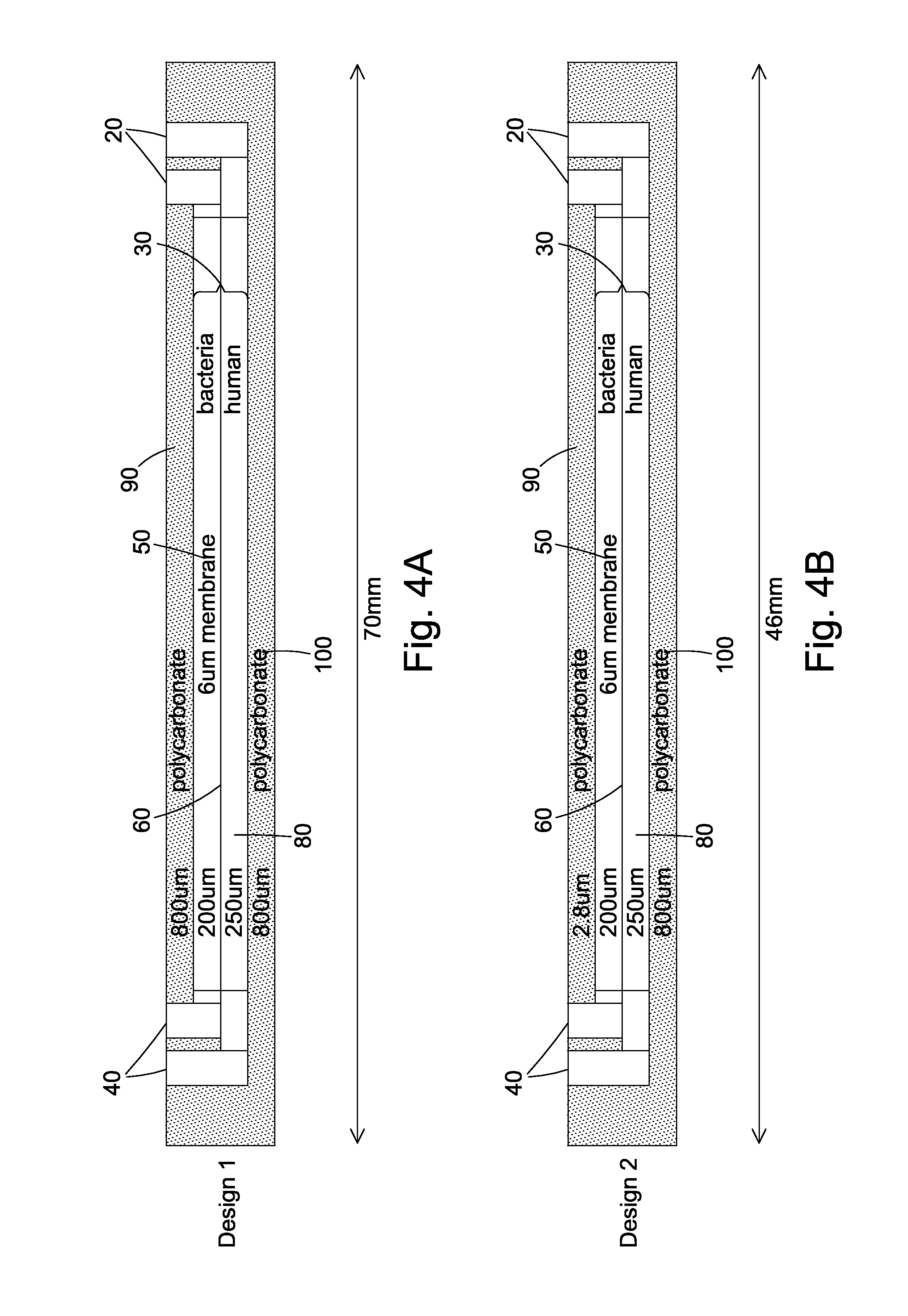
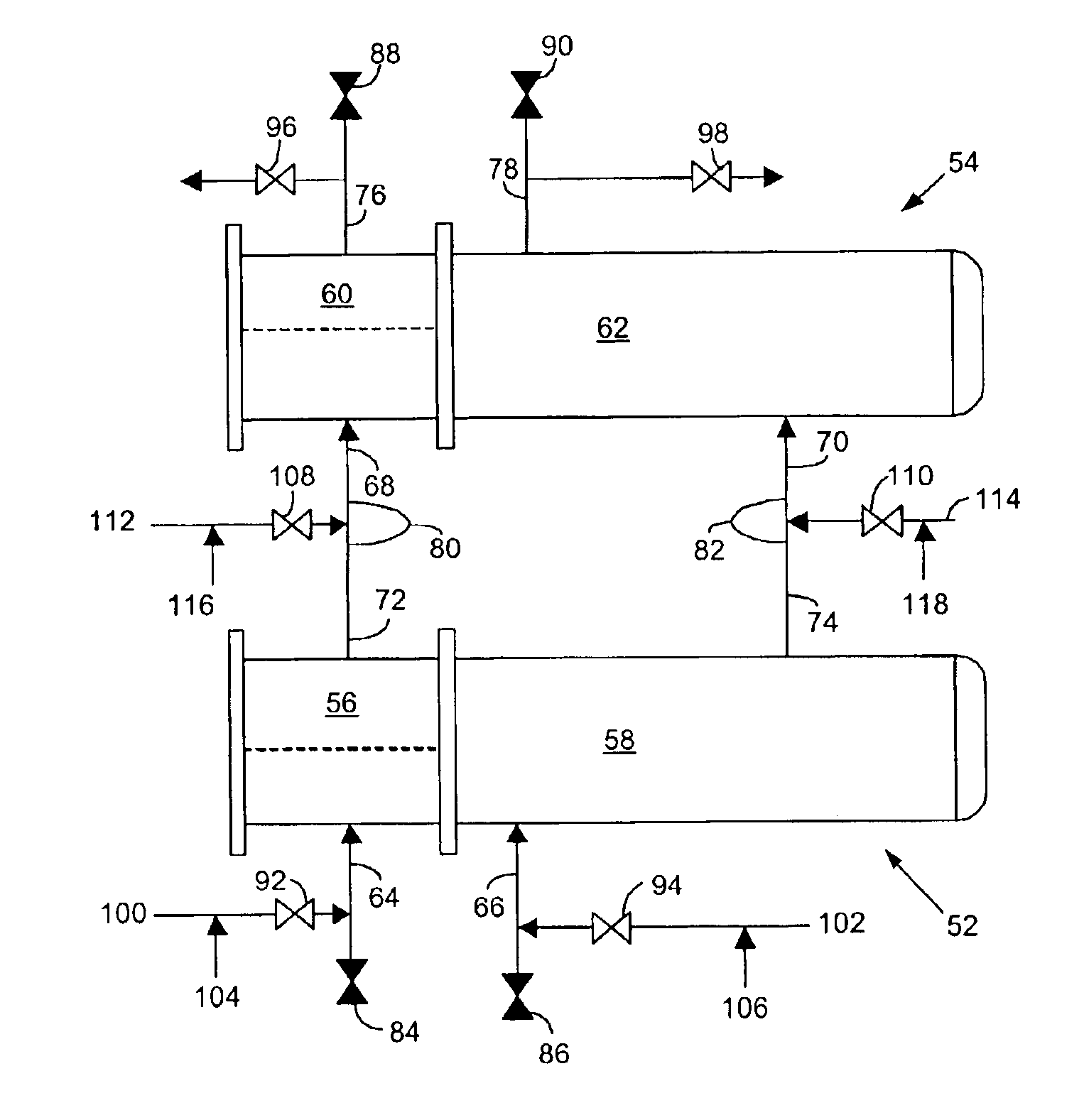
Cell culture apparatus and culture methods using same
InactiveUS20150072413A1Improve surface adhesionEffective medium supplyBioreactor/fermenter combinationsBiological substance pretreatmentsMicrobial fuel cellSemipermeable membrane
Cell culture apparatus comprising at least two adjacent cell cultivation channels separated by a permeable or semipermeable membrane, wherein at least one channel, for the majority of its length, has a cross sectional area of no more than 1 mm2, said channel being provided with entrance and exit means to permit the passage of media therethrough, allows co-culture of separate cell types, e.g. human and microbial cells, without mingling, allowing monitoring of cell cultures and chemical exchanges between the respective cell cultures.
Owner:THE ARIZONA BOARD OF REGENTS ON BEHALF OF THE UNIV OF ARIZONA +2
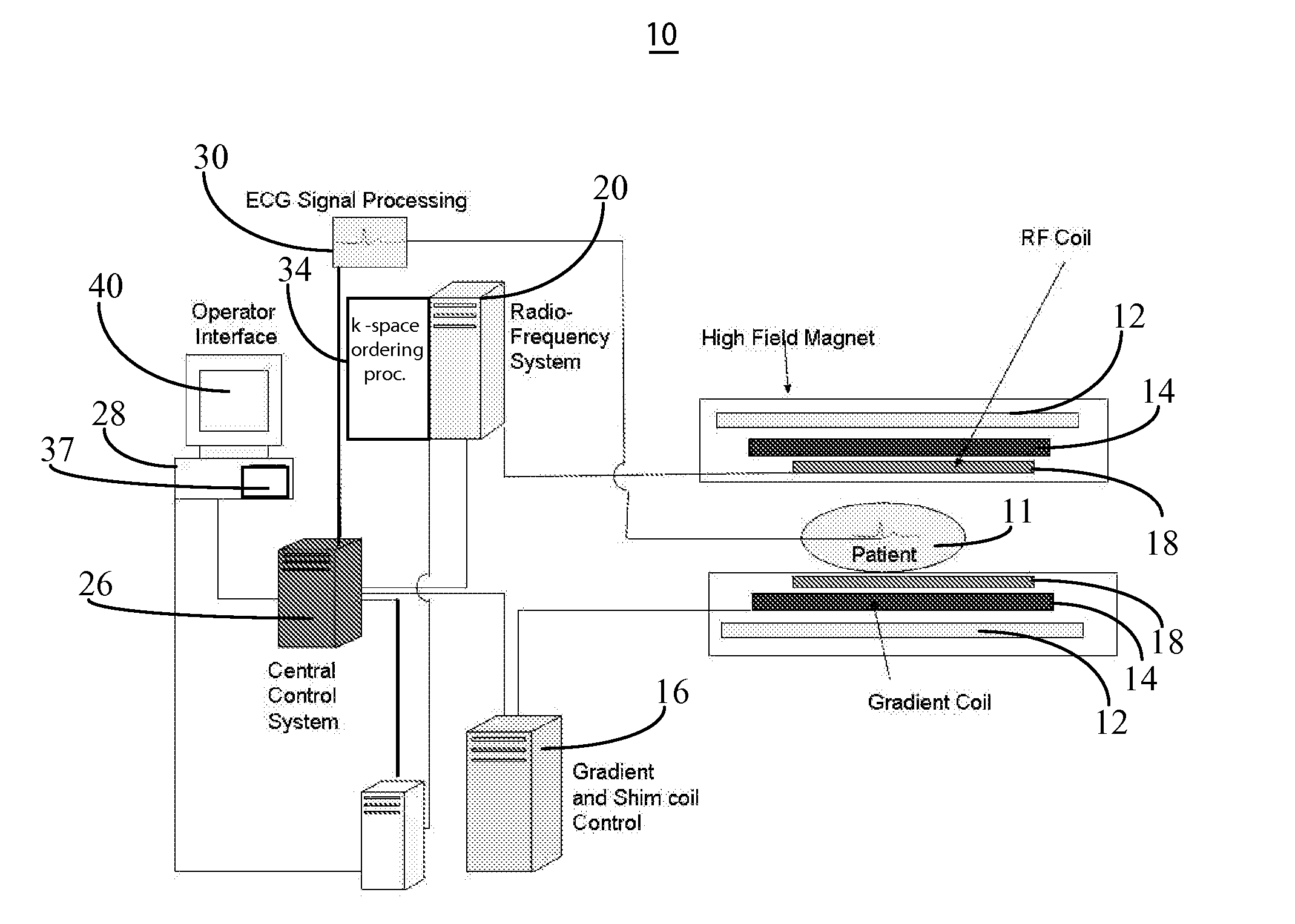
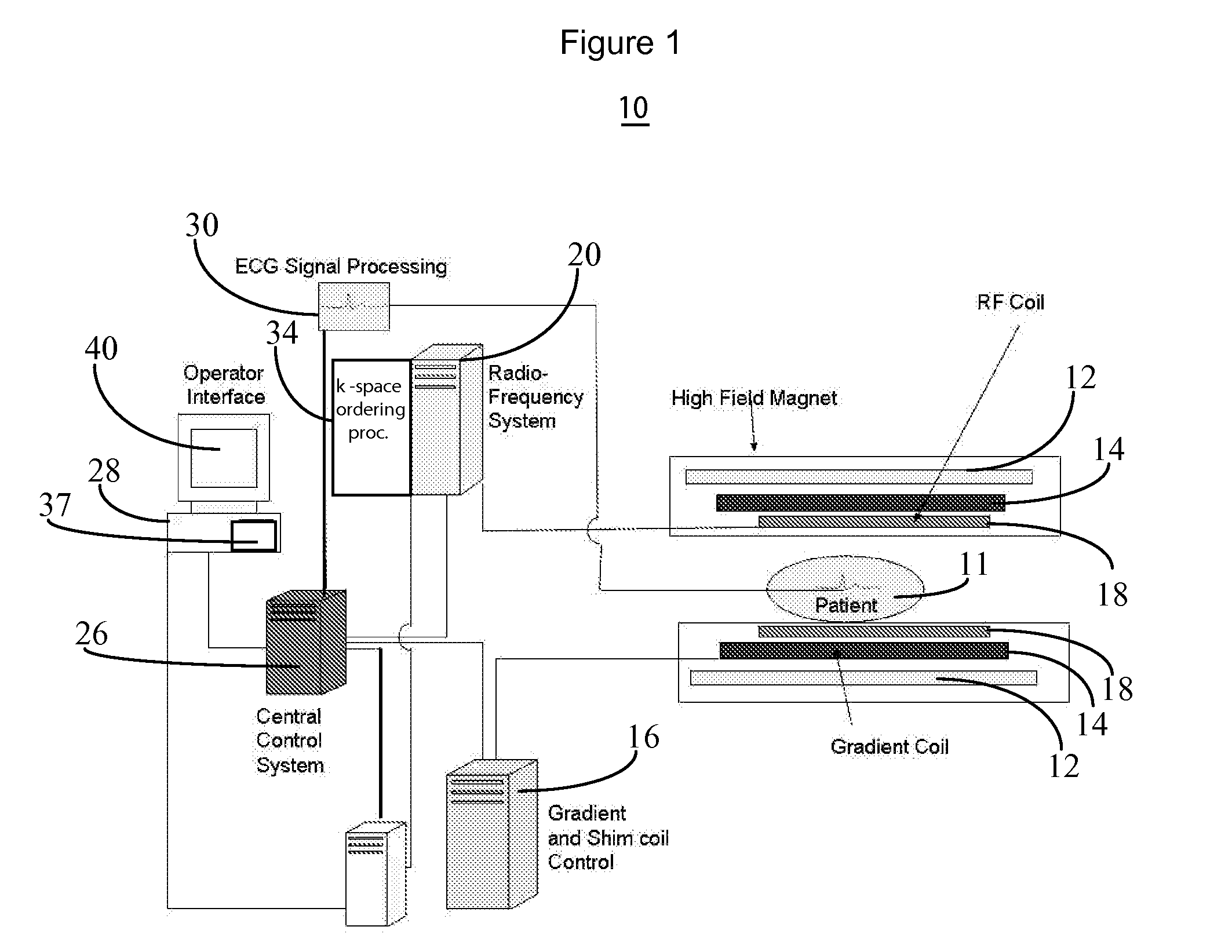
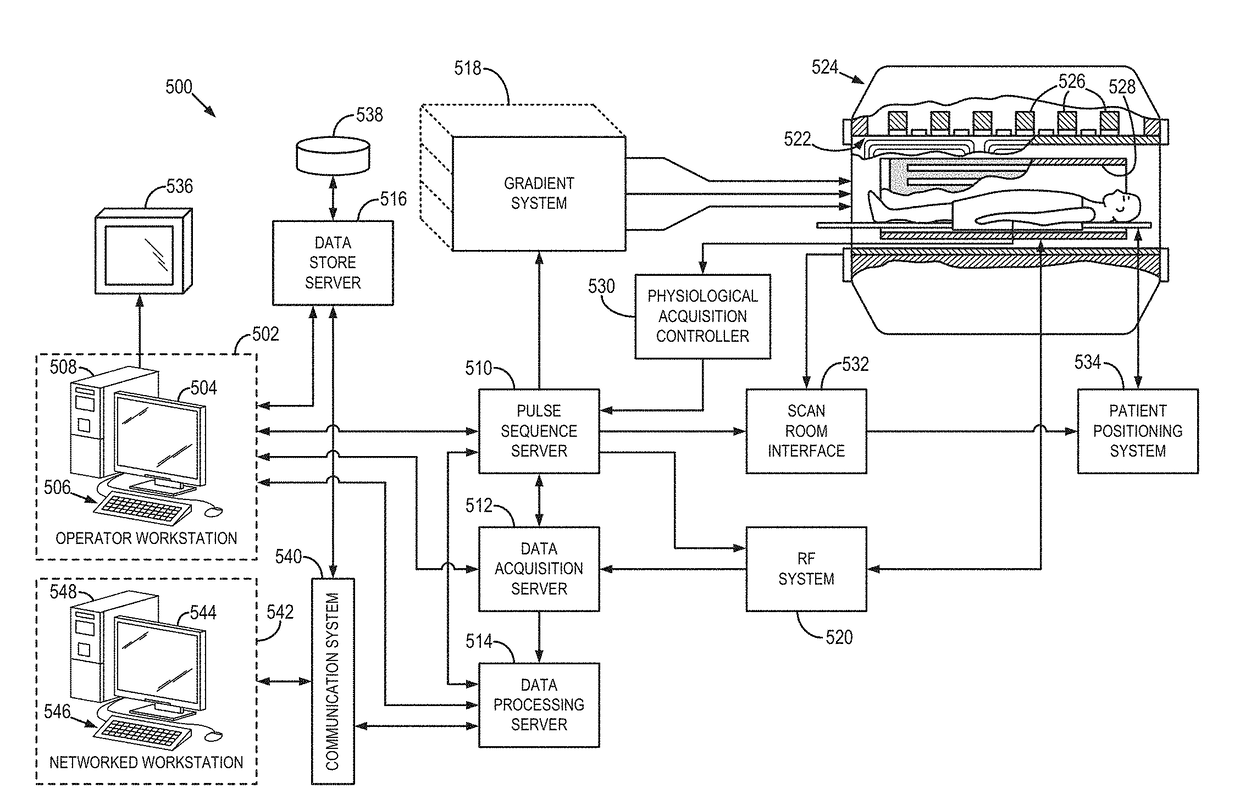
MR imaging using nested pulse sequence involving IR pulse
InactiveUS6850793B1Improve signal-to-noise ratioSignificant positive effectDiagnostic recording/measuringSensorsCross relaxationImage contrast
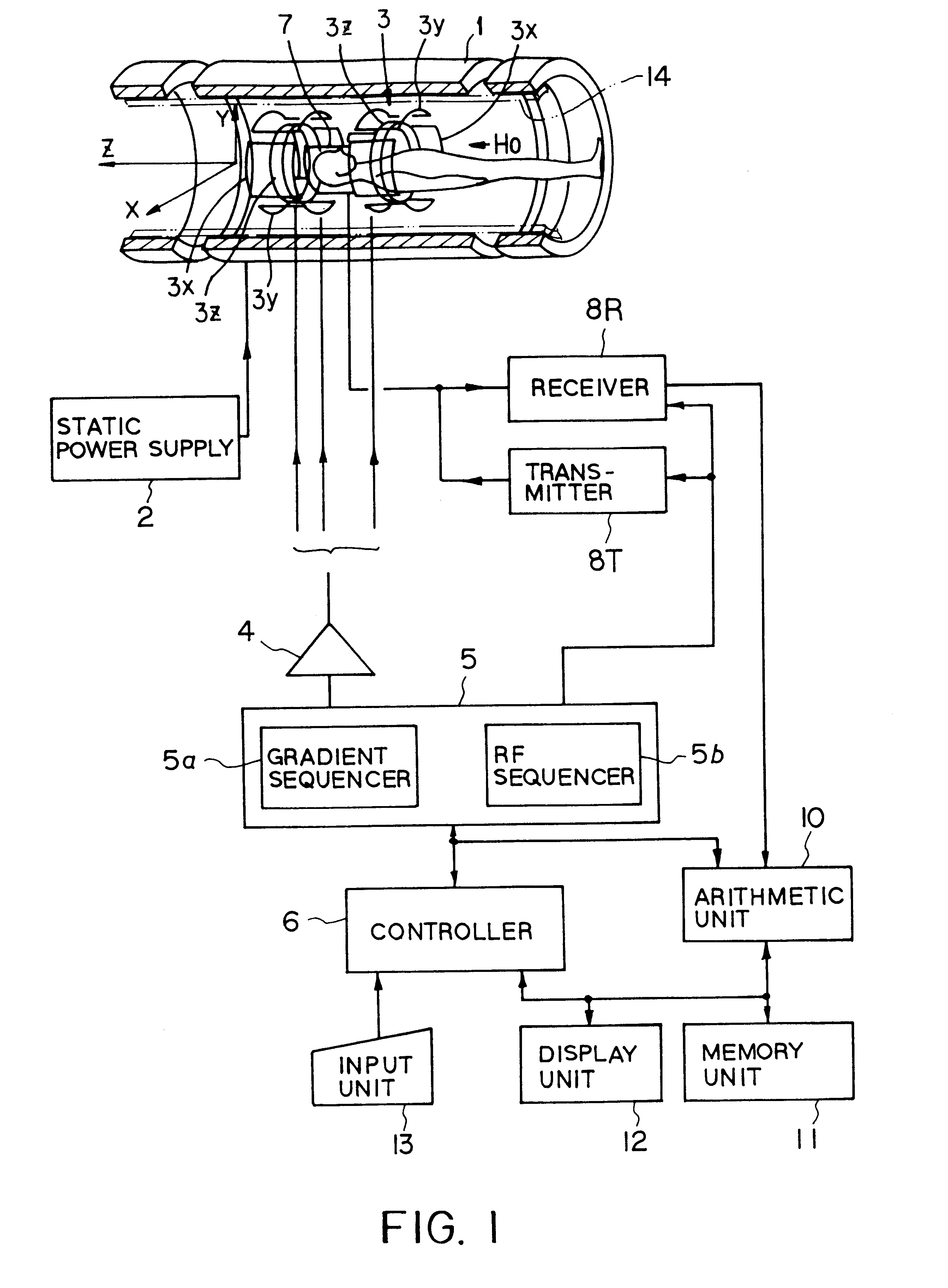
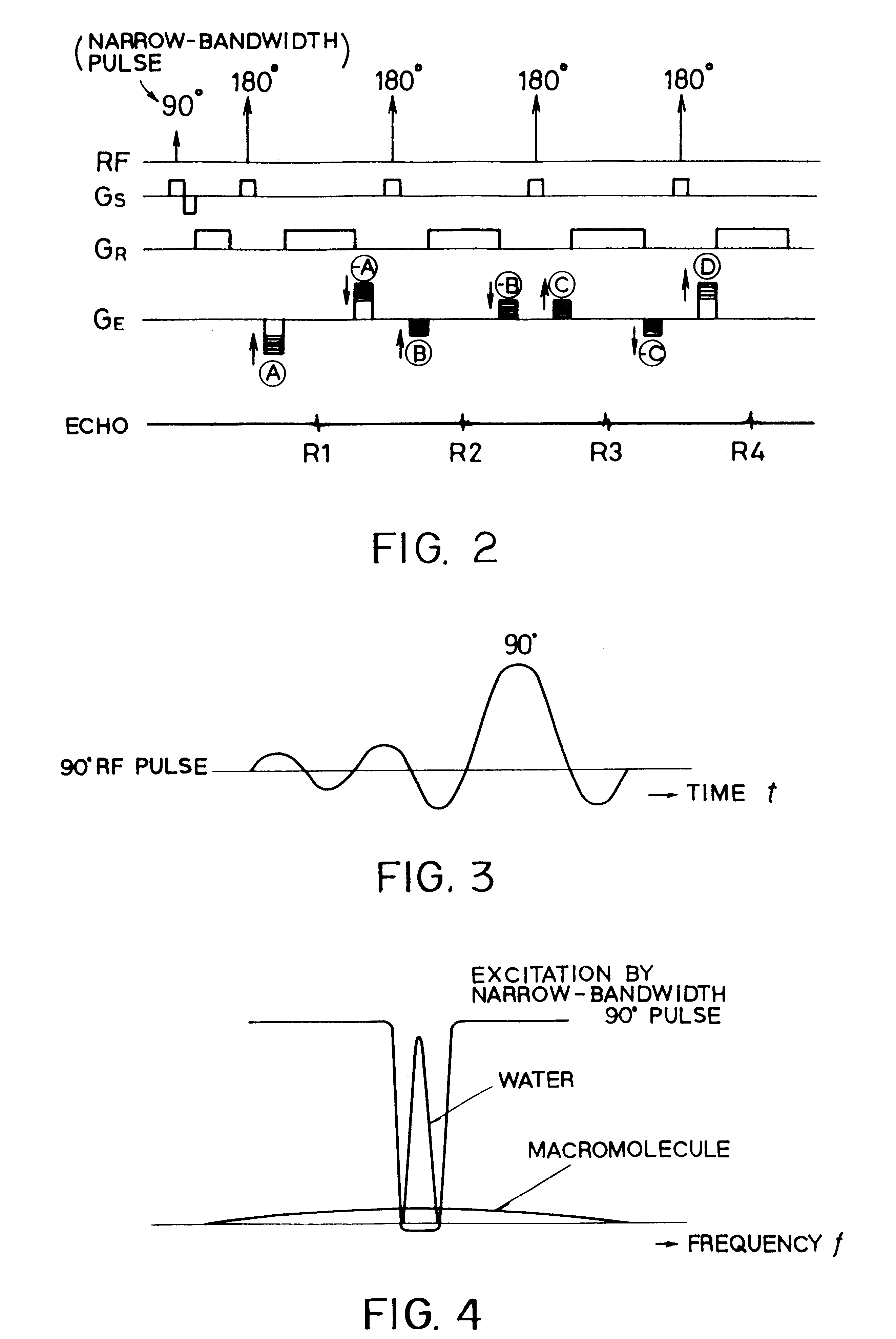
In addition to the known MT (magnetization transfer) effect, an RMT (reverse MT) is newly found, which increases a detected MR signal strength. Both the MT and RMT effects can be explained with mutual interaction, such as phenomena of chemical exchange and / or cross relaxation, acted between a pool of water proton spins and another pool of macromolecule proton spins, for example, within an object. In order to enhance the MT or RMT effect, the frequency bandwidths of RF pulses, such as a 90° RF exciting pulse in a SE or FSE method, an inversion pulse in a FLAIR or fast FLAIR method, and others, are controlled. To enhance the MT effect, the bandwidth is controlled into a wider value (approx. more than 1250 Hz) than the normally (conventionally) used bandwidth, while to obtain the RMT effect, the bandwidth is controlled into a narrower value (approx. less than 1000 Hz) than the normally used bandwidth. Actively controlling the MT or RMT effect permits changed image contrast in MR imaging.
Owner:KK TOSHIBA
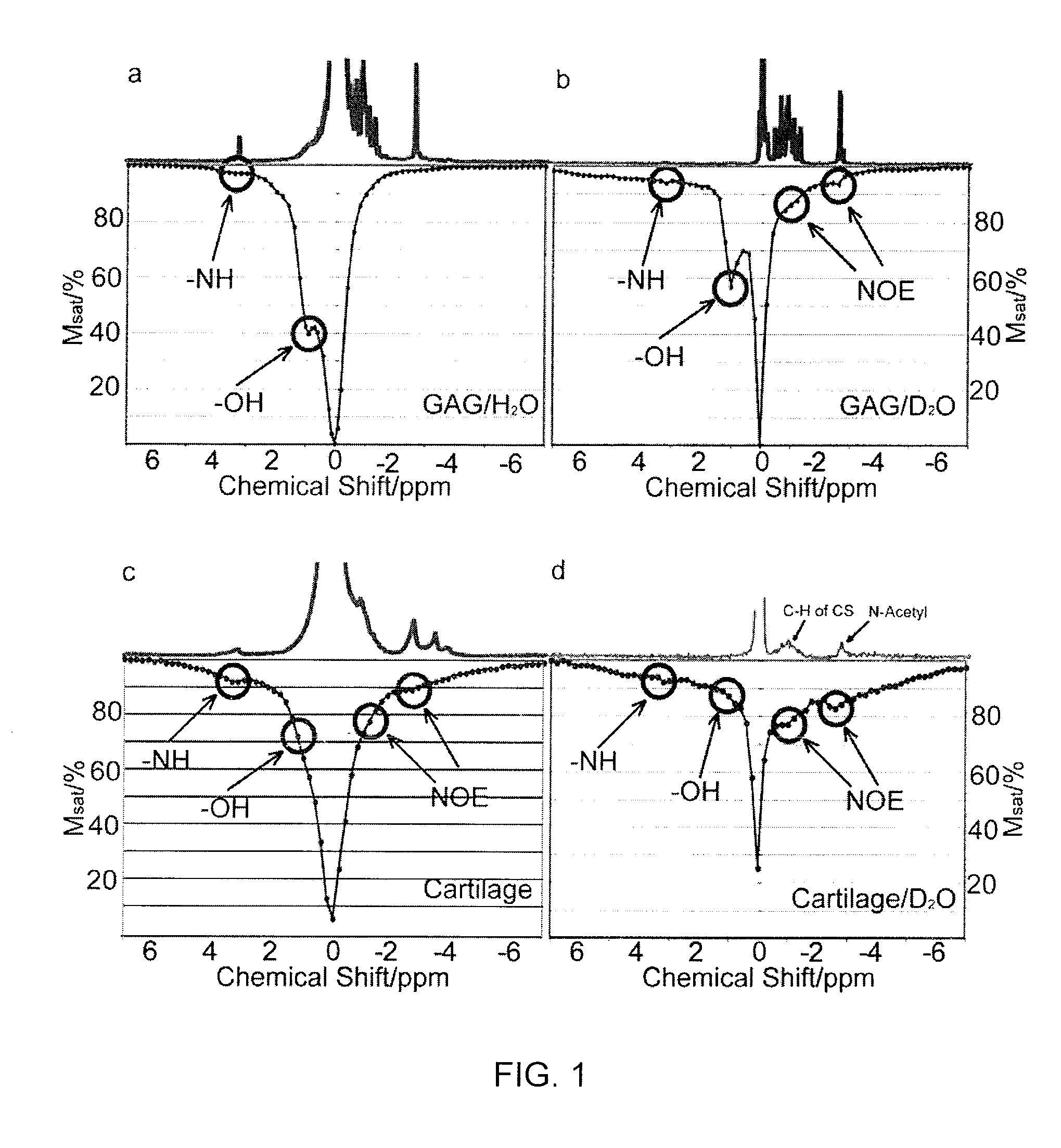
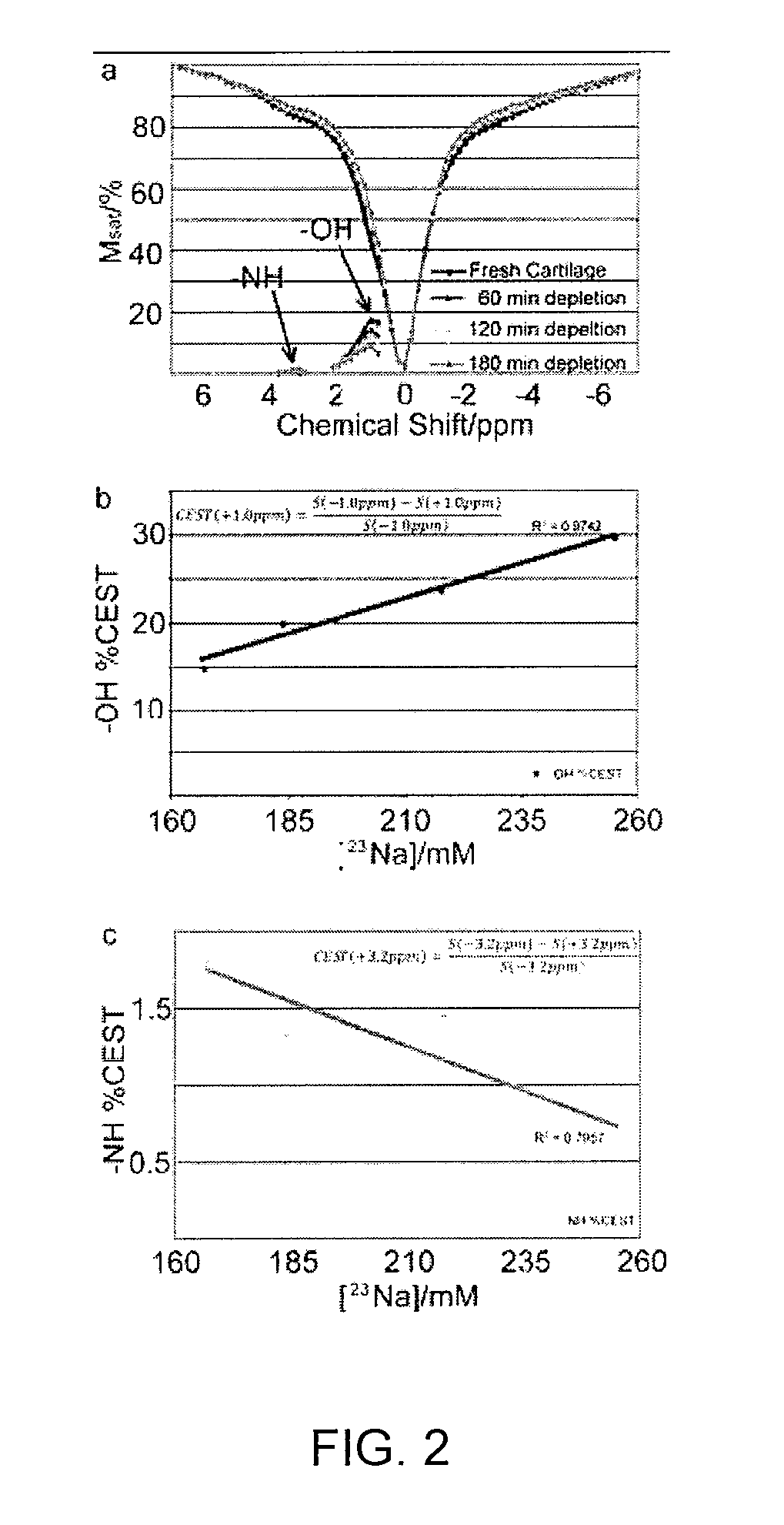
Method, System, and Computer-Accessible Medium for Assessment of Glycosaminoglycan Concentration in Vivo by Chemical Exchange Saturation Transfer
InactiveUS20110054299A1Measurements using NMR spectroscopyDiagnostic recording/measuringCross relaxationResonance
An exemplary methodology, procedure, system, method and computer-accessible medium can be provided to determine one or more particular frequencies of cross-relaxation between at least one molecule and at least one particular compound, determine a chemical exchange based on magnetic resonance data using a further frequency which is different from the one or more particular frequencies, and derive particular information about the anatomical region of interest based on the chemical exchange.
Owner:NEW YORK UNIV
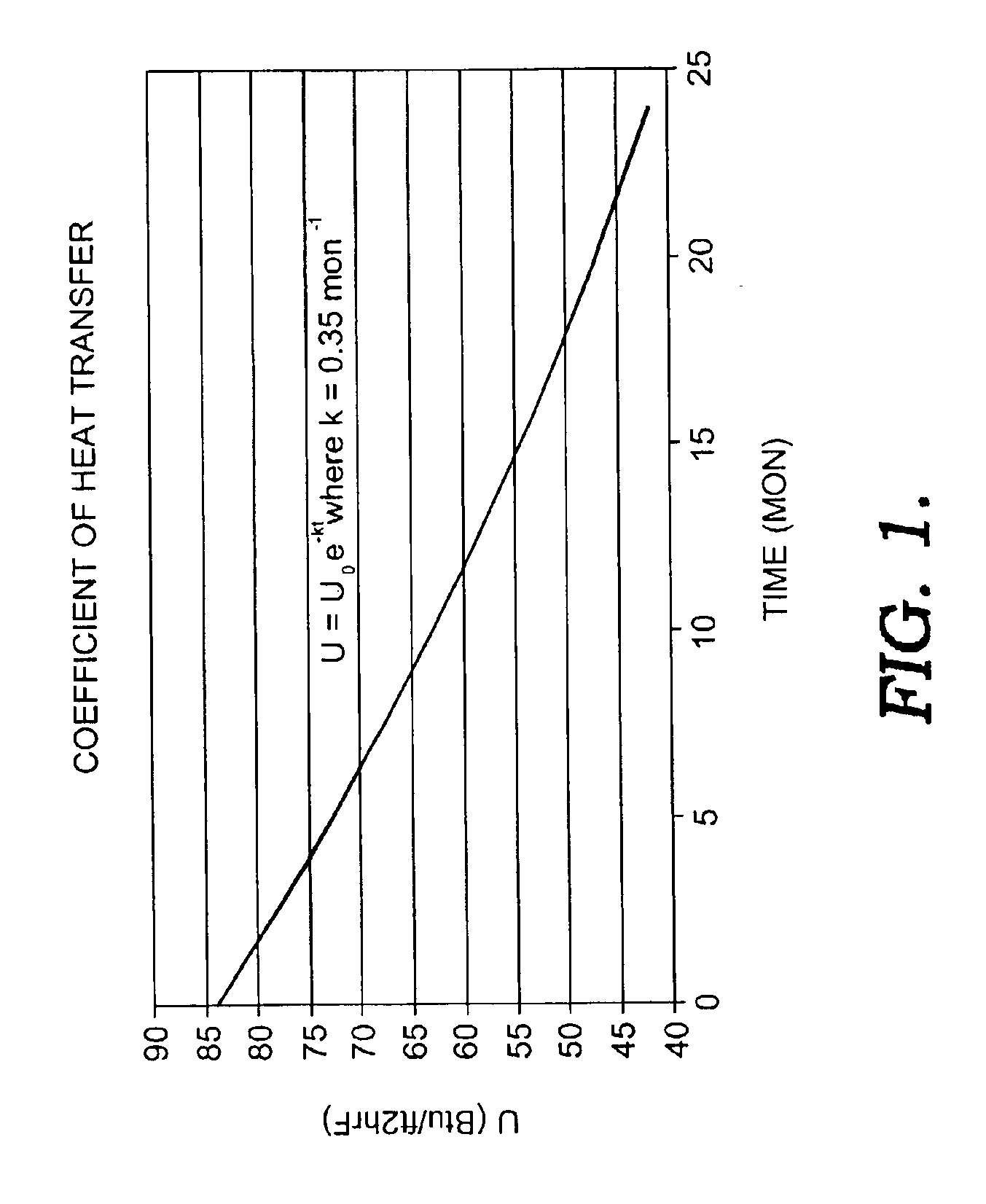
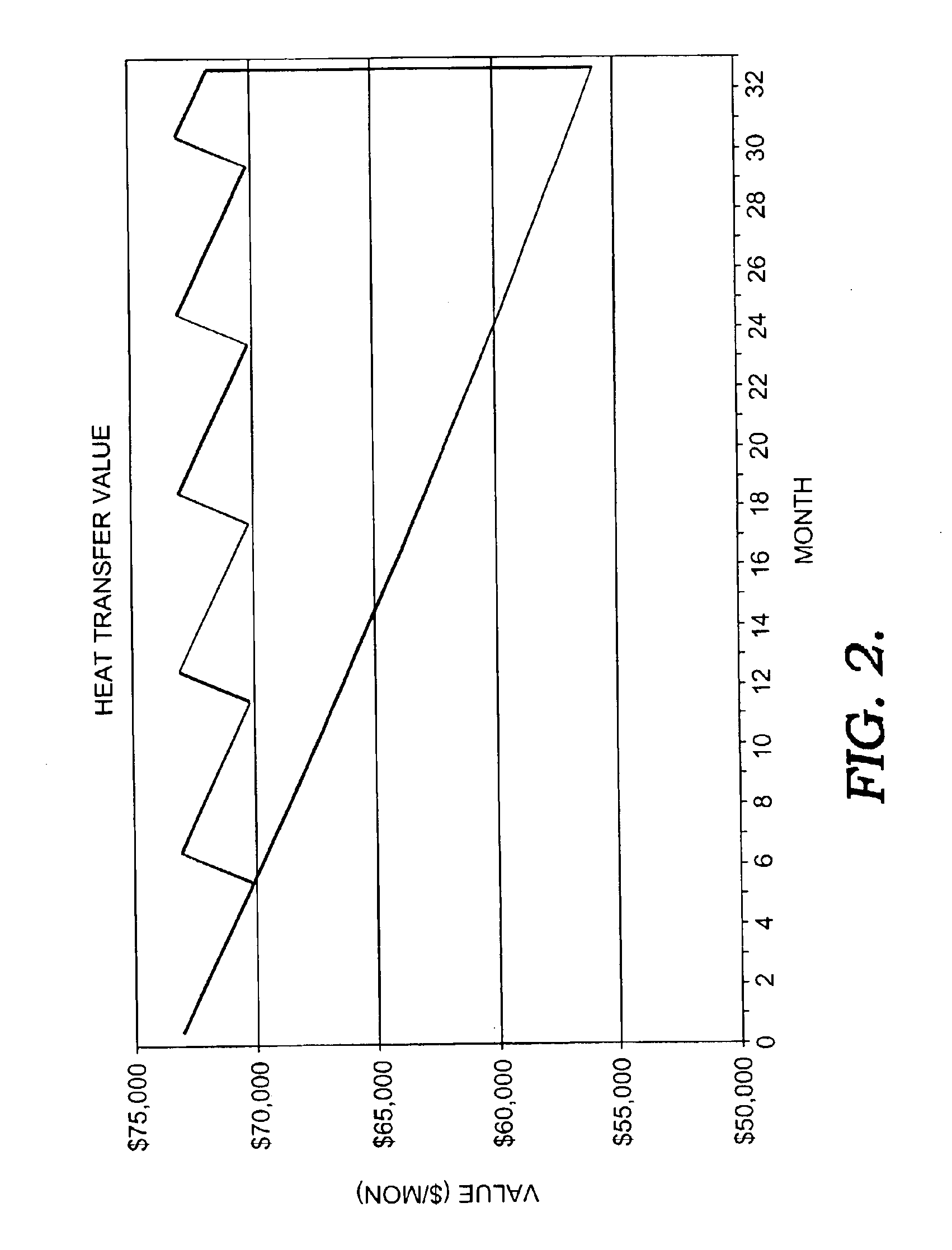
Heat exchanger cleaning process
InactiveUS6936112B2Cationic surface-active compoundsOrganic detergent compounding agentsCompound (substance)Engineering
Disclosed is a novel process for cleaning and restoring the operating efficiency of organic liquid chemical exchangers in a safe and effective manner and in a very short period of time, without a need to disassemble the equipment and without the need to rinse contaminate from the equipment after cleaning. Used is a formulation of monocyclic saturated terpene mixed with a non-ionic surfactant package specifically suited to oil rinsing. The terpene-based chemical is injected into organically contaminated exchangers using a novel process involving high-pressure steam to form a very effective cleaning vapor.
Owner:REFINED TECH
Semiconductor manufacturing apparatus and chemical exchanging method
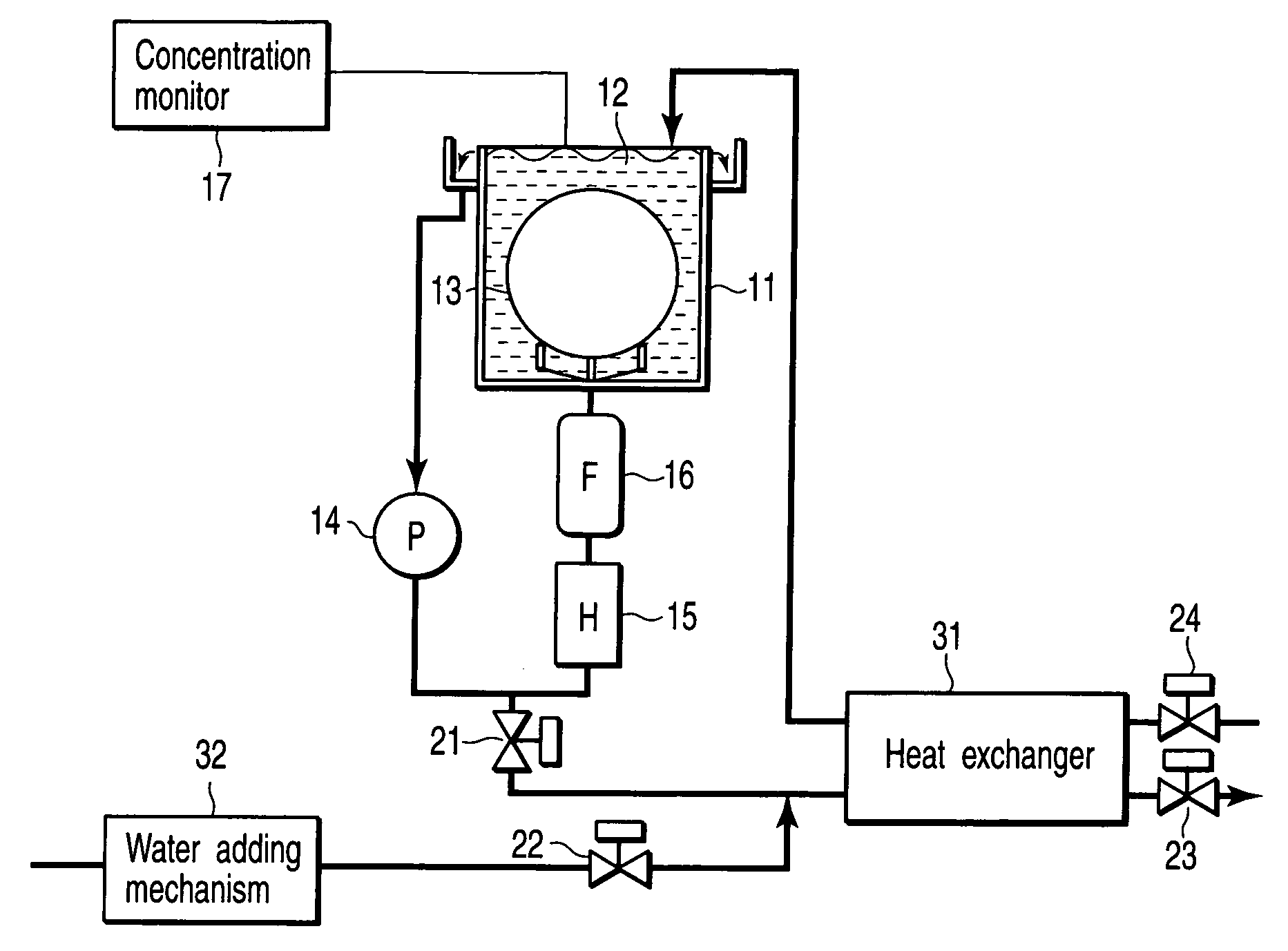
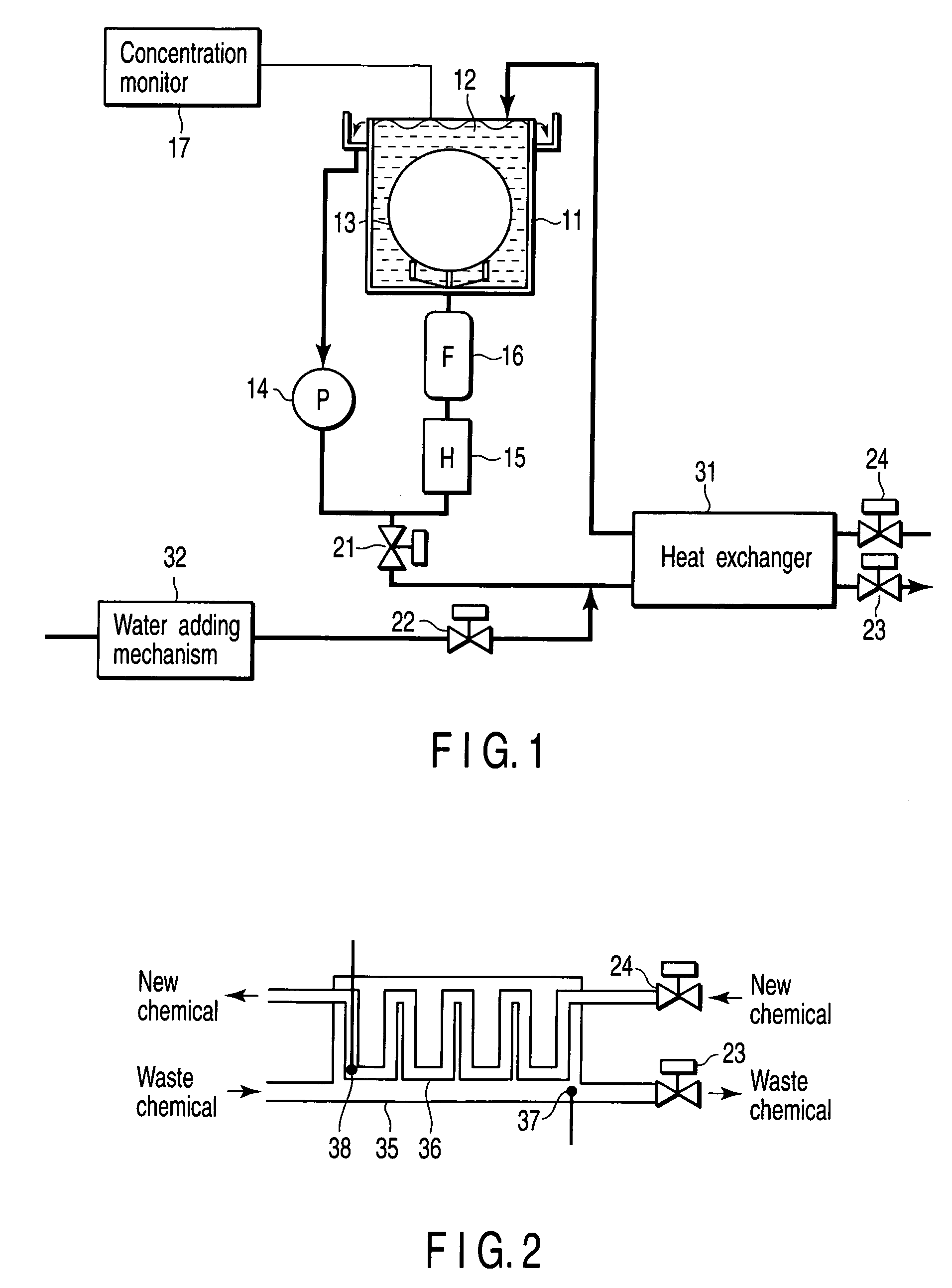
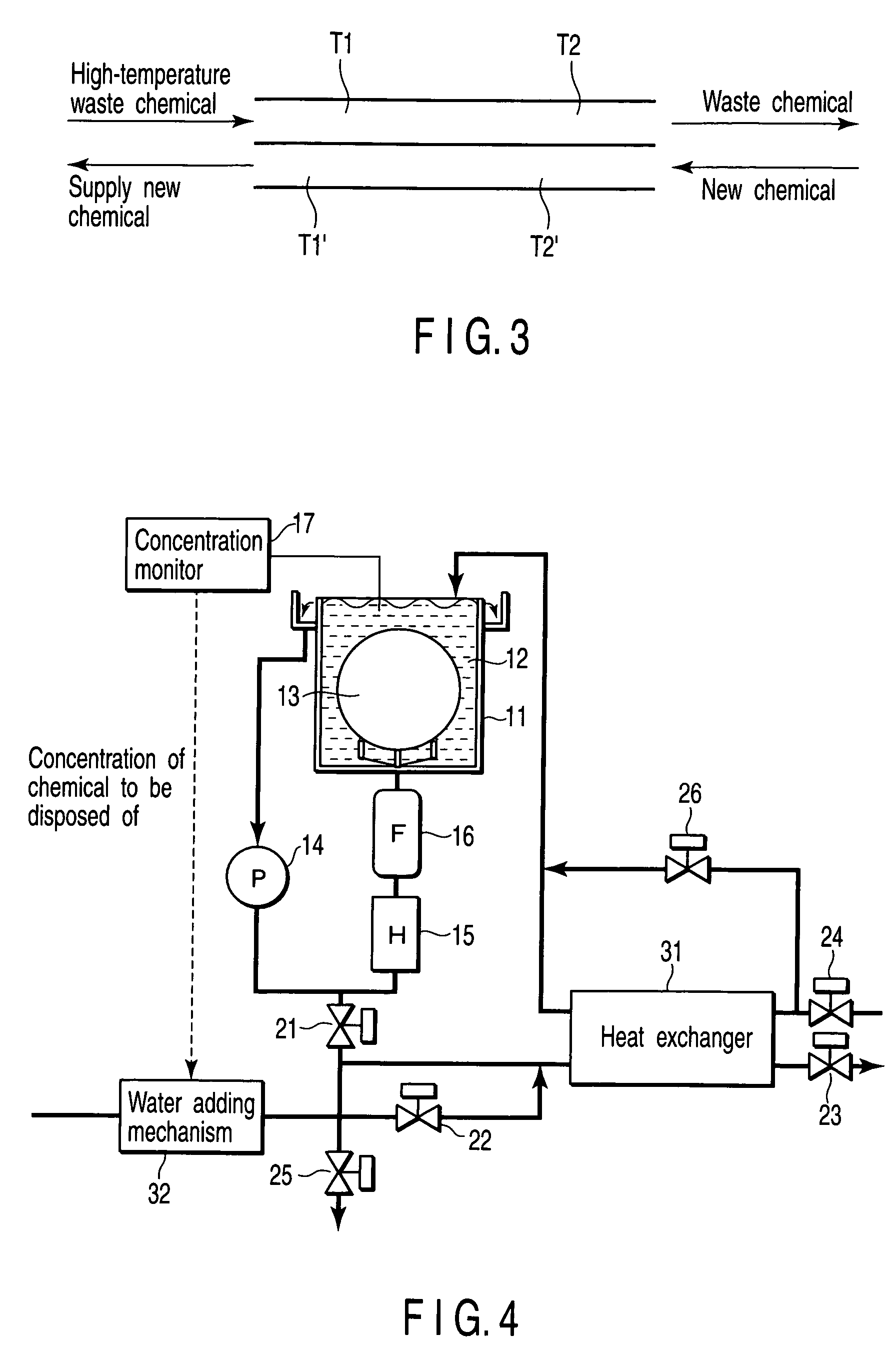
InactiveUS20060042756A1Increase temperatureLighting and heating apparatusDecorative surface effectsCompound (substance)Manufactured apparatus
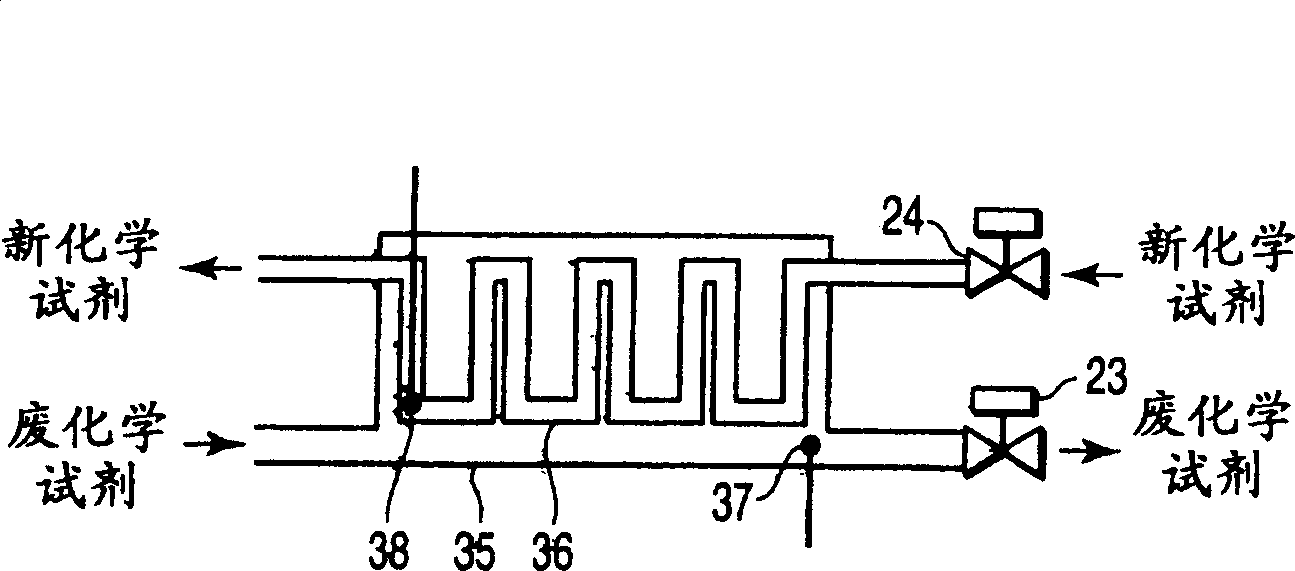
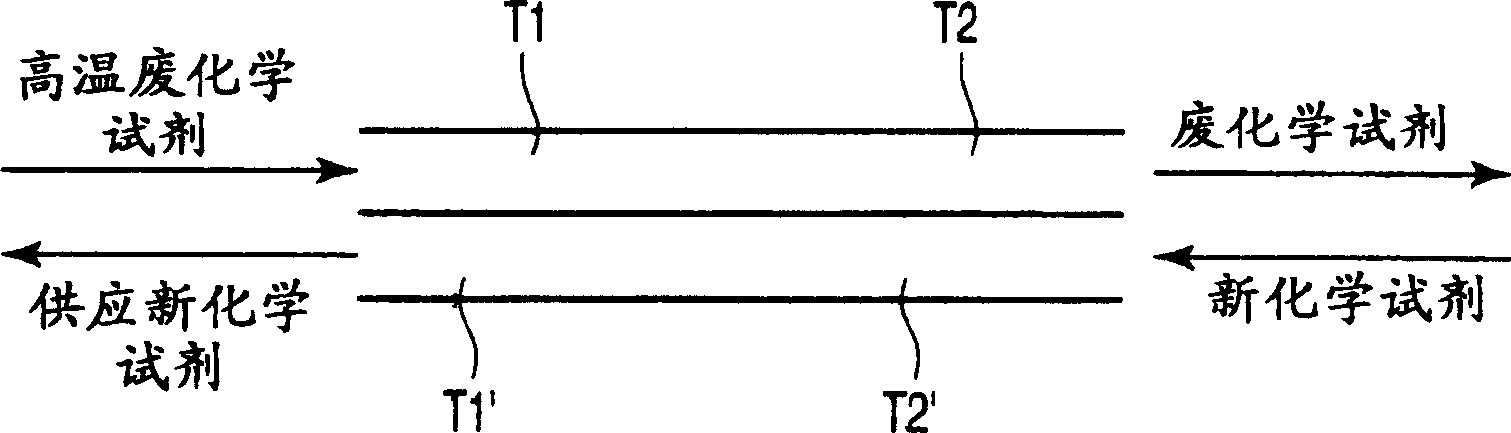
A semiconductor manufacturing apparatus for cleaning a semiconductor substrate comprises a high-temperature circulation type chemical bath which is filled with a chemical to be used for cleaning of a semiconductor substrate and in which the chemical is circulated and reused, a draining mechanism which drains the chemical in the chemical bath therefrom, an auxiliary fluid supplying mechanism which adds to the drained chemical regarded as a waste chemical an auxiliary fluid, and thereby heats the waste chemical, a heat exchanger in which the heated waste chemical is stored temporarily and a new chemical is allowed to flow, and which cools the waste chemical and raises temperature of the new chemical by heat exchange, and a pipe in which the new chemical having the temperature raised in the heat exchanger is supplied to the chemical bath.
Owner:KK TOSHIBA +1
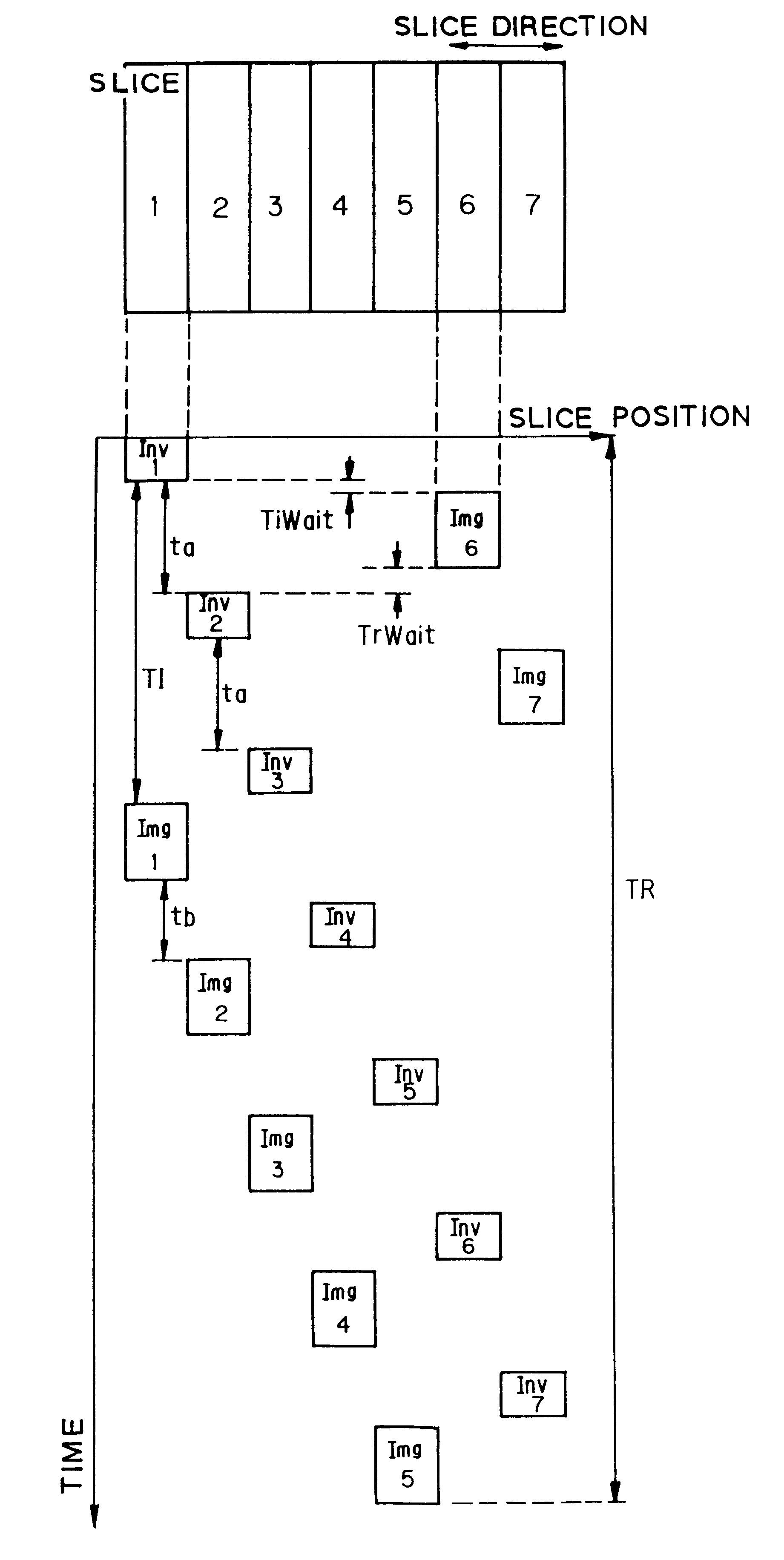
MR Parallel Imaging System Reducing Imaging Time
ActiveUS20140070803A1Good effectIncreased RF pulse sequence duty cycleMeasurements using NMR imaging systemsElectric/magnetic detectionParallel imagingPulse sequence
An MR imaging system uses multiple RF coils, for reducing image acquisition time, suitable for chemical exchange saturation transfer (CEST) imaging. Multiple RF (Radio Frequency) coils provide CEST imaging preparation in an anatomical volume by providing multiple interleaved RF pulses. The multiple interleaved RF pulses provide substantially increased RF pulse sequence duty cycle in the multiple RF coils relative to a duty cycle provided by a single coil of the multiple RF coils. The multiple RF coils subsequently provide RF excitation pulses in a reduced anatomical volume using k-space undersampling in an accelerated imaging method using the multiple RF coils and enable subsequent acquisition of associated RF echo data for deriving a CEST image.
Owner:CEDARS SINAI MEDICAL CENT +1
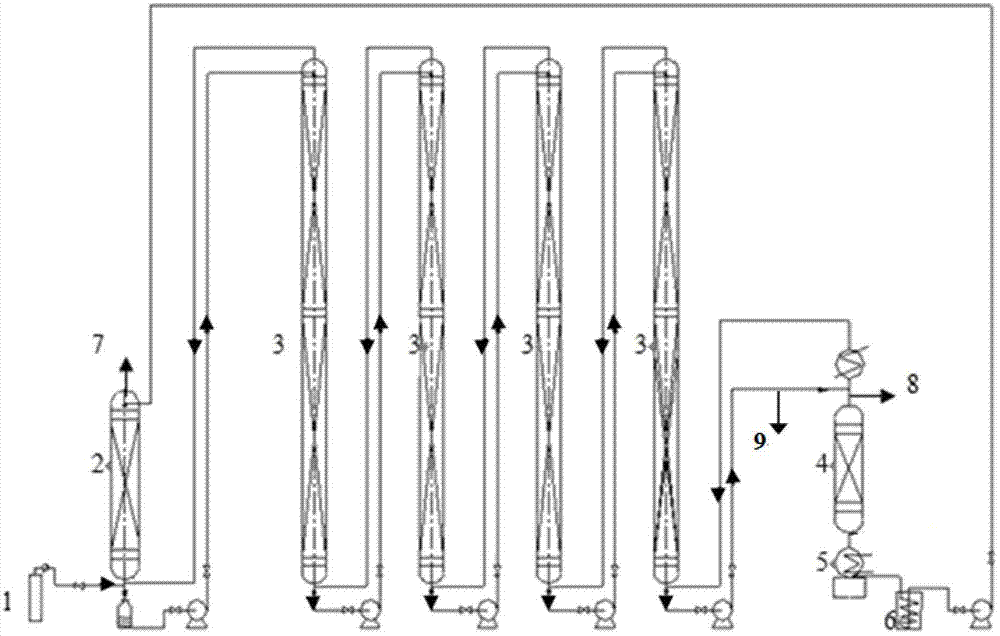
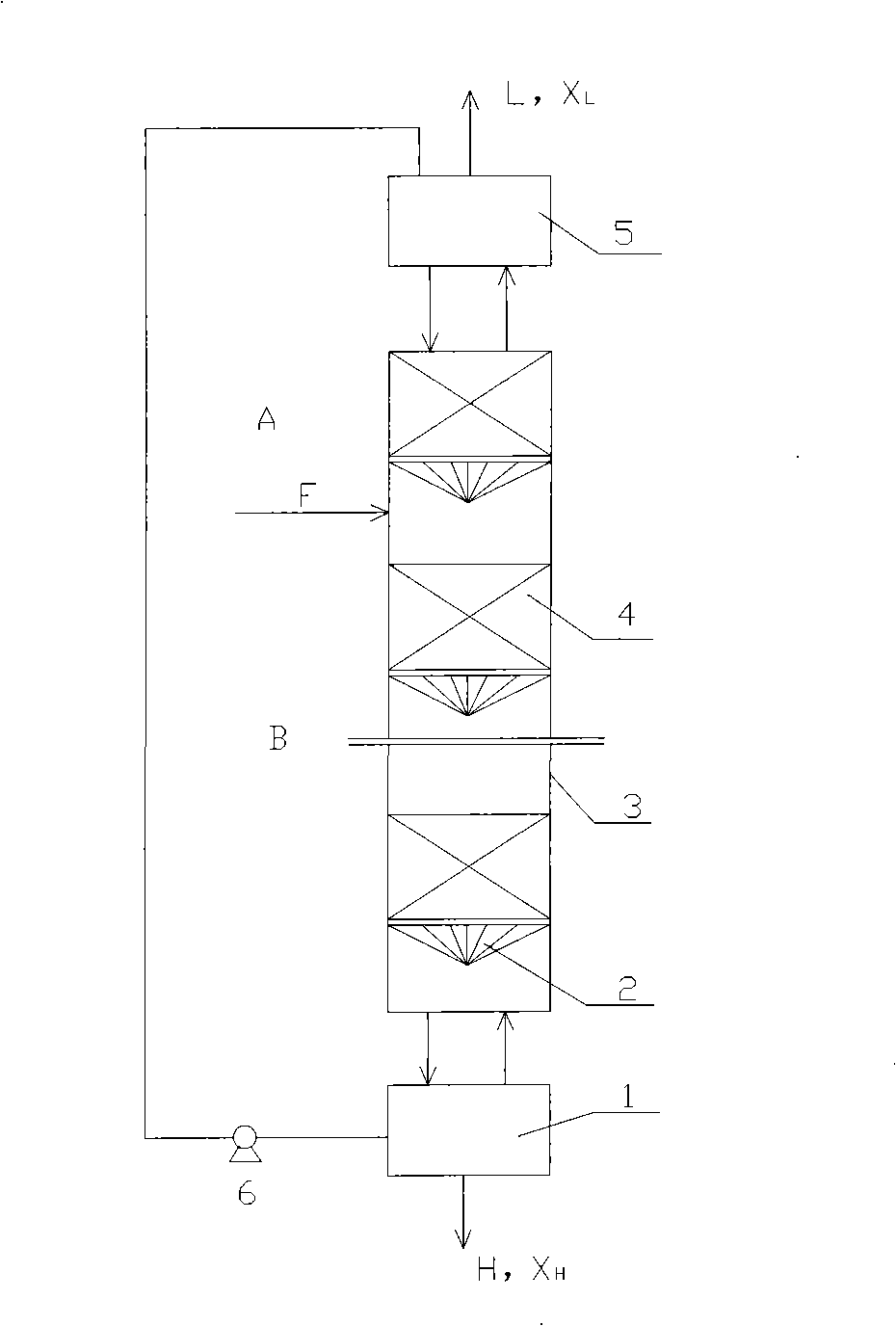
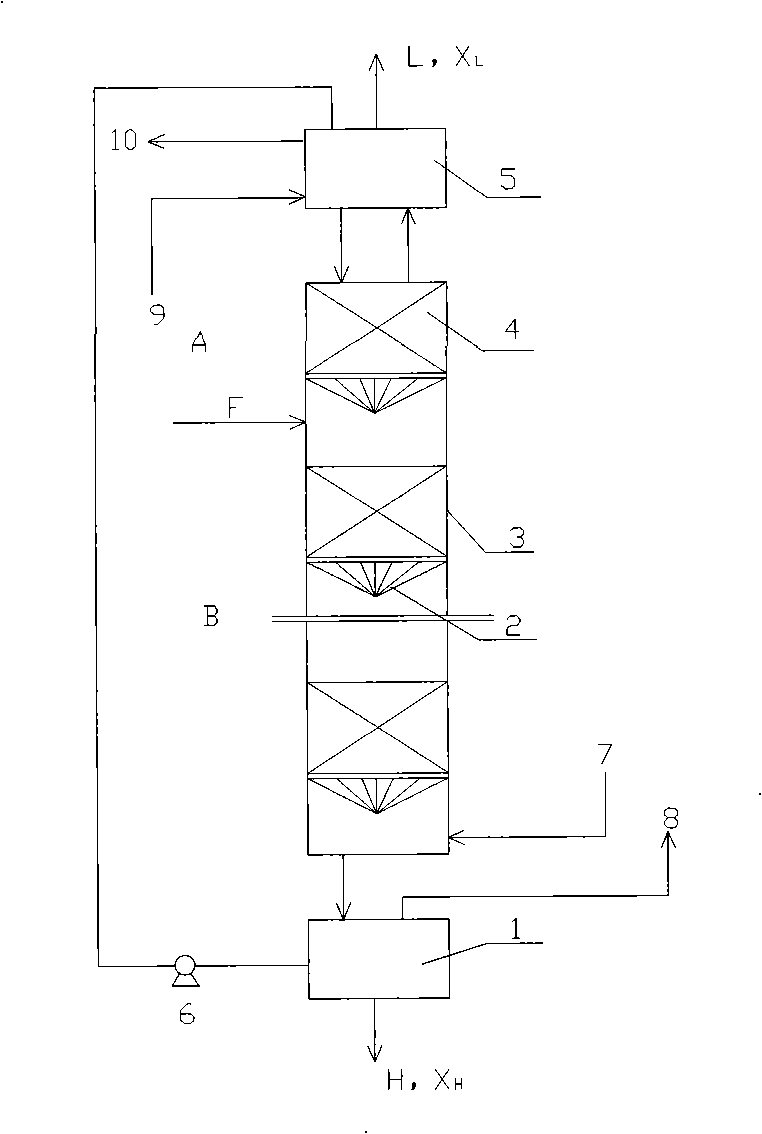
Method and device for separately producing enriched boron-10 (10B) by using multiple serial towers
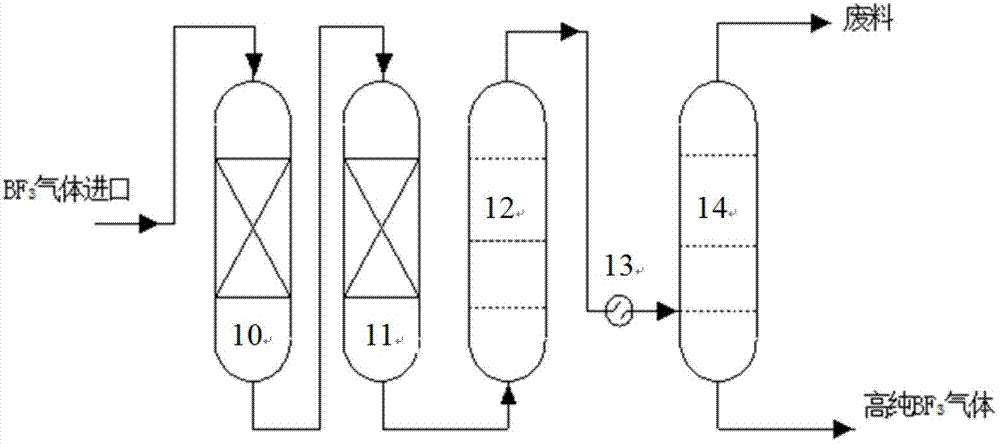
InactiveCN102773016ASatisfy the abundance requirementIsotope separationBoron halogen compoundsNuclear powerContinuous flow
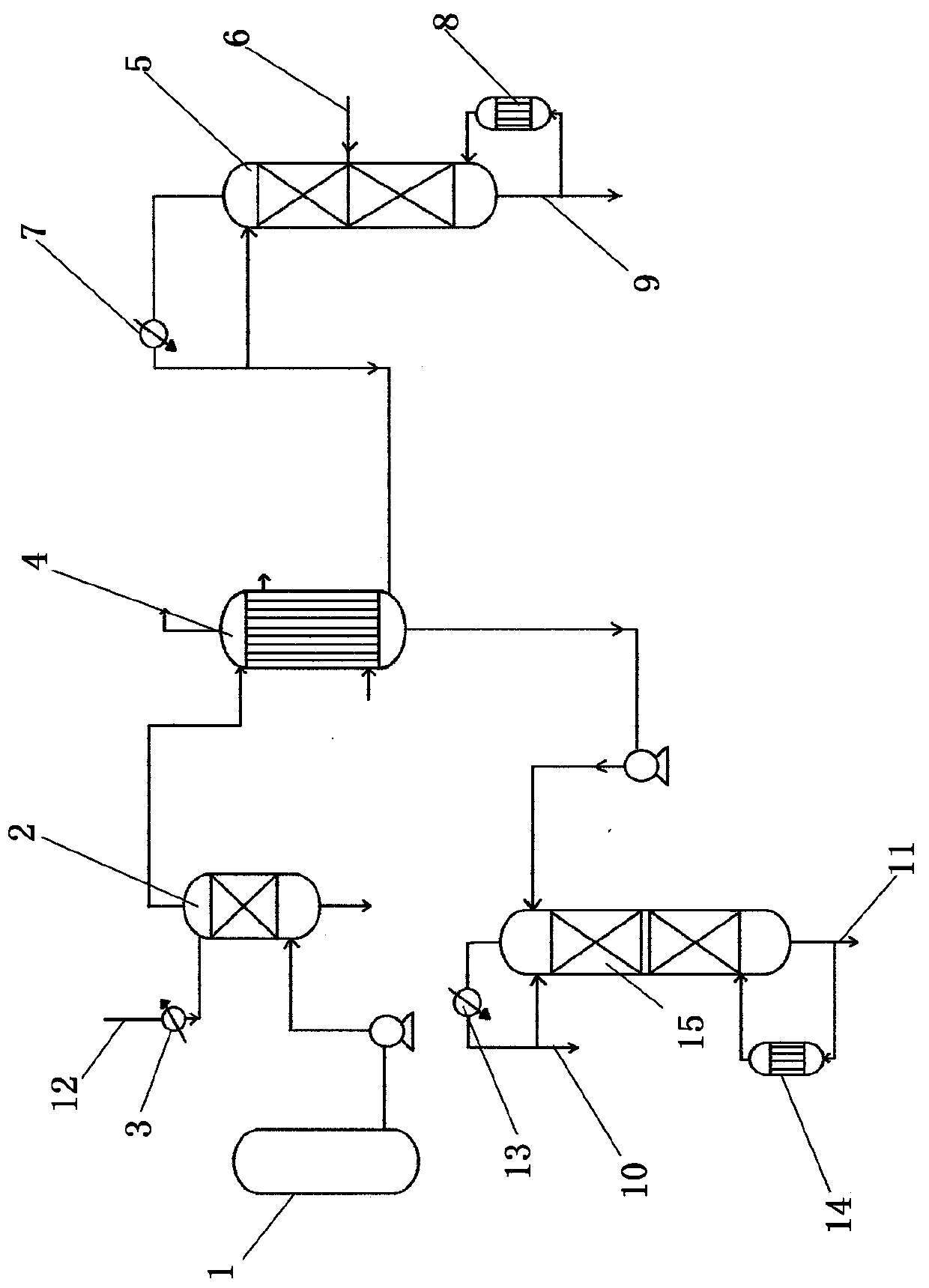
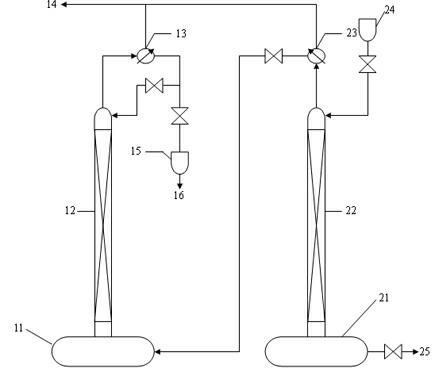
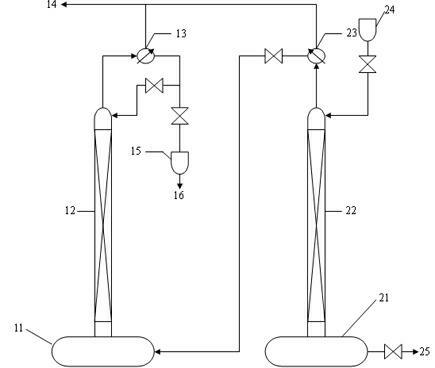
The invention relates to a method and device for separately producing enriched boron-10 (10B) by using multiple serial towers. In the method, enriched production of 10B is realized through multiple chemical exchange towers which are connected in series. The production device mainly comprises a complexing tower, serial chemical exchange towers, and a cracking tower, wherein the tower top of the cracking tower is provided with a condenser; the tower bottom of the cracking tower is provided with a heating kettle; and continuous flow of a liquid phase is realized among the complexing tower, each chemical exchange tower and the cracking tower through a pump. A liquid phase sprayed out of the complexing tower is pumped to the top of the first chemical exchange tower through a pump, and is pumped to the top of the second chemical exchange tower after flowing out of the bottom of the first chemical exchange tower till the liquid phase flowing out of the bottom of the last exchange tower is pumped to the top of the cracking tower; and a liquid phase flowing out of the bottom of the cracking tower is subjected to heat exchange through a heat exchanger, and is pumped to the top of the complexing tower through a pump, so that circular flow of the liquid phase among serial towers is formed. Due to the adoption of the method and the device, production of enriched 10B is realized through multiple serial towers, the abundance of the boron-10 is over 95 percent, and the abundance requirement of an enriched 10B product in the fields of nuclear power, military industry, aerospace and the like is met.
Owner:TIANJIN UNIV
Method for producing enriched boric-10 acid from trifluoride-anisole complex and application thereof
The invention relates to a method for producing enriched boric-10 acid from a trifluoride-anisole complex and application thereof. The method comprises the following steps: reacting a boron trifluoride-anisole complex with excessive sodium methoxide methanol solution, operating in an ice bath for 5-25 minutes, carrying out thermostatic water bath, reacting for 40-60 hours while keeping the reaction temperature within the range of 40-60 DEG C, stopping heating, and carrying out centrifugal stratification; fractionating the centrifugated supernatant: heating the mixture, starting to collect the fraction when the temperature rises to 50 DEG C, and stopping collecting the fraction when the temperature rises to 60 DEG C; and carrying out salting-out stratification on the collected fraction, mixing with deionized water, carrying out vacuum filtration on the mixture to obtain a solid, and drying to obtain the boric acid. The enriched boric-10 acid is used in the field of production of 10B-isotope-enriched downstream boric acid products, nuclear-grade boric acids and other enriched boric-10 acids by an anisole chemical exchange fractionation process. The production raw materials are from a closed system; and the invention has the impurity removal link, so the product purity is higher, thereby lowering the difficulty of subsequent boric acid production.
Owner:TIANJIN UNIV
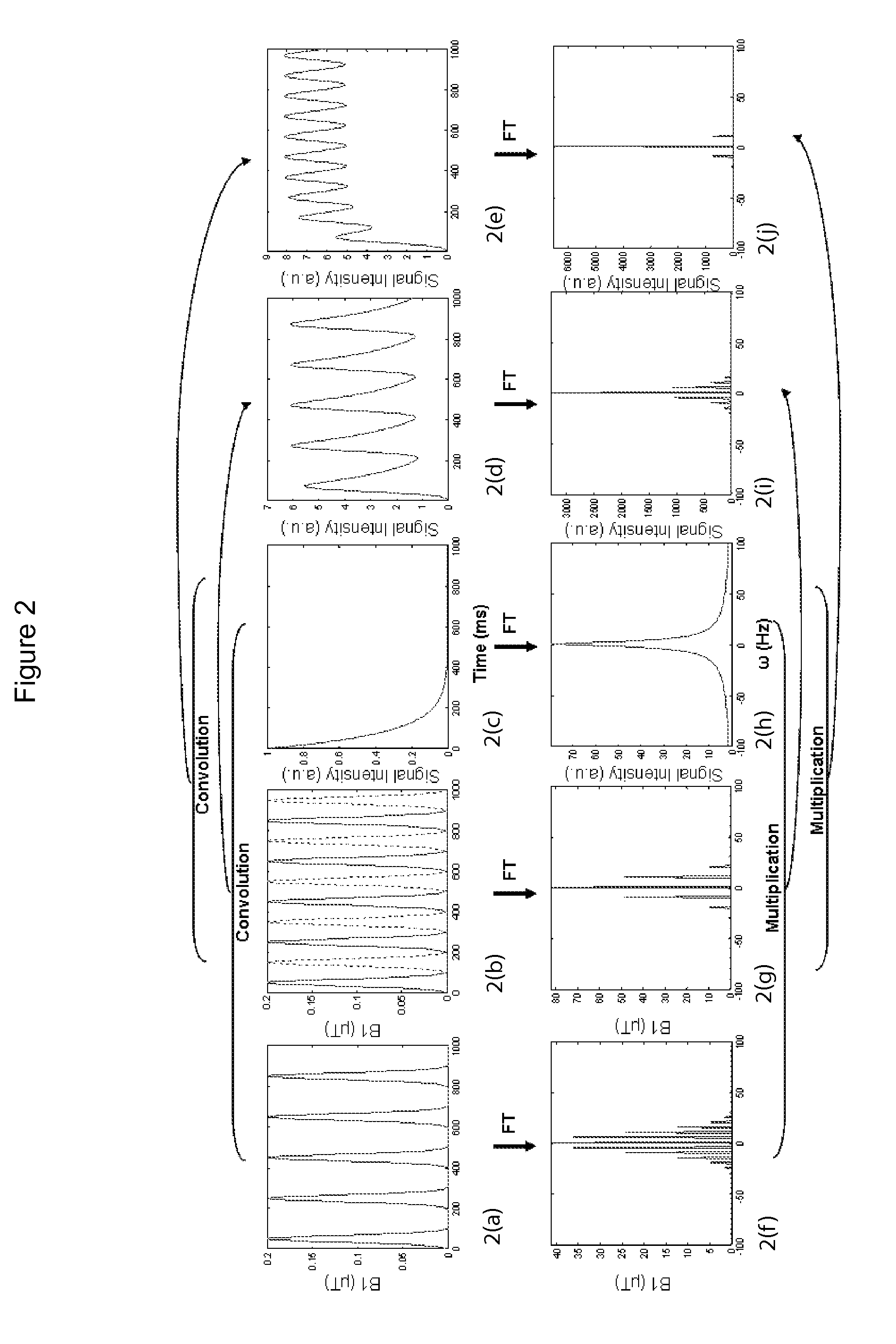
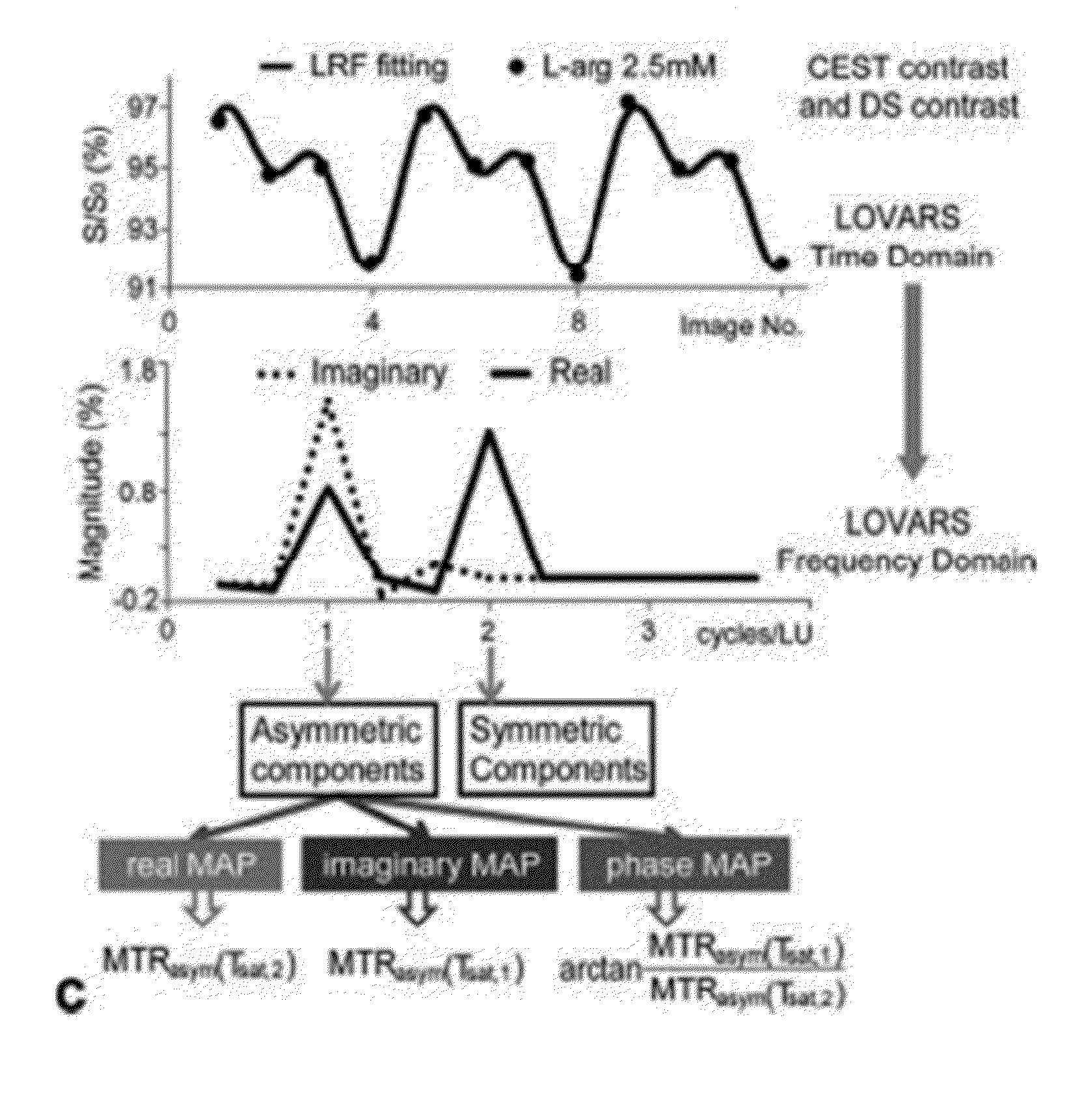
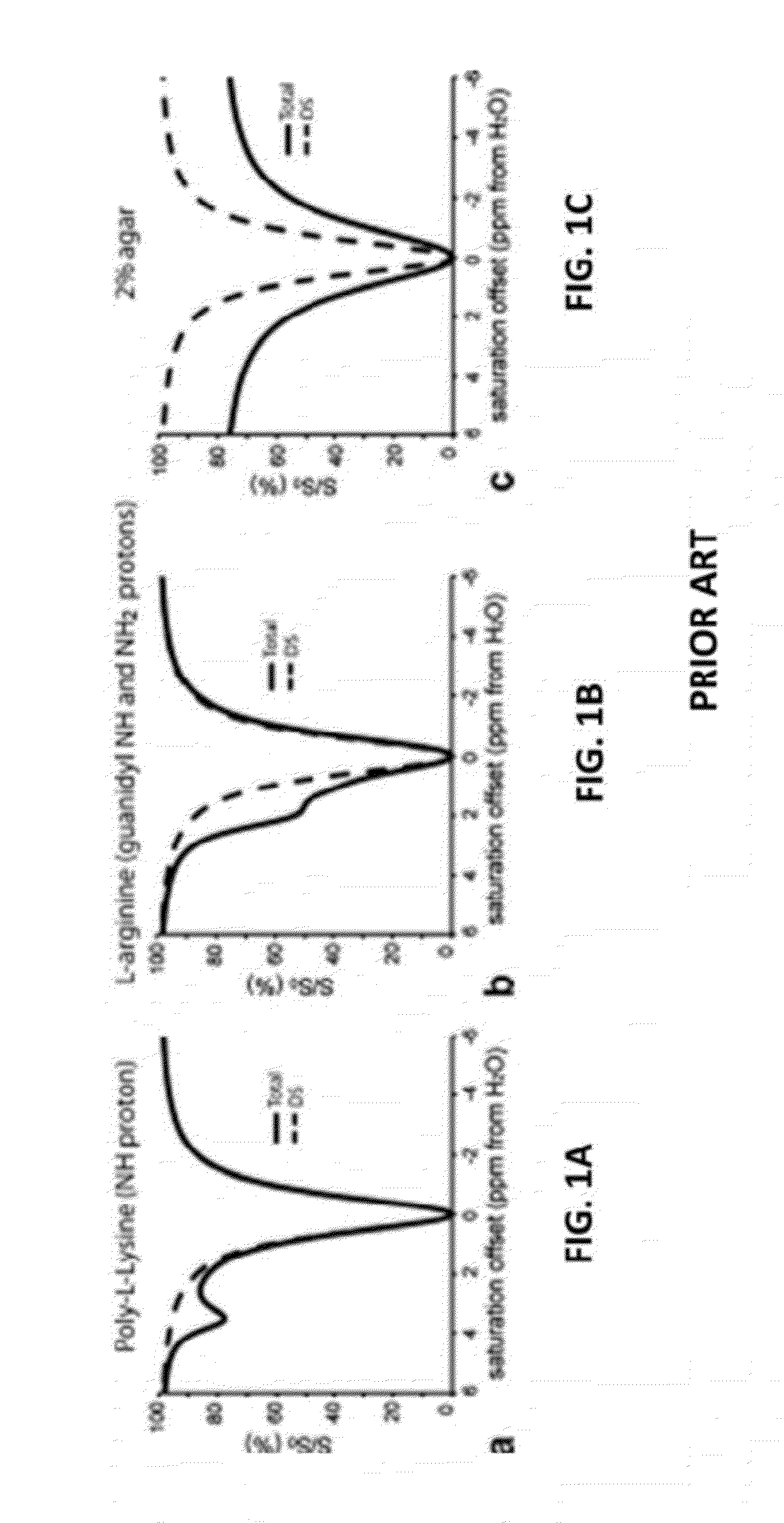
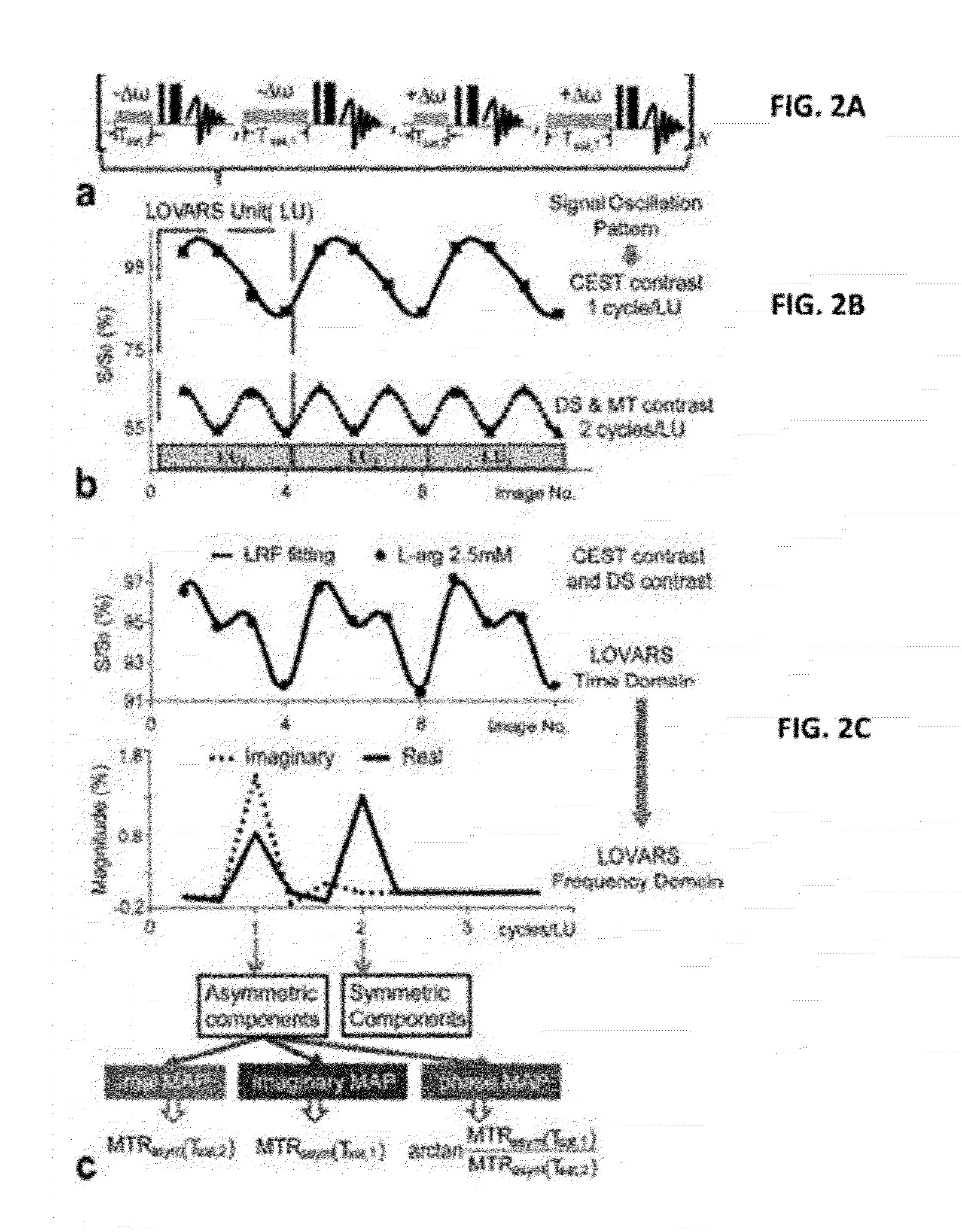
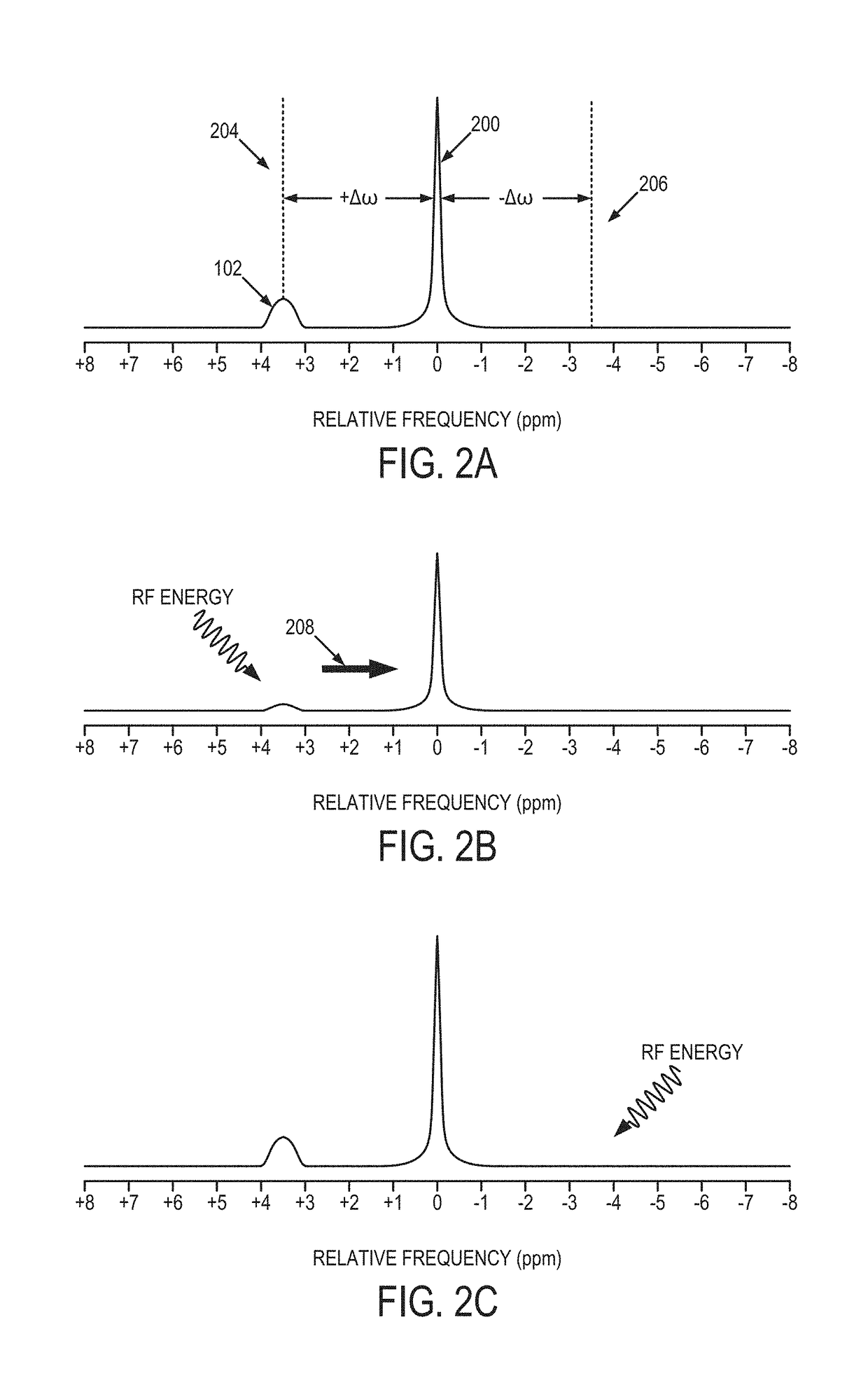
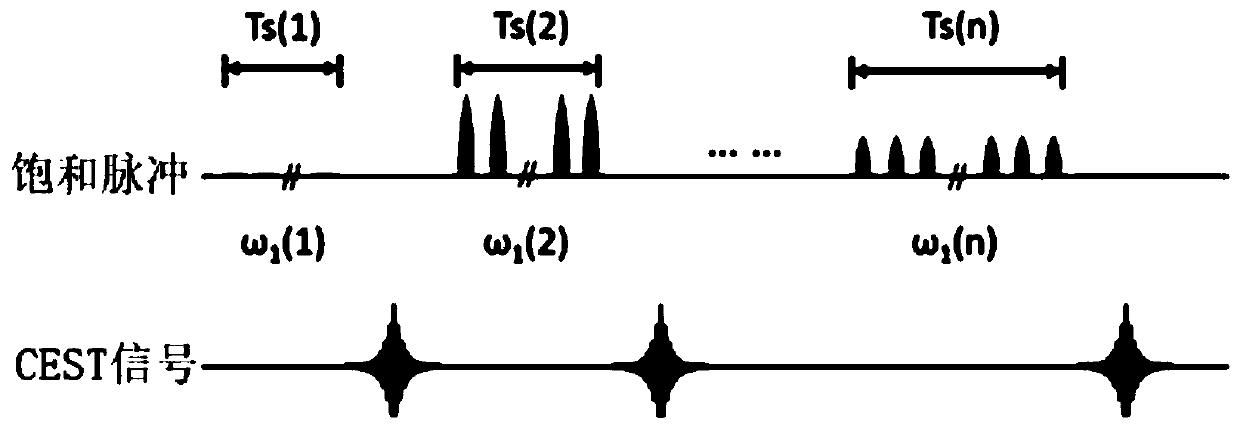
CEST Phase and Magnitude Imaging Using a Multi-Parametric Varied Saturation Scheme
An embodiment in accordance with the present invention provides a method for obtaining a magnetic resonance image (MRI) or spectrum. The method includes a step of performing a chemical exchange saturation transfer (CEST) or magnetization transfer (MT) magnetic labeling experiment of a subject using an MRI machine. When performing the CEST or MT magnetic labeling experiment aspects of a saturation pulse or a serial saturation pulse sequence, such as length (tsat), number (Nsat), offset (Δω), modulation frequency (ωs) and power (B1) can be varied in specific-designed schemes. Data is generated from the CEST magnetic labeling experiment and is transmitted to a data processing unit. The data is processed to generate a visual representation of the data.
Owner:THE JOHN HOPKINS UNIV SCHOOL OF MEDICINE +1
Device and method for producing boron trifluoride-11 electronic specific gas
InactiveCN102774845AFulfil requirementsMeet production requirementsBoron halogen compoundsCounter flowBoron trifluoride
The invention relates to a device and a method for producing boron trifluoride-11 (11BF3) electronic specific gas, wherein boron trifluoride raw gas is fed from the bottom of a synthesizer, anisole is downwards sprayed from the top of the synthesizer, and at the operation temperature of 10-25 DEG C, complex reaction is carried out to produce a boron trifluoride-anisole complex; a BF3 gas which is cracked from a cracking device is fed from the bottom of a chemical exchange tower, the gas and the liquid counter flow to fully contact with each other, and at the operation temperature of 15-30 DEG C, the chemical exchange reaction is carried out; heavier 11B isotope is enriched at the top of the tower in a gaseous state, and lighter 10B isotope is enriched in the liquid complex in the bottom of the tower; the enriched 11BF3 gas enters the synthesizer from the bottom, and is synthesized with the anisole again to form the liquid complex; the liquid complex of the enriched 10B isotope enters a decomposer, is heated to decompose into 11BF3 lean gas at 140-170 DEG C, and enters the chemical exchange tower to be subjected to chemical exchange; and the operation is repeated until the 11B abundance of the 11BF3 gas reaches above 99.7%, and the product is recovered from an 11BF3 product outlet.
Owner:TIANJIN UNIV
Preparation method and zpplication of super light water
The invention relates to the water treatment technical field, in particular to a preparing method and application of an ultra light water, wherein sulfureted hydrogen two-temperature exchange method or other alternative methods are adopted to produce ultra light water; sulfureted hydrogen two-temperature exchange method realizes separation of hydrogen isotopes through adopting multistage concatenation of cooling tower and thermal tower by use of isotope exchange reaction between sulfureted hydrogen and water; liquid raw material water enters from the top of the cooling tower and flows through the cooling tower and the thermal tower from above to below, while gas material sulfureted hydrogen is added from the part between the cooling tower and the thermal tower and circulates inside the cooling tower and the thermal tower from bottom to top inside; heavy components inside the cooling tower is transferred towards liquid phase through chemical exchange so as to be concentrated inside the liquid phase; liquid material with concentrated the heavy components completes chemical exchange with ascending gas during flowing through the thermal tower, thereby, the heavy components are transferred towards gas phase; finally, finished product with lower content of heavy hydrogen can be obtained after liquid phase material discharged from the thermal tower enters into a degassing column to removing sulfureted hydrogen. The invention has the health effects such as delaying aging, radiation resisting, resisting cell mutation, activating immunocyte, improving basal metabolism level of human body, improving sleep, enhancing physical function of male, beauty and body slimming.
Owner:丛峰松 +1
Synthesis method of crizotinib
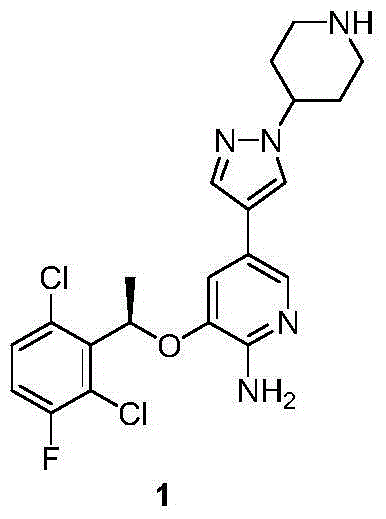
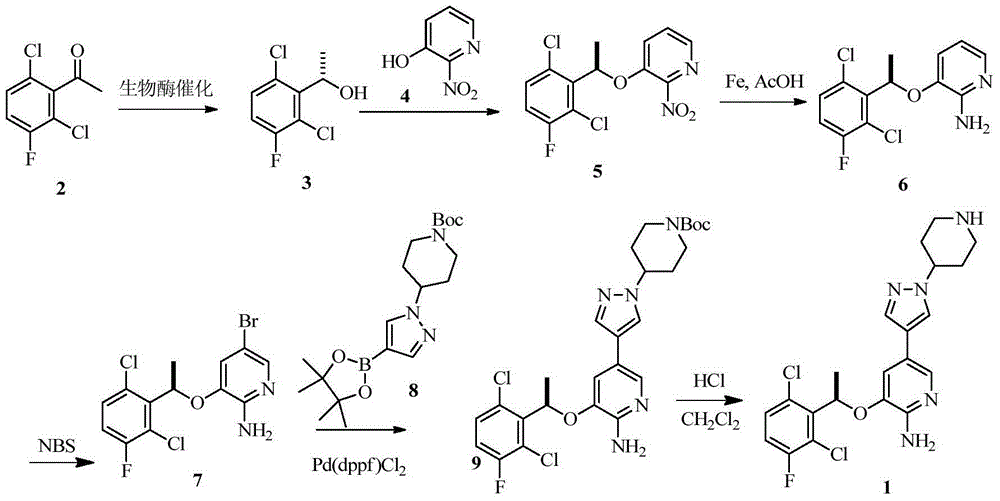
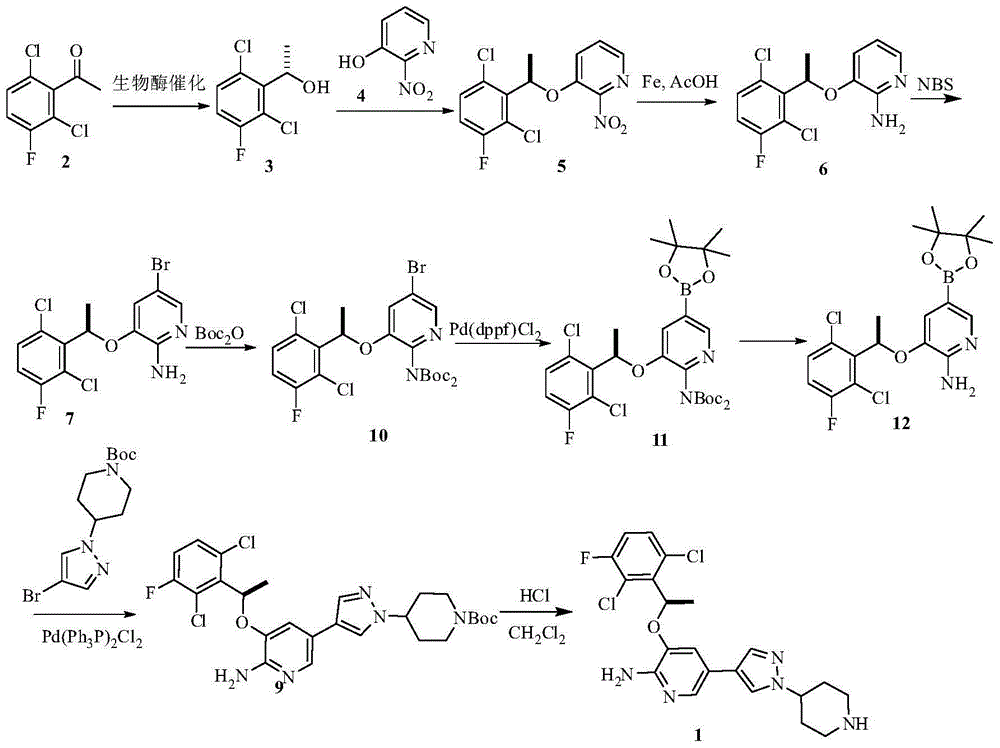
InactiveCN104693184AHigh purityReduce dosageOrganic chemistrySynthesis methodsCombinatorial chemistry
The invention provides a synthesis method of crizotinib. The method comprises the following steps: performing chemical exchange reaction for a compound (10) to obtain an intermediate compound (11); adding a compound (6) to react to obtain a compound (12); performing deprotection reaction for the compound (12) to obtain crizotinib. The method is simple in steps, short in line, simple in after-processing, low in cost, and particularly suitable for industrial production; the reaction line is shown in the specification, wherein X is Br or I; Nu is MgX or ZnX; R1 and R2 are independently selected from Boc or H.
Owner:ARROMAX PHARMATECH
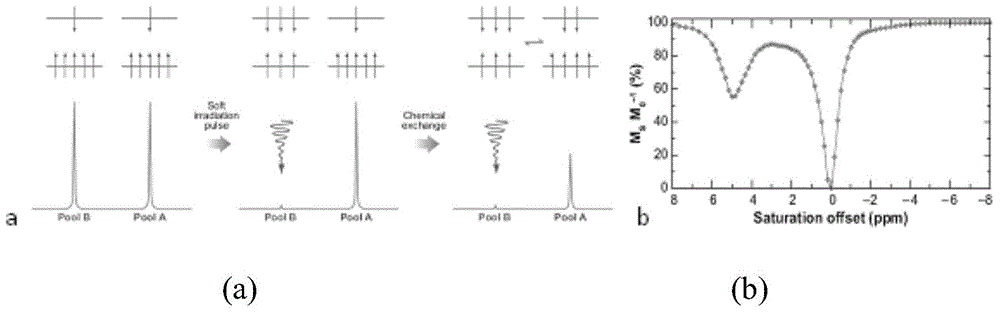
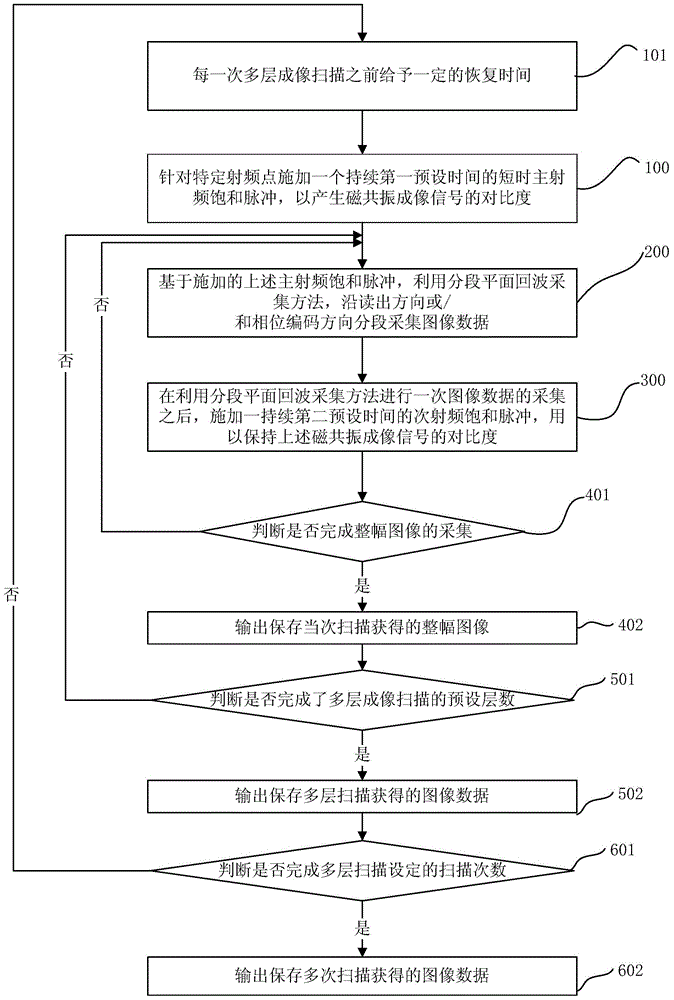
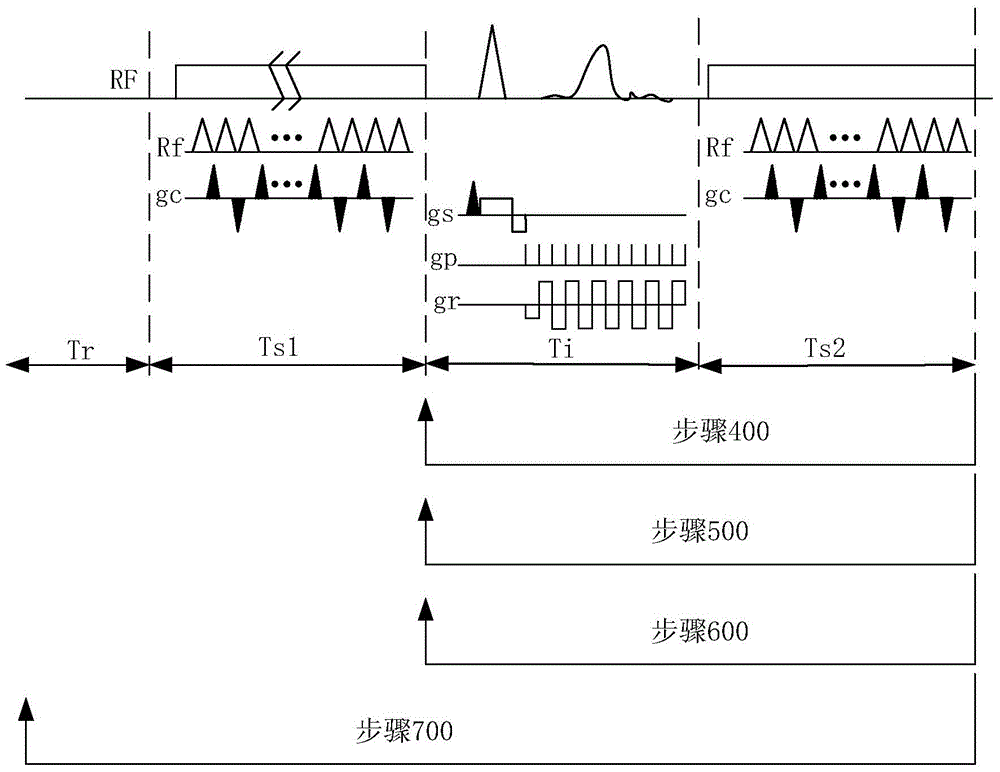
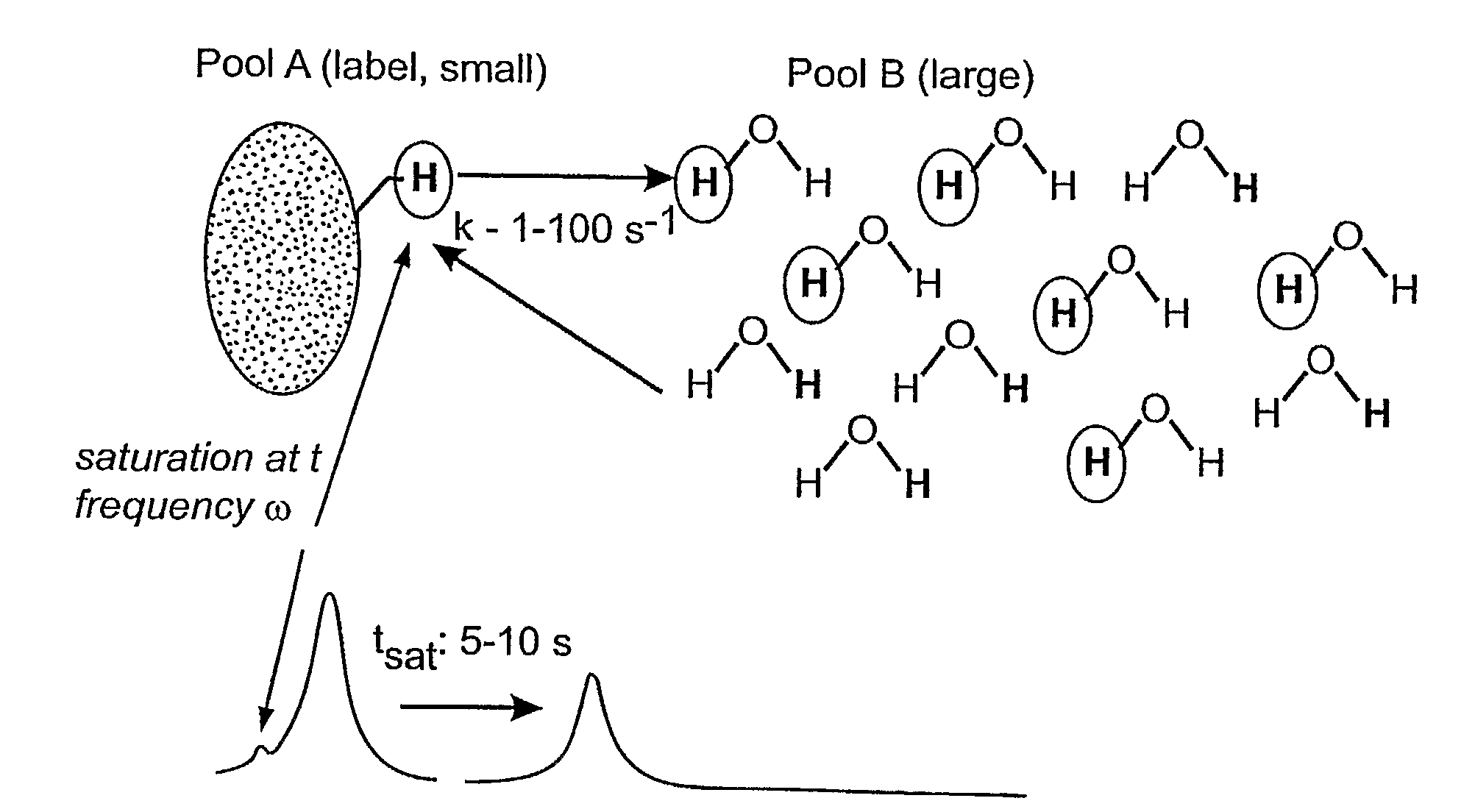
Magnetic resonance chemical exchange saturation transfer imaging method and magnetic resonance chemical exchange saturation transfer imaging system
ActiveCN105572613AHigh strengthImprove signal-to-noise ratioMeasurements using NMR imaging systemsResonanceData acquisition
The invention provides a magnetic resonance chemical exchange saturation transfer imaging method and a magnetic resonance chemical exchange saturation transfer imaging system. The magnetic resonance chemical exchange saturation transfer imaging method comprises a main RF pulse generating step, applying a short-time main RF saturation pulse which lasts for a first preset time for aiming at a specific RF point, thereby generating the contrast of a magnetic resonance imaging signal; an image acquisition step, segmentally acquiring image data in a reading-out direction or / and a phase encoding direction based on the applied main RF saturation pulse by means of a segmental planar echo acquisition method; and a sub-RF pulse generating step, after one-time image data acquisition by means of the segmental planar echo acquisition method, applying a sub-RF saturation pulse which lasts for a second preset time, thereby keeping the contrast of the magnetic resonance imaging signal. The magnetic resonance chemical exchange saturation transfer imaging method and the magnetic resonance chemical exchange saturation transfer imaging system settle problems of low signal sensitivity and low imaging efficiency in existing magnetic resonance chemical exchange saturation transfer imaging technology.
Owner:SHENZHEN INST OF ADVANCED TECH CHINESE ACAD OF SCI
Chemical Exchange Saturation Transfer Based Mri Using Reporter Genes and Mri Methods Related Thereto
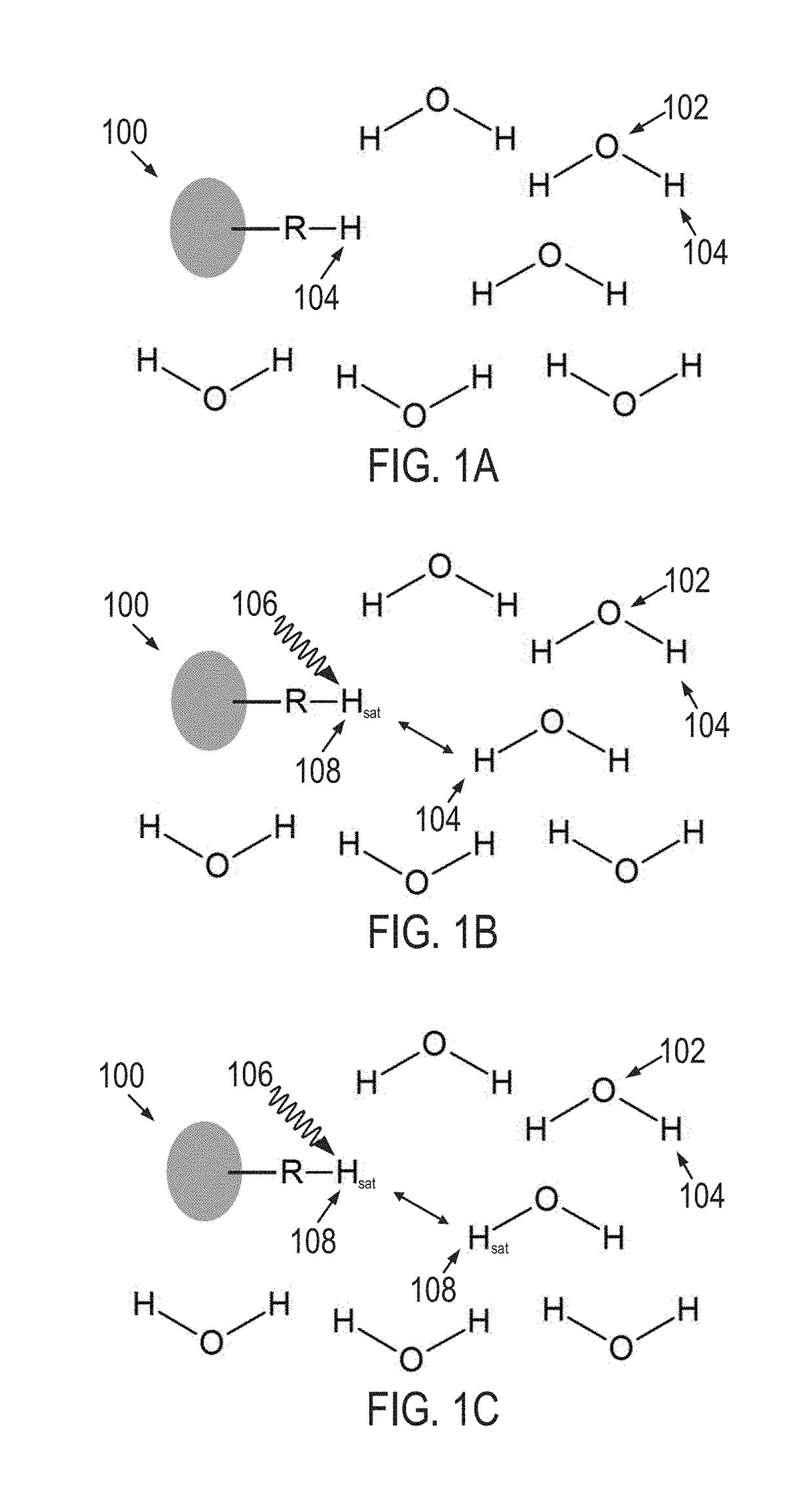
ActiveUS20080284427A1Advantageous biodistributionEliminate needSugar derivativesMicrobiological testing/measurementMr contrastRadio frequency
Featured are a new class of reporter genes including reporter compositions as well as methods, MRI systems and MRI imaging kits related thereto. The genes according to the present invention provide MR contrast when the sample / subject is irradiated at a specific off-resonance radio-frequency (RF frequency), where the contrast mechanism utilizes chemical exchange saturation transfer (CEST) technique for imaging.
Owner:THE JOHN HOPKINS UNIV SCHOOL OF MEDICINE
Drug carrier providing MRI contrast enhancement
Described are drug carriers useful in magnetic resonance imaging (MRI)-guided drug release comprising a shell capable of releasing an enclosed biologically active agent as a result of a local stimulus, e.g. energy input, such as heat, wherein the shell encloses a 19F MR contrast agent. Preferably, the carrier also acts as a contrast enhancement agent for MRI based on the principle of Chemical Exchange-dependent Saturation Transfer (CEST). To this end the shell encloses a cavity that comprises a paramagnetic chemical shift reagent, a pool of proton analytes, and the 19F contrast agent, and wherein the shell allows diffusion of the proton analytes.
Owner:KONINKLIJKE PHILIPS ELECTRONICS NV
Apparatus, system, method and computer-readable medium for isolating chemical exchange saturation transfer contrast from magnetization transfer asymmetry under two-frequency RF irradiation
ActiveUS20130166226A1Eliminate contributionMagnetic measurementsChemical machine learningDual frequencyFrequency spectrum
Apparatus, system, method and computer-readable medium for isolating chemical exchange saturation transfer contrast from magnetization transfer asymmetry under two-frequency RF irradiation. A two-pool model for magnetization transfer (MT) can be established fully based on Provotorov's theory of saturation, and then extended to the situation of simultaneous two-frequency RF irradiation. Numerical simulations and experimental results demonstrate that two-frequency RF irradiation can make MT effects independent of irradiation frequency over a wide range, and thus can suppress MT asymmetry. Exemplary embodiments can be provided to isolate chemical exchange saturation transfer (CEST) contrast from MT asymmetry contrast by using the two-frequency RF irradiation technique. A further embodiment can isolate a narrow-frequency spectrum MT mechanism from a broad-frequency spectrum MT mechanism.
Owner:NEW YORK UNIV
Chemical exchange saturation transfer magnetic resonance imaging with gating synchronized acquisition
Methods and systems for producing a magnetic resonance (MR) image of a subject include acquiring a first physiological monitoring signal related to a first physiological process of the subject and acquiring a second physiological monitoring signal related to a second physiological process of the subject. The method also includes analyzing the first physiological monitoring signal and the second physiological monitoring signal to identify at least a first trigger point and a second trigger point and, upon identifying the first trigger point, applying a radiofrequency (RF) saturation module at a selected frequency to saturate a selected spin species in the subject. Upon identifying the second trigger point, the method includes performing a chemical exchange striation transfer (CEST) readout to acquire CEST data and then reconstructing the CEST data to produce a CEST image of the subject.
Owner:THE GENERAL HOSPITAL CORP
Magnetic resonance image processing method and device
ActiveCN107133957AShort processReduce complexityImage enhancementImage analysisImaging processingResonance
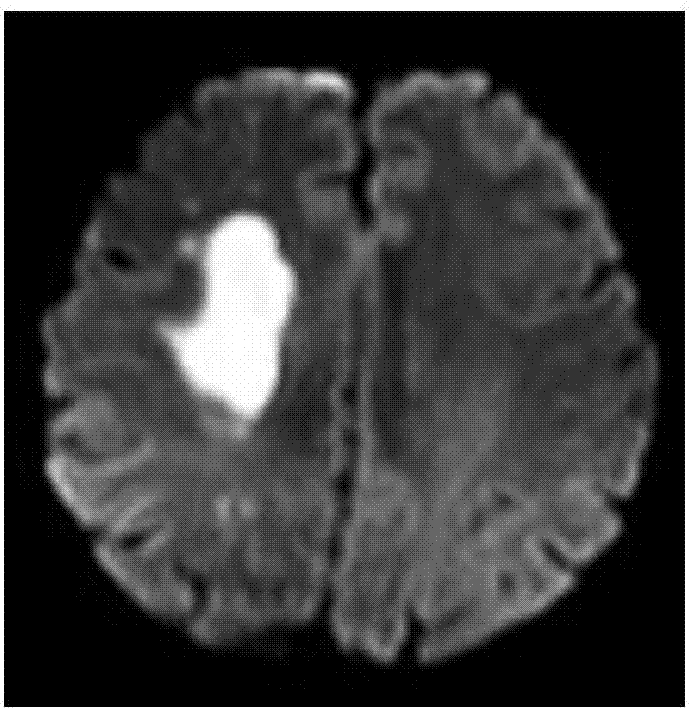
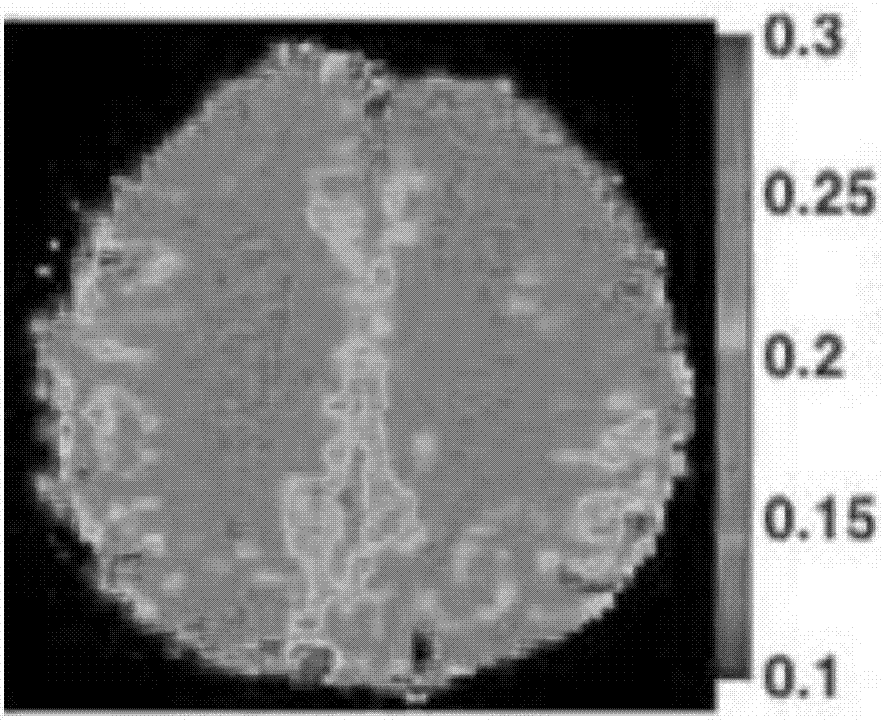
The embodiment of the invention discloses a magnetic resonance image processing method and device. The method comprises the steps that diffusion weighted imaging scanning and pulse parameter modulation imaging scanning based on chemical exchange saturation transfer are performed on the area of interest of the brain, and a diffusion weighted image and a chemical exchange saturation transfer image are acquired; when the chemical exchange saturation transfer image presents low signal shadow in comparison with the preset benchmark template, arterial spin mark imaging scanning is performed on the area of interest of the brain so as to acquire an arterial spin mark image; the diffusion weighted image is compared with the arterial spin mark image so as to determine a first mismatch area; the diffusion weighted image is compared with the chemical exchange saturation transfer image so as to determine a second mismatch area; and the second mismatch area is removed out of the first mismatch area, and the residual area of the first mismatch area is determined as an ischemic penumbra area. The accuracy of the ischemic penumbra can be enhanced, the image processing flow can be saved, the complexity can be reduced and the safety can be enhanced.
Owner:SIEMENS HEALTHINEERS LTD +2
Method for producing stable isotope of C-13 with chemical catalysis sorting by exchanging
InactiveCN101314109AQuick responseNo pollution in the processIsotope separationCarbamateOrganic solvent
The invention relates to a method for producing a stable isotope <13>C by adopting a chemical catalysis exchange method. The method adopts a CO2 / carbamate gas-liquid countercurrent chemical catalysis exchange system to perform chemical exchange and separation in a packing tower, wherein, a gas phase is the CO2, and a liquid phase is an amic organic solvent; the packing tower is regular packing or random high-efficiency packing; and a separating system realizes the separation of the stable isotope <13>C in a multi-tower concatenation connection. Compared with the prior art, the method has the characteristics of simple process, low production cost, normal-temperature normal-pressure operation and so on.
Owner:SHANGHAI RES INST OF CHEM IND
Method for separating boron-10 isotopic element using reducing tower
InactiveCN101502758AIncreased chance of convective exposureIncrease exposureIsotope separationFractional distillationRefluxGas phase
The invention discloses a method for separating boron-10 isotopes by a reducing tower. Chemical exchange distillation is carried out on complex compound of boron triflouride by three rectifying towers which have the same height and progressively decreased diameters and are connected with each other in series; the operating condition of each rectifying tower is that the vacuum degree of the tower system is 20-200mmHg, the temperature at the top of the towers is 50-100DEG C, the temperature of a still is 60-120DEG C, the reflux ratio is 400-150:1. The rectifying towers with progressively decreased diameters directly change the flow of liquid phase and gas phase, effectively increase chances of convection contact between the liquid phase and the gas phase, increase the speed so that the system achieves balance in 72-75 hours, and increase the separation efficiency of products simultaneously, the abundance ratio of boron-10 in the final product is up to 90%. The invention has the advantages of simple structure, easy operation, rapid speed for achieving balance, high separation efficiency and little energy consumption.
Owner:DALIAN TAIRUI CHEM IND
Method for using CEST contrast agents in MRI
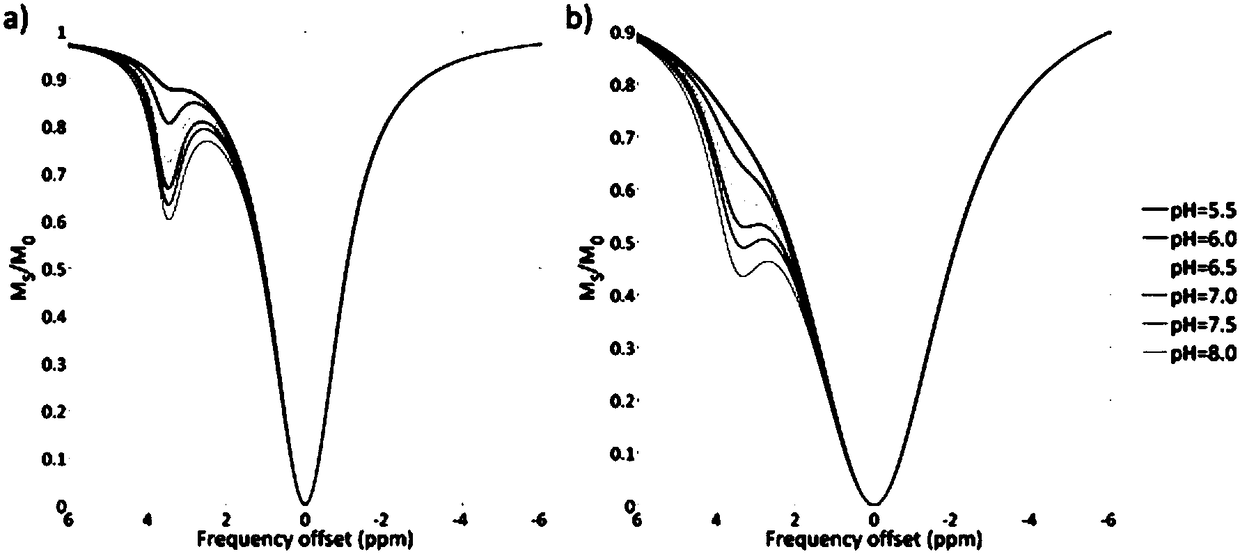
InactiveCN101166987AEliminate dependenciesElimination of concentration dependenceMeasurements using NMR imaging systemsMagnetic variable regulationMedicineProton
The invention relates to a method for mapping of a physico-chemical parameter such as pH value, temperature, PO2 or metabolite concentration, using a chemical exchange saturation transfer contrast agent in Magnetic Resonance Imaging. This method may be used with agents having only one exchangeable entity pool, e.g. proton pool, by applying two different RF frequencies for pre-saturation of the contrast agent.
Owner:KONINKLIJKE PHILIPS ELECTRONICS NV
Targeted mr imaging agents
InactiveUS20070237721A1Weakening rangeEfficient switchingIn-vivo radioactive preparationsDispersion deliveryParticulatesMRI contrast agent
MRI contrast agents that employ paramagnetic agents and chemical exchange saturation transfer (paraCEST) and which are coupled to targeted particulate delivery vehicles provide sufficient concentration of the paraCEST contrast agents to obtain useful images of target tissues or organs. In addition, the image contrast may be switched on or off with a presaturation radio frequency pulse, avoiding the necessity obtaining pre-injection and post-injection images.
Owner:BARNES JEWISH HOSPITAL
Ph-weighted MRI using fast amine chemical exchange saturation transfer (CEST) imaging
ActiveUS20180164393A1Easy to optimizeReduce scan timeDiagnostic recording/measuringAnalysis using nuclear magnetic resonanceMetaboliteNon invasive
A pH-weighted chemical exchange saturation transfer (CEST) magnetic resonance imaging (MRI) method and system are provided that works by indirectly measuring the NMR signal from amine protons found on the backbones of amino acids and other metabolites, which resonate at a frequency of +2.8-3.2 ppm with respect to bulk water protons. The technique uses a modified magnetization transfer radiofrequency saturation pulse for the generation of image contrast. A train of three 100 ms Gaussian pulses at high amplitude (6 uT) or Sinc3 pulses are played at a particular frequency off-resonance from bulk water prior to a fast echo planar imaging (EPI) readout, with one full image acquired at each offset frequency. This non-invasive pH-weighted MRI technique does not require exogenous contrast agents and can be used in preclinical investigations and clinical monitoring in patients with malignant glioma, stroke, and other ailments.
Owner:RGT UNIV OF CALIFORNIA
Semiconductor manufacturing apparatus and chemical exchanging method
InactiveCN1741249AExothermal chemical reaction heat productionSemiconductor/solid-state device manufacturingManufactured apparatusCompound (substance)
A semiconductor manufacturing device for cleaning a semiconductor substrate is provided with a high temperature circulation-type chemical tank 11 which is filled with a chemical supplied for cleaning the semiconductor substrate in a state that a temperature is raised to a processing temperature, in which the chemical after cleaning is circulated and reused, a bulb 21 discharging the chemical 12 in the chemical tank 11, an auxiliary liquid addition mechanism 32 heating waste liquid by adding auxiliary liquid generating heat by mixing with waste liquid to waste liquid being discharged liquid, a heat exchanger 31 in which heated waste liquid is temporarily stored and new liquid is circulated, waste liquid is cooled and the temperature of new liquid is raised by the heat exchange of waste liquid and new liquid, and piping supplying new liquid whose temperature is raised through the heat exchanger 31 into the chemical tank 11.
Owner:SEIKO EPSON CORP
Industrial production method of boron-10 isotope
ActiveCN109942005AReduce moisture contentLess side effectsBoron halogen compoundsComing outChemical synthesis
The invention provides an industrial production method of a boron-10 isotope and belongs to the field of chemical synthesis and separation. The industrial production method is invented mainly for solving the problem that boron-10 isotope cannot be continuously and stably produced at present. Methyl ether is pressurized to become liquid, a molecular sieve is used for adsorbing the methyl ether liquid, boron trifluoride gas is rectified by a low-temperature rectifying column and then comes out of a column top, and the methyl ether and the boron trifluoride are subjected to complexation reactionin a complexation reactor. The generated complex enters an exchange reaction distillation column. By heating the complex through a reboiler, the complex is heated to a temperature for decomposition, vapor is completely condensed as a liquid to reform a boron trifluoride-methyl ether complex, the refluxing liquid and the vapor are in convective contact for chemical exchange reaction to enable borontrifluoride-10 to enter a liquid phase from a gas phase successively and enter a column kettle with the downward flowing liquid, and the complex of boron trifluoride-11 is also continuously subjectedto exchange reaction and is transferred from the liquid phase to the gas phase to the top of the column. The industrial production method has the advantage of continuous and stable production.
Owner:刘禹超 +1
pH value testing method
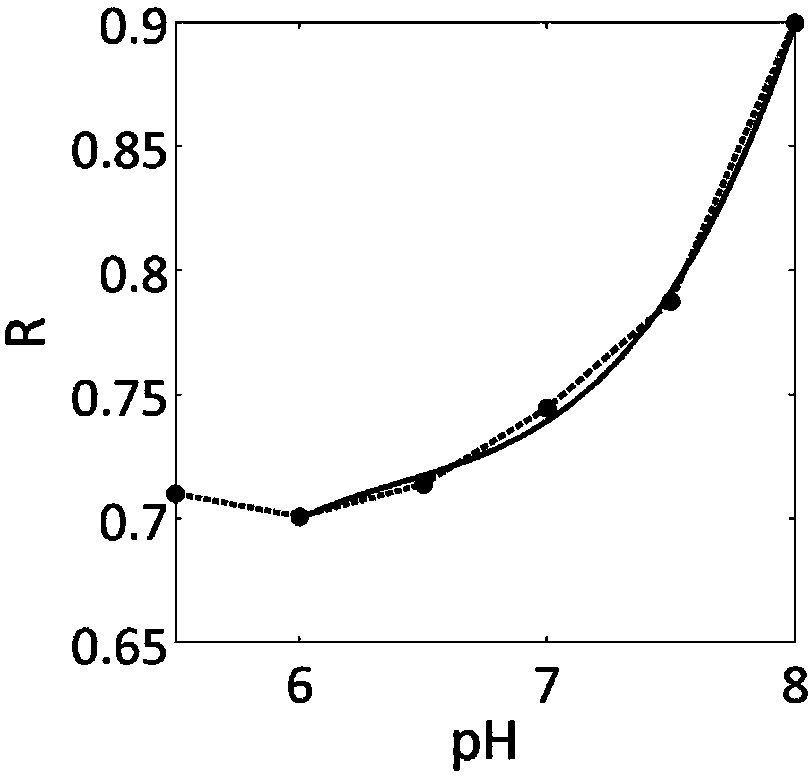
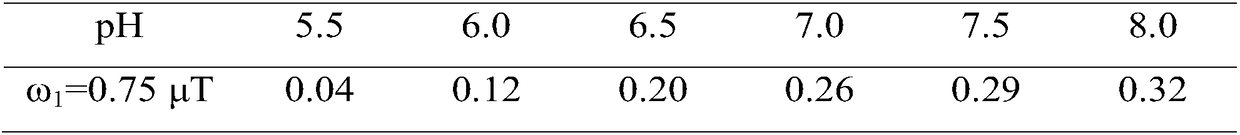
ActiveCN108195867AAccurate measurementEasy to measureMagnetic measurementsAnalysis using nuclear magnetic resonanceResonanceProton
The invention relates to the field of biological medicines, in particular to a pH value testing method which comprises the following steps: with an amino proton as an endogenous contrast agent, testing corresponding chemical exchange saturated transferring effect ratios R of amino protons of known pH value amino proton source solutions under different saturated pulse intensities, establishing function relationships of pH values and the ratios R corresponding to the different known pH value amino proton source solutions according to the different known pH value amino proton source solutions andtested corresponding ratios R, and finally calculating expected pH values according to the function relationships of values Ri tested through experiments and the function relationships. By adopting the method, influence of concentrations can be eliminated, parameters such as exchangeable proton concentrations and longitudinal relaxation times of water do not need to be estimated or measured, so that pH values can be relatively accurately, conveniently and non-destructively measured. In addition, since amino is relatively low in chemical exchange velocity, stable imaging under magnetic resonance field intensity of common clinical application can be achieved, and thus the method has good application prospects.
Owner:SHENZHEN INST OF ADVANCED TECH
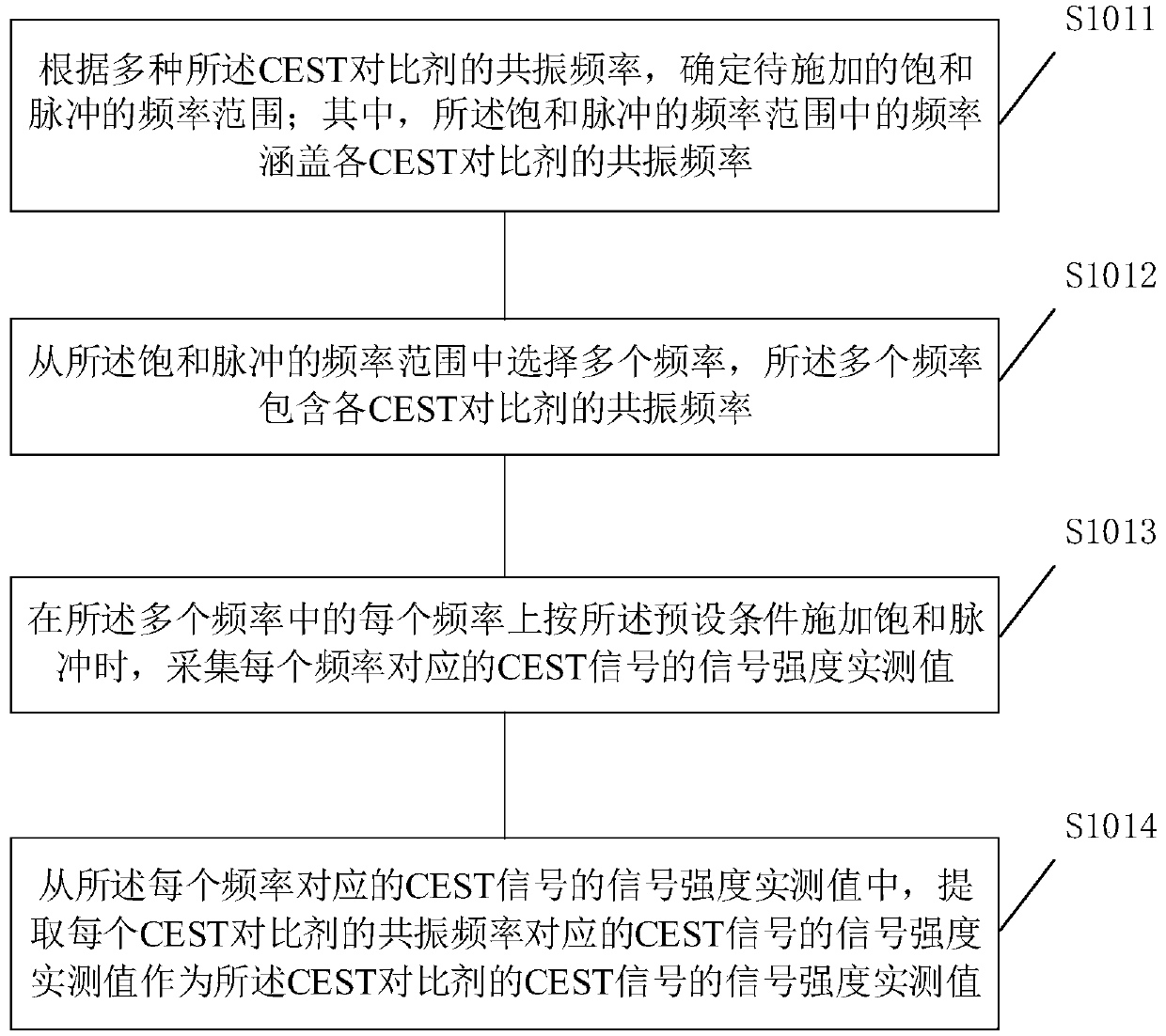
Chemical exchange characteristic quantification method and equipment
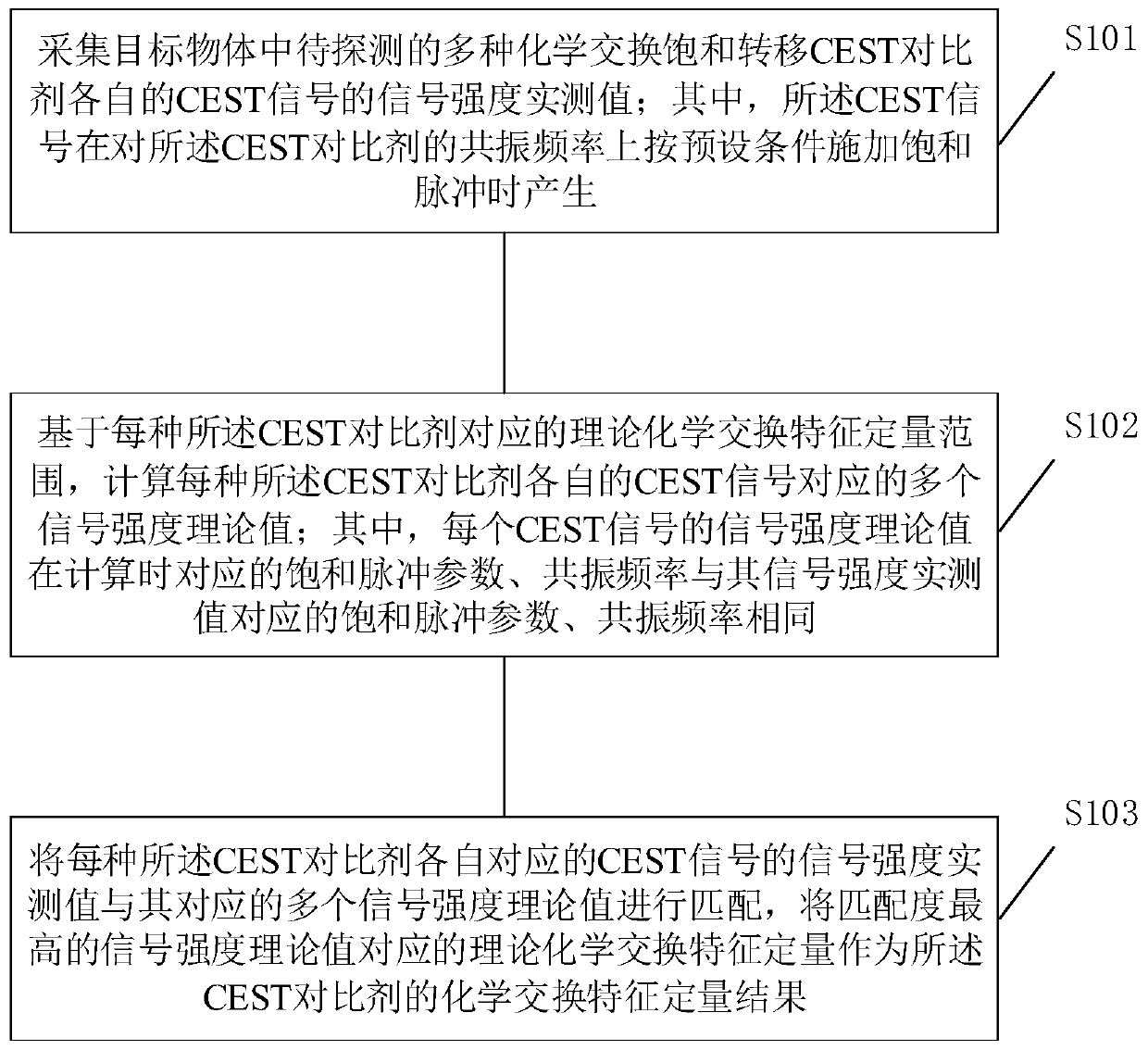
ActiveCN110824398AExact matchImprove accuracyMagnetic measurementsChemical physicsPhysical chemistry
The invention is suitable for the field of biomedical engineering, and provides a chemical exchange characteristic quantification method and chemical exchange characteristic quantification equipment.The chemical exchange characteristic quantification method comprises the steps of: acquiring signal intensity measured values of CEST signals of various CEST contrast agents; respectively calculatingcorresponding signal intensity theoretical values under the same saturation pulse parameter and resonance frequency as the signal intensity measured values based on a theoretical chemical exchange characteristic quantitative range of each CEST contrast agent; and regarding theoretical chemical exchange characteristic quantification corresponding to the theoretical value with the highest matching degree as a chemical exchange characteristic quantification result of the CEST contrast agents. According to the chemical exchange characteristic quantification method and the chemical exchange characteristic quantification equipment, the chemical exchange characteristic quantification process does not need to select a reference signal, thus is not susceptible to other chemical exchange effects, the theoretical chemical exchange characteristic quantification corresponding to the matching result can represent the actual chemical exchange specific quantity more precisely, and the precision of thechemical exchange specific quantity is effectively improved.
Owner:SHENZHEN INST OF ADVANCED TECH
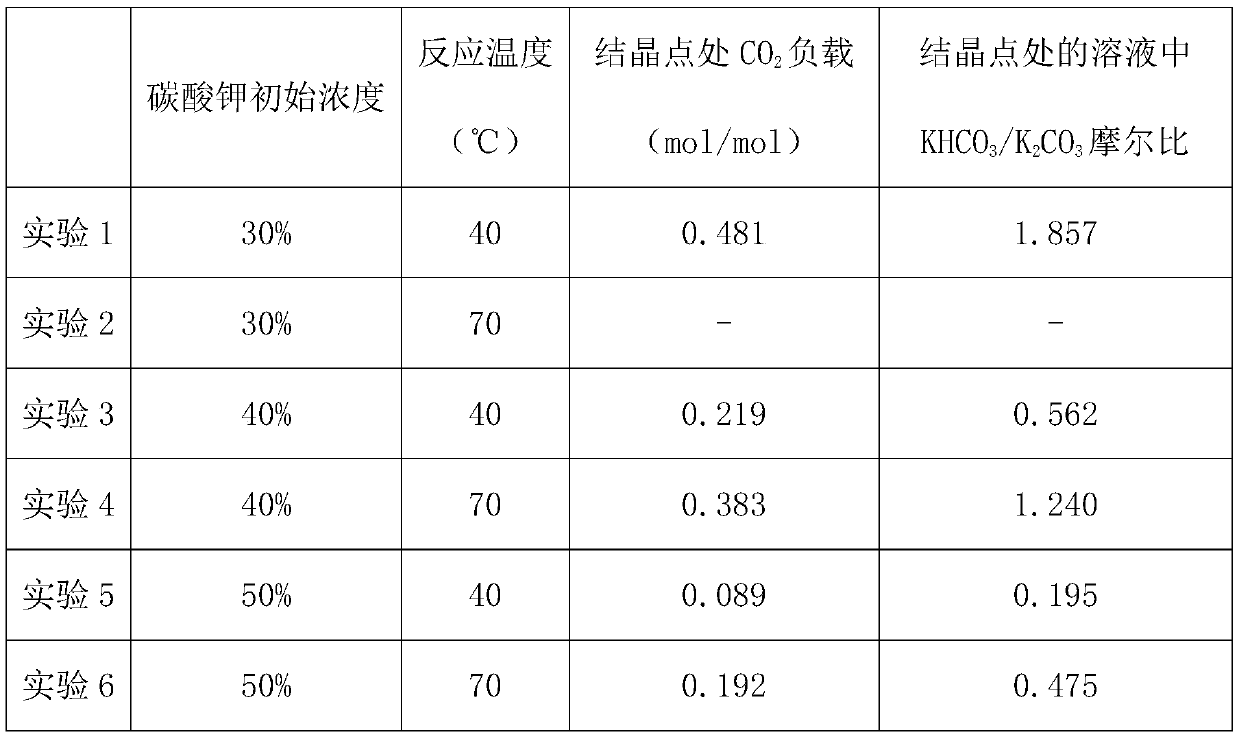
Liquid-solid phase change absorbent used for carbon dioxide capture and application of absorbent
InactiveCN109701362AStrong toleranceReduce volatilityDispersed particle separationAir quality improvementHigh concentrationSolubility
The invention provides a liquid-solid phase change absorbent used for carbon dioxide capture and an application of the absorbent. The absorbent comprises the following components, in percentages by mass: 10%-60% of a carbonate, 2%-40% of a bicarbonate, 1%-20% of an absorption activator, 0.01%-10% of an absorption auxiliary agent, and the balance of water. According to the absorbent provided by theinvention, precipitation of the bicarbonate is realized by utilizing solubility difference of the carbonate and the bicarbonate in an aqueous solution and using a chemical exchange or cooling crystallization manner, then the high-concentration bicarbonate crystal slurry is subjected to pyrolysis regeneration, so that participation of water during regeneration can be reduced, solvent heating sensible heat during the enriched-liquid regeneration and water gasification latent heat can be reduced, and CO2 regeneration heat consumption and capture costs can be reduced.
Owner:HUANENG POWER INTERNATIONAL +1
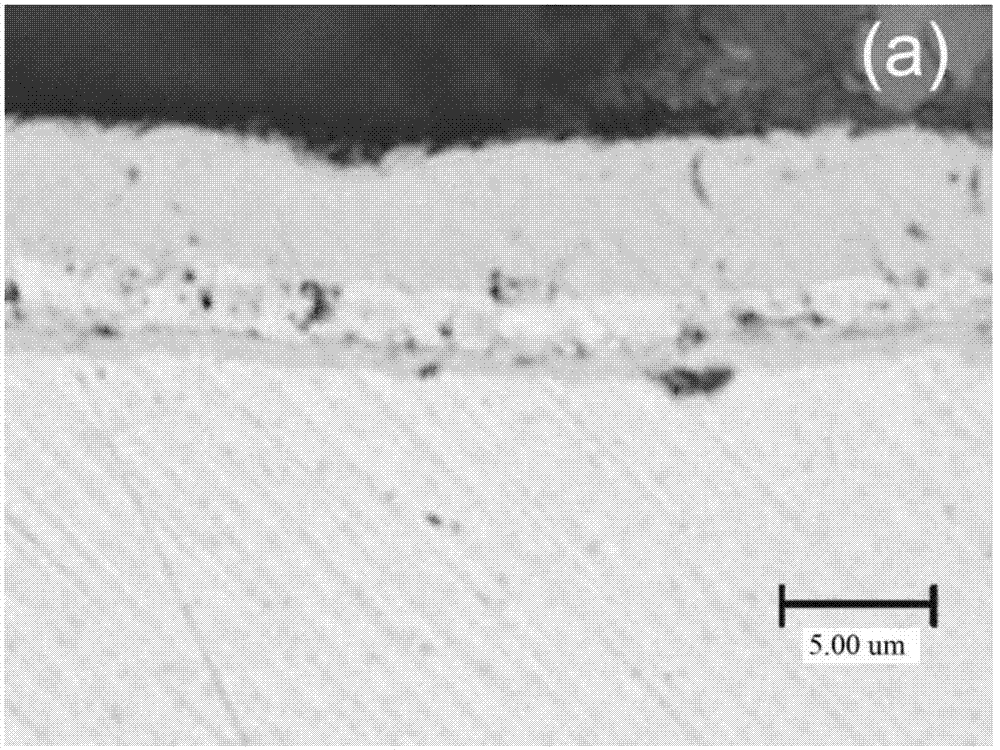
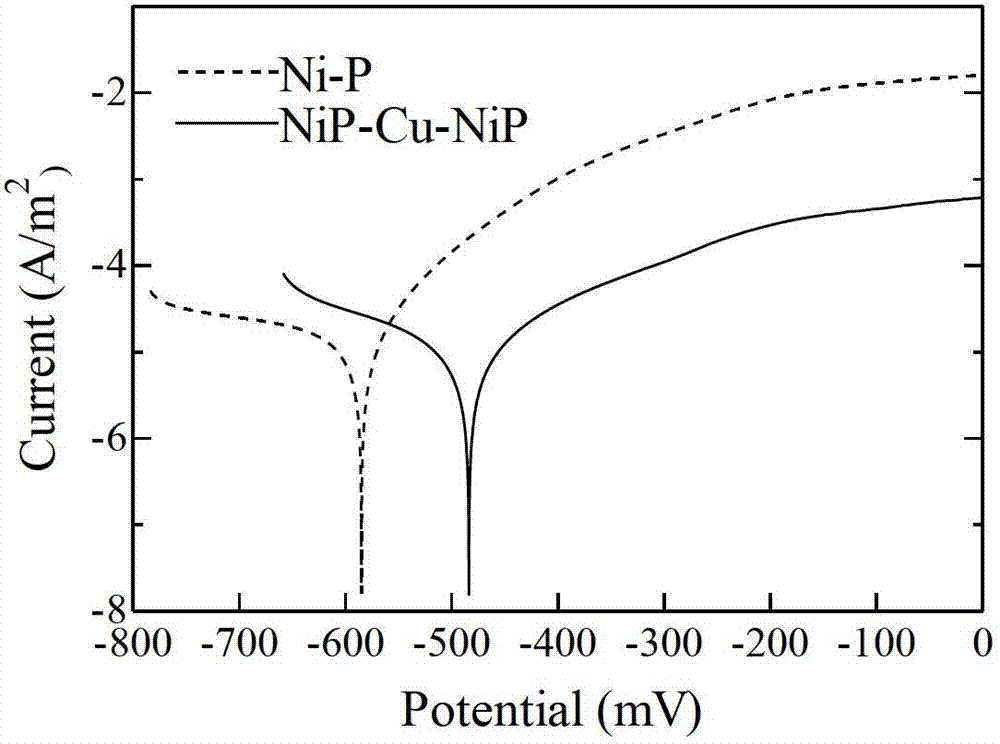
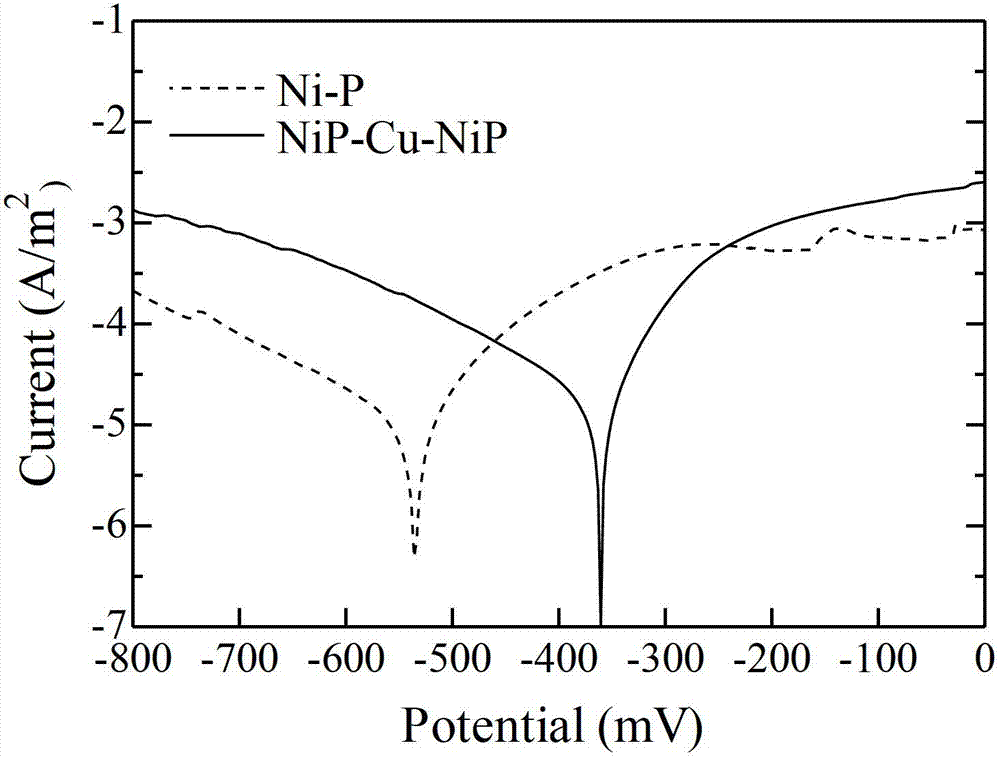
Method for improving corrosion resistance of nickel-phosphorus chemical coating
InactiveCN102899642AReduce bond strengthGuaranteed bonding strengthLiquid/solution decomposition chemical coatingPorosityCopper plating
The invention relates to a method for improving corrosion resistance of a nickel-phosphorus chemical coating. The method comprises the following steps: plating a thin layer of nickel and phosphorus on the surface of a carbon steel substrate, and plating a layer of copper using a chemical exchange method; and then plating a layer of nickel and phosphorus. According to the invention, chemical copper plating is adopted for sealing holes and improving corrosion potential; in the process of the primary nickel and phosphorus plating, nickel and phosphorus are plated on most of the carbon steel substrate, so that the possibility that lower bonding strength of the coating is caused by reaction between copper and iron in a large amount during the secondary copper plating step is reduced, thereby ensuring the bonding strength of the coating. The method is suitable for occasions that the corrosion resistance of the coating is reduced due to small thickness and high porosity of the coating. By using the method, the corrosion resistance effect can be greatly improved under the condition that the thin coating is obtained.
Owner:SHANDONG UNIV
Preparation method of high-purity enriched 11B boron trifluoride gas
ActiveCN102115093AEasy to integrateHigh densityBoron halogen compoundsBoron trifluorideSodium tetrafluoroborate
The invention relates to a high-purity enriched 11B boron trifluoride gas and a preparation method thereof. The preparation method comprises the step of enriching the 11B in a double-tower in-series high-efficiency rectifying tower by a chemical exchange method by using a mass of 11B boron trifluoride methyl ether generated during producing the enriched 11B and taking the part of a byproduct as a raw material. The abundance of the 11B is higher than 95% (at.), and the purity is higher than 99wt%. Therefore, the 11B boron trifluoride methyl ether coordination compound is directly reacted with the sodium fluoride to obtain the 11B sodium fluoborate, so that the technical process is simple, the product purity is high, and the yield of the 11B sodium fluoborate is greatly improved. The 11B sodium fluoborate is directly heated at 600-700DEG C to be thermally decomposed so as to obtain the 11B boron trifluoride gas. The abundance of the prepared boron trifluoride gas 11B is higher than 95% (at.), and the purity is higher than 99.99vol%.
Owner:DALIAN BORONTEN SCI & TECH
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com