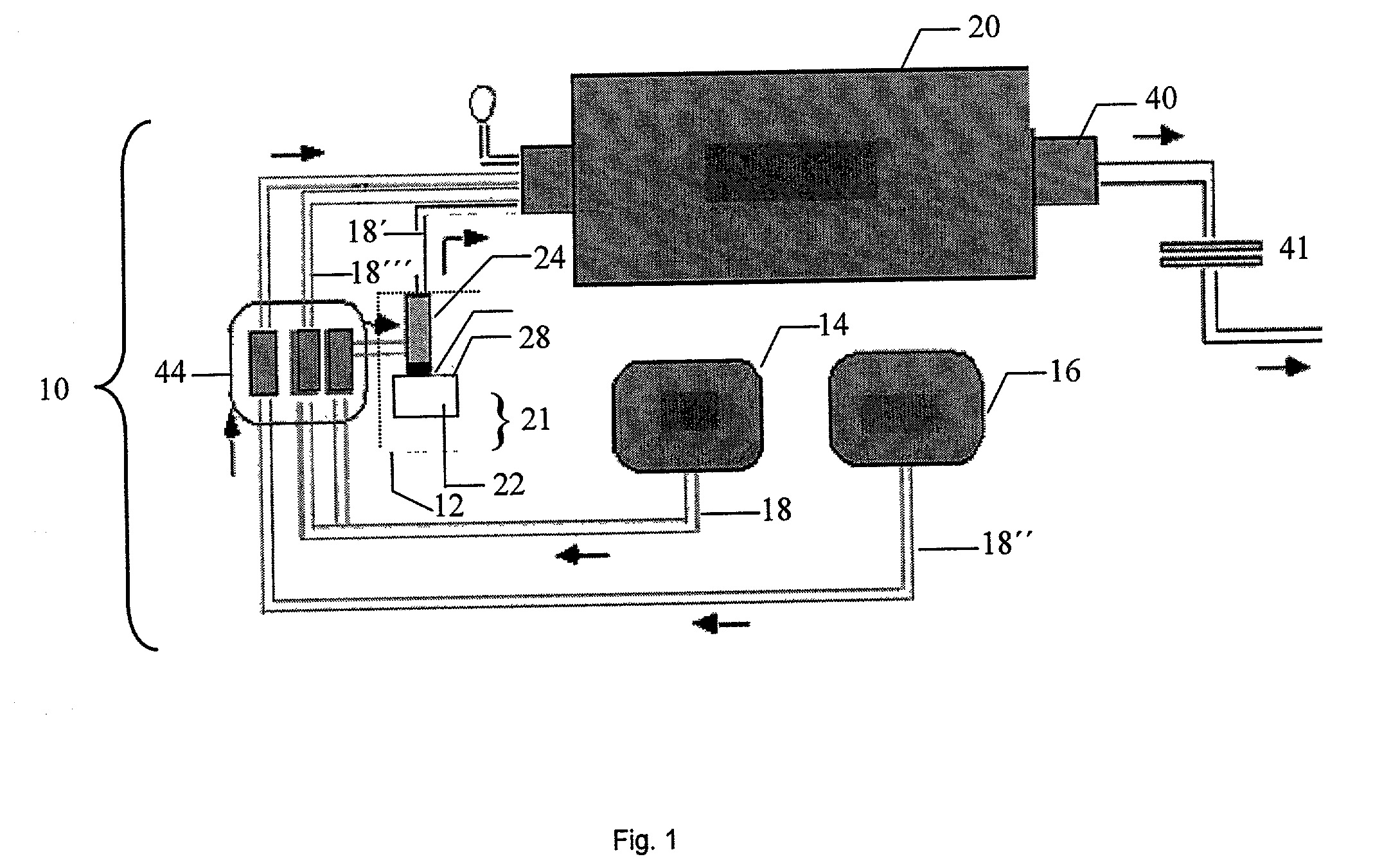
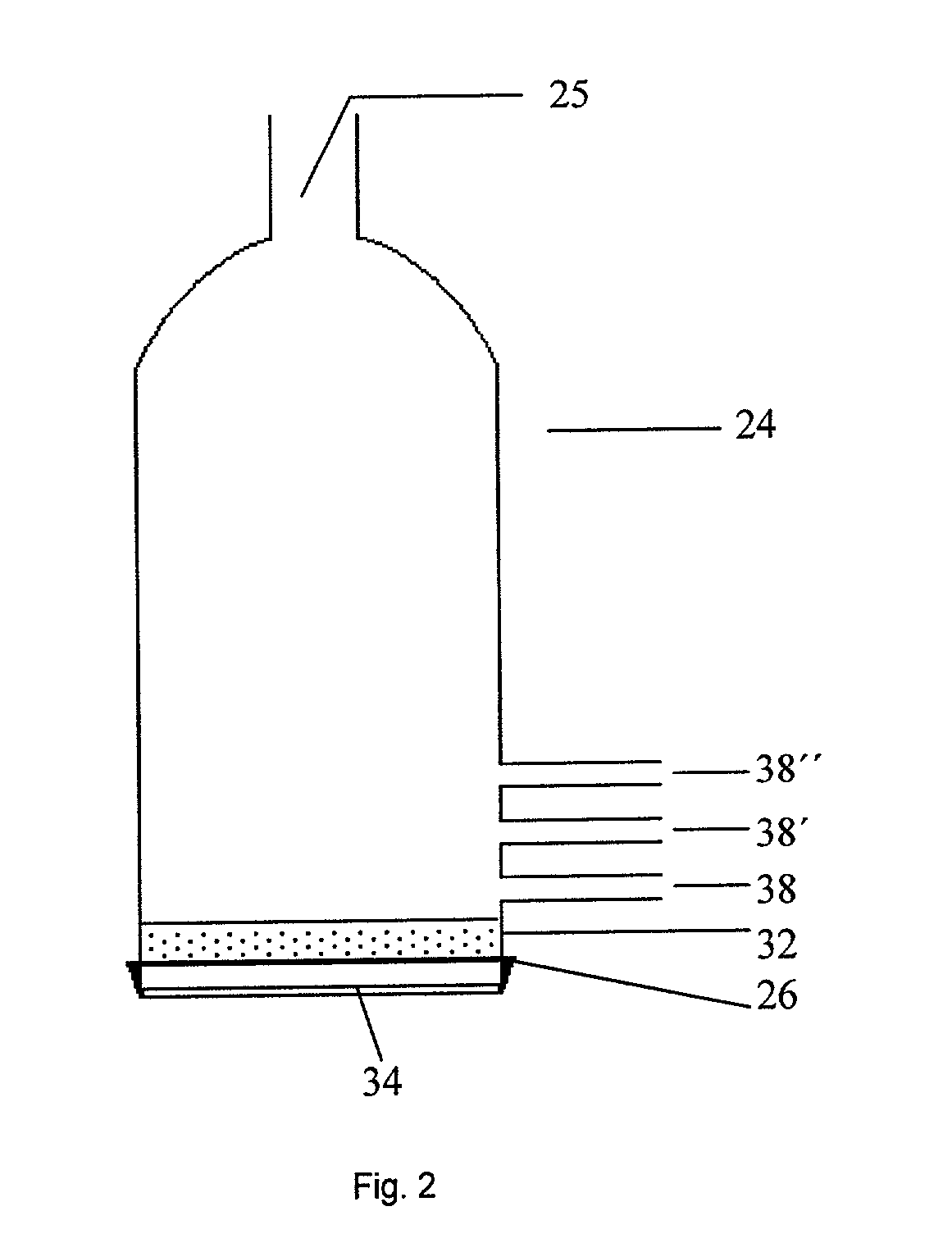
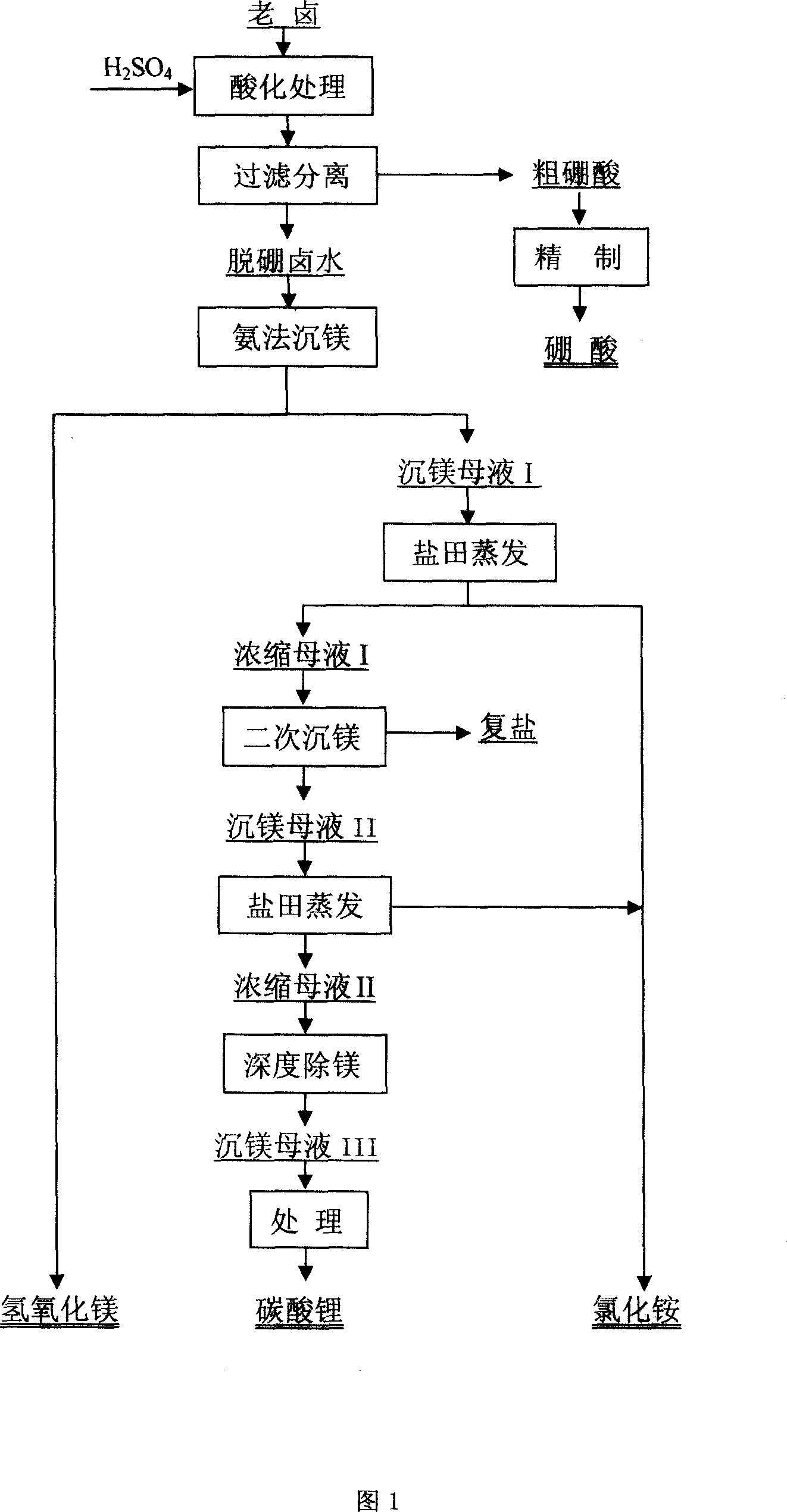
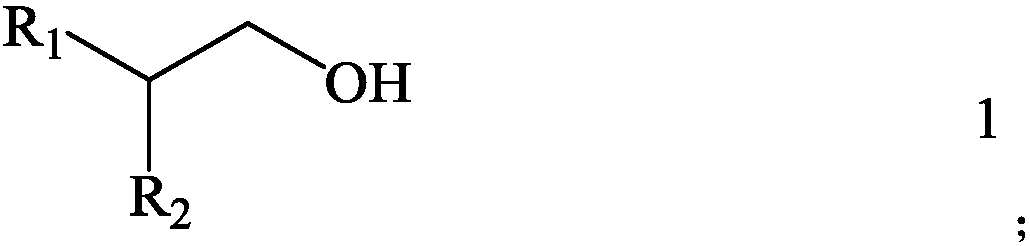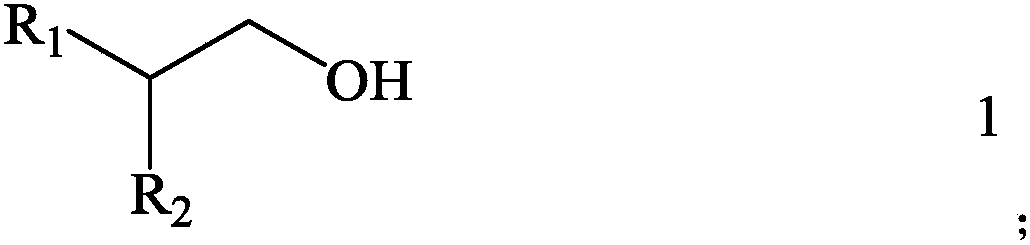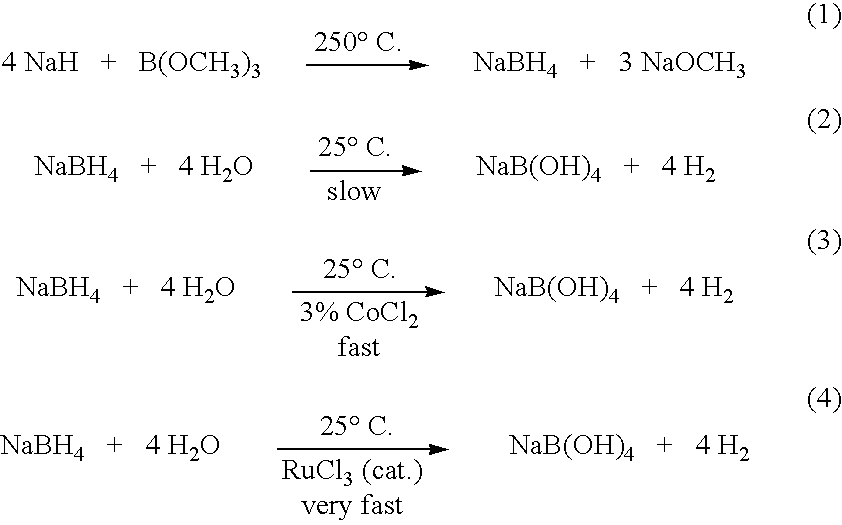Patents
Literature
319results about "Boron-oxygen compounds" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
Catalysts for petrochemical catalysis
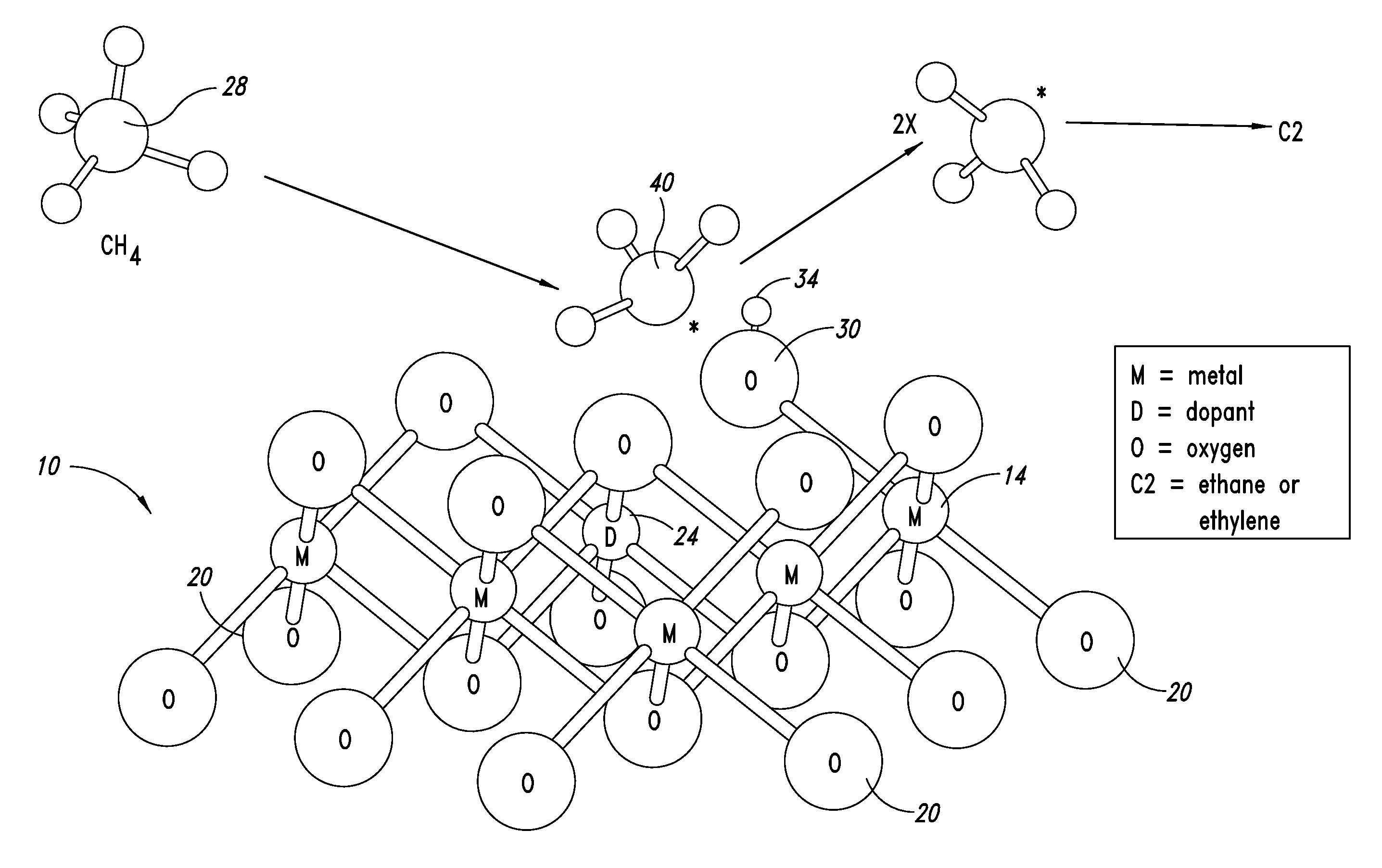
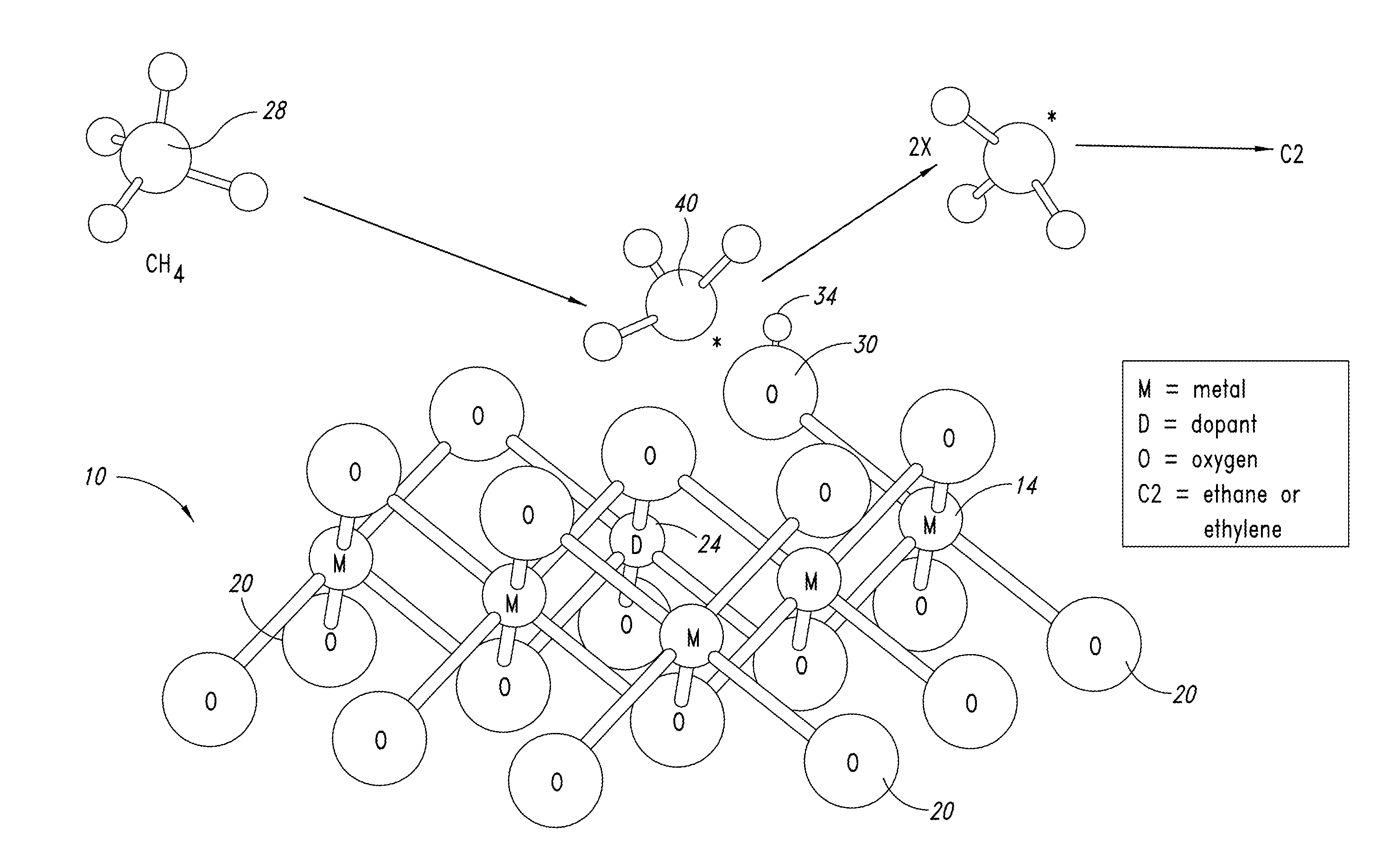
ActiveUS20130023709A1Sequential/parallel process reactionsManganese oxides/hydroxidesDopantPetrochemical
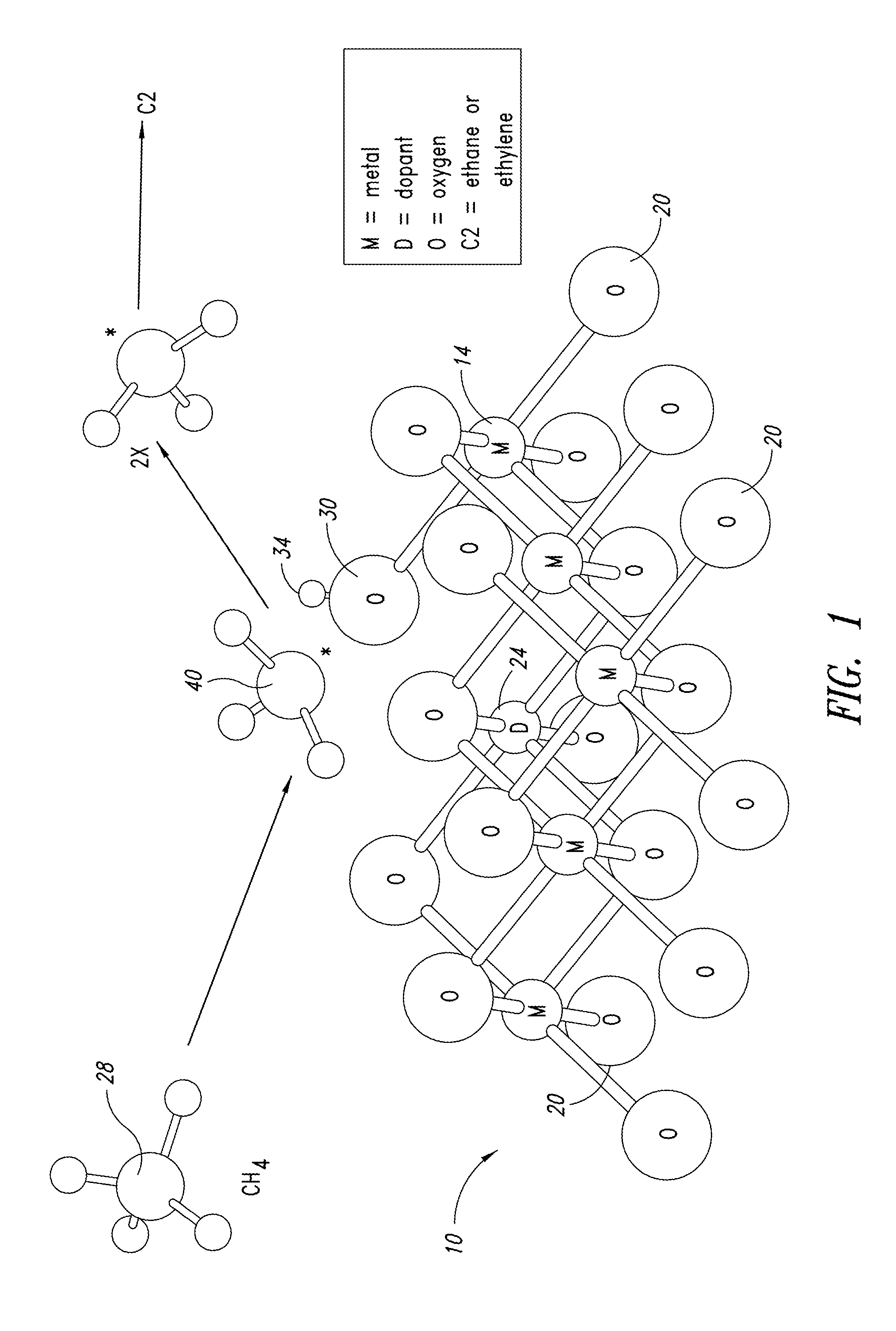
Metal oxide catalysts comprising various dopants are provided. The catalysts are useful as heterogenous catalysts in a variety of catalytic reactions, for example, the oxidative coupling of methane to C2 hydrocarbons such as ethane and ethylene. Related methods for use and manufacture of the same are also disclosed.
Owner:SILURIA TECH INC
Nanowire catalysts and methods for their use and preparation
ActiveUS20130165728A1Material nanotechnologyManganese oxides/hydroxidesNanowireOxidative coupling of methane
Nanowires useful as heterogeneous catalysts are provided. The nanowire catalysts are useful in a variety of catalytic reactions, for example, the oxidative coupling of methane to C2 hydrocarbons. Related methods for use and manufacture of the same are also disclosed.
Owner:LUMMUS TECH LLC
Solid electrolyte, method for preparing the same, and battery using the same
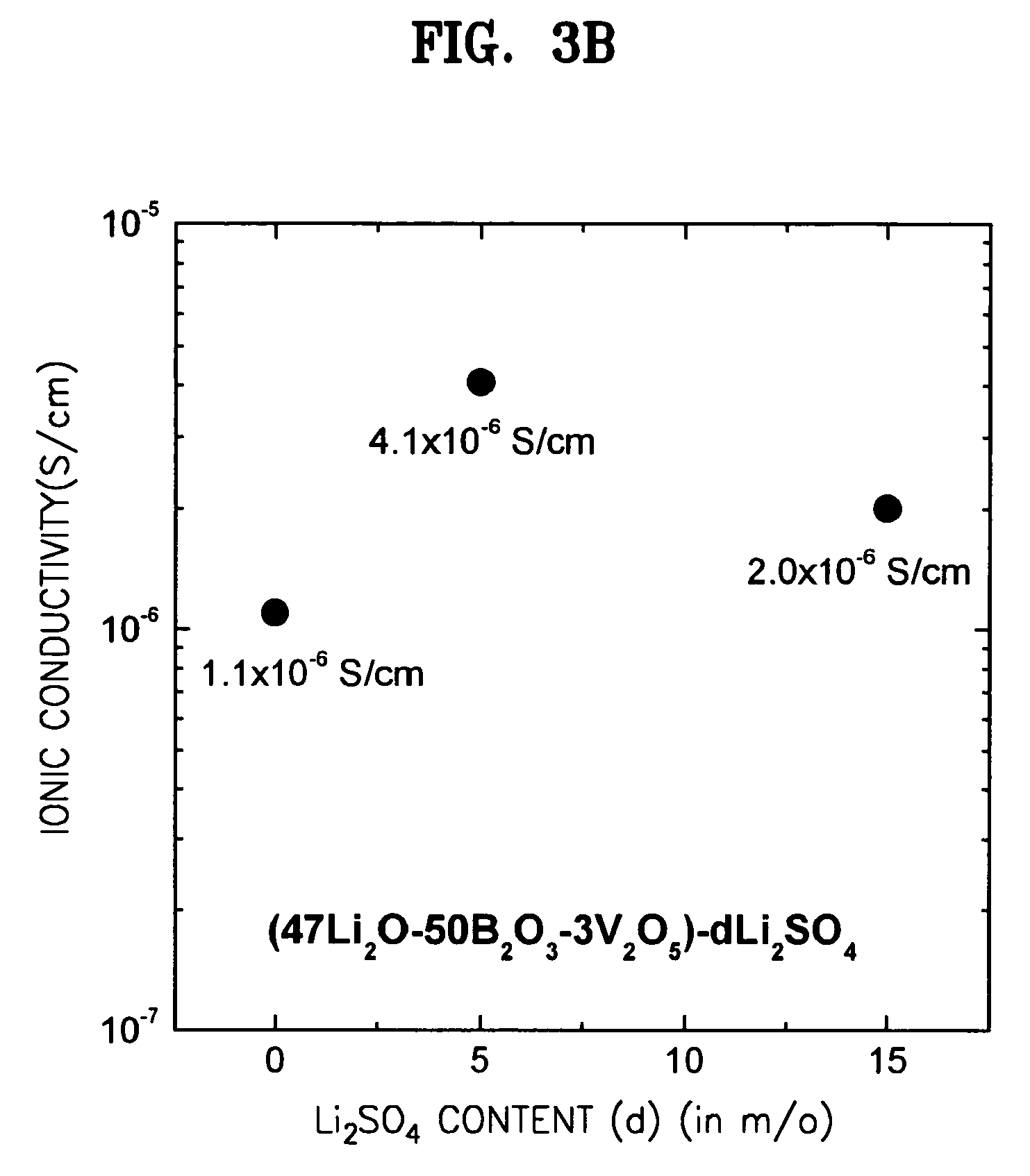
ActiveUS7273682B2Improve ion conductionImproved in charge/discharge rateNon-metal conductorsElectrolytic capacitorsLithiumPhysical chemistry
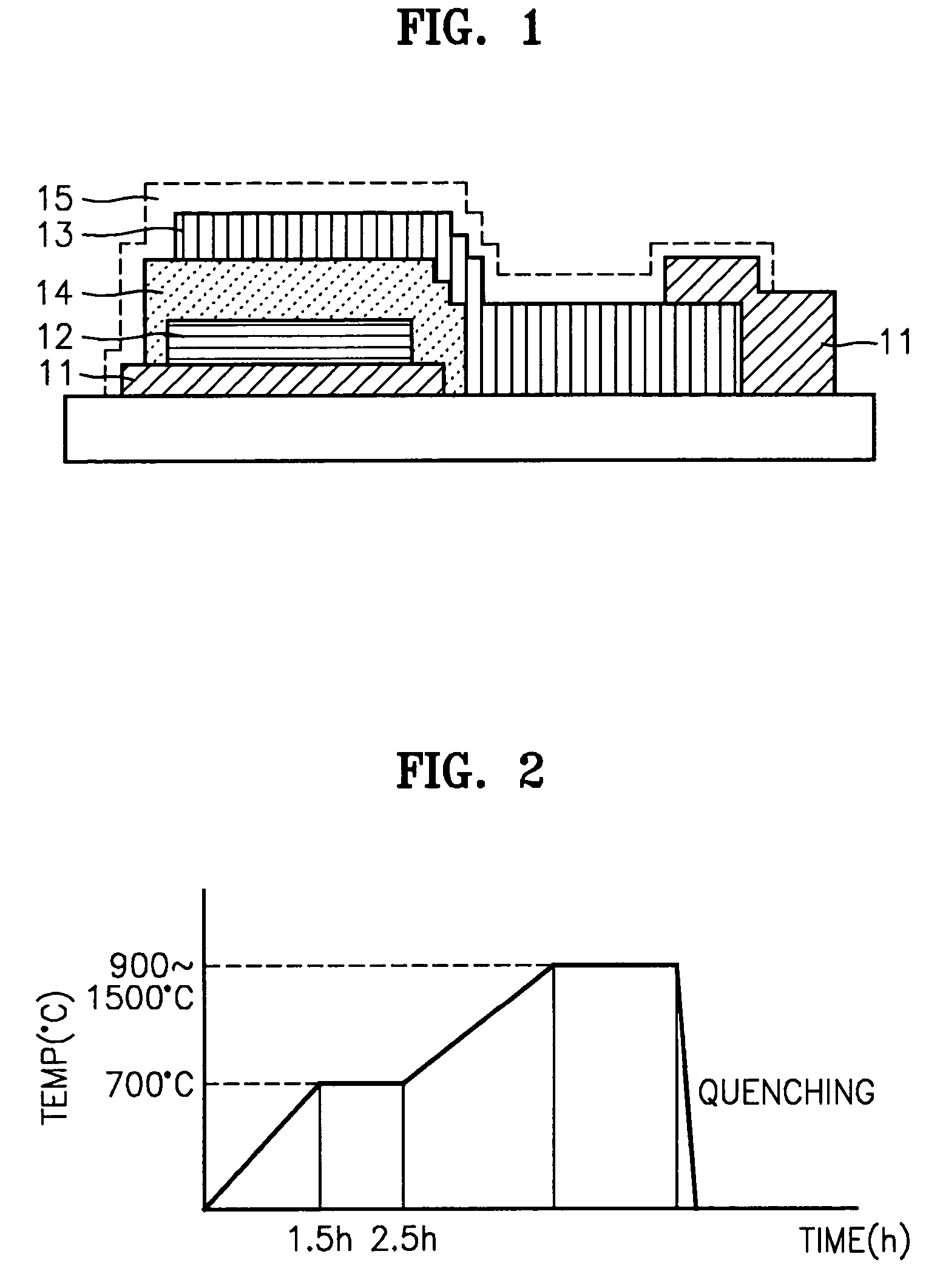
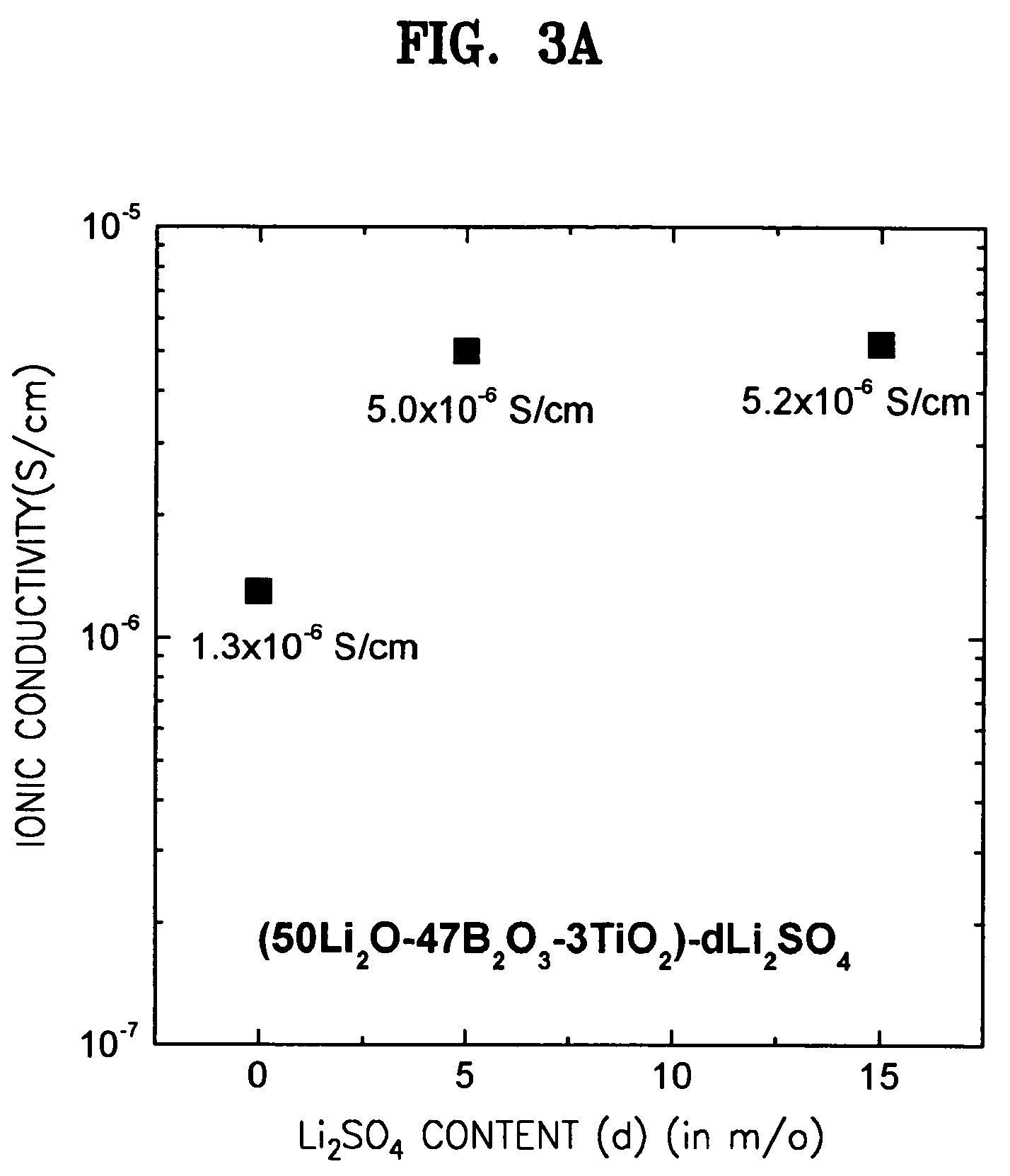
A solid electrolyte including a composition represented by Formula 1 below is provided: aLi2O-bB2O3-cM-dX (1) wherein M is at least one selected from the group consisting of TiO2, V2O5, WO3, and Ta2O5; X is at least one selected from LiCl and Li2SO4; 0.4<a<0.55; 0.4<b<0.55; 0.02<c<0.05; a+b+c=1, and 0≦d<0.2. A method for preparing the solid electrolyte and a battery using the solid electrolyte are also provided. The solid electrolyte exhibits high ionic conductivity. Lithium and thin film batteries using the solid electrolyte are improved in charge / discharge rate, power output, and cycle life.
Owner:SAMSUNG SDI CO LTD
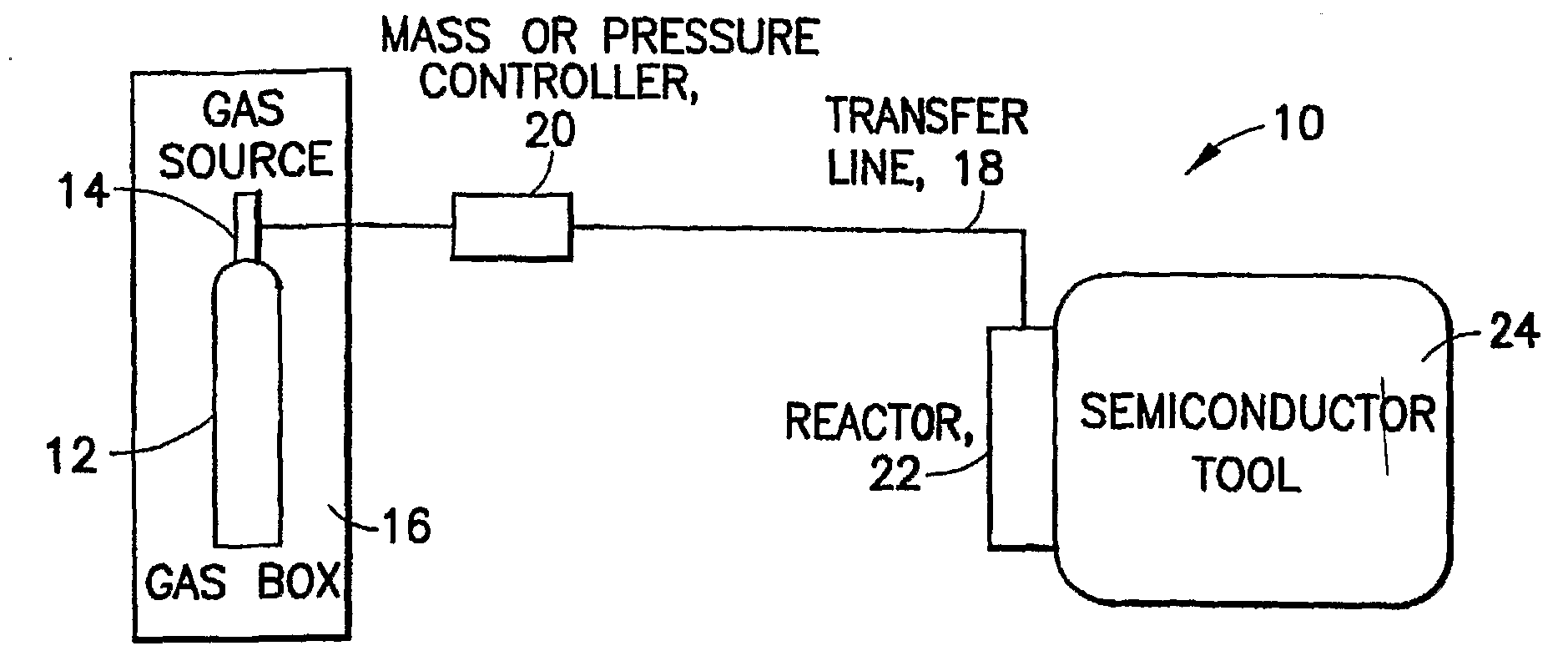
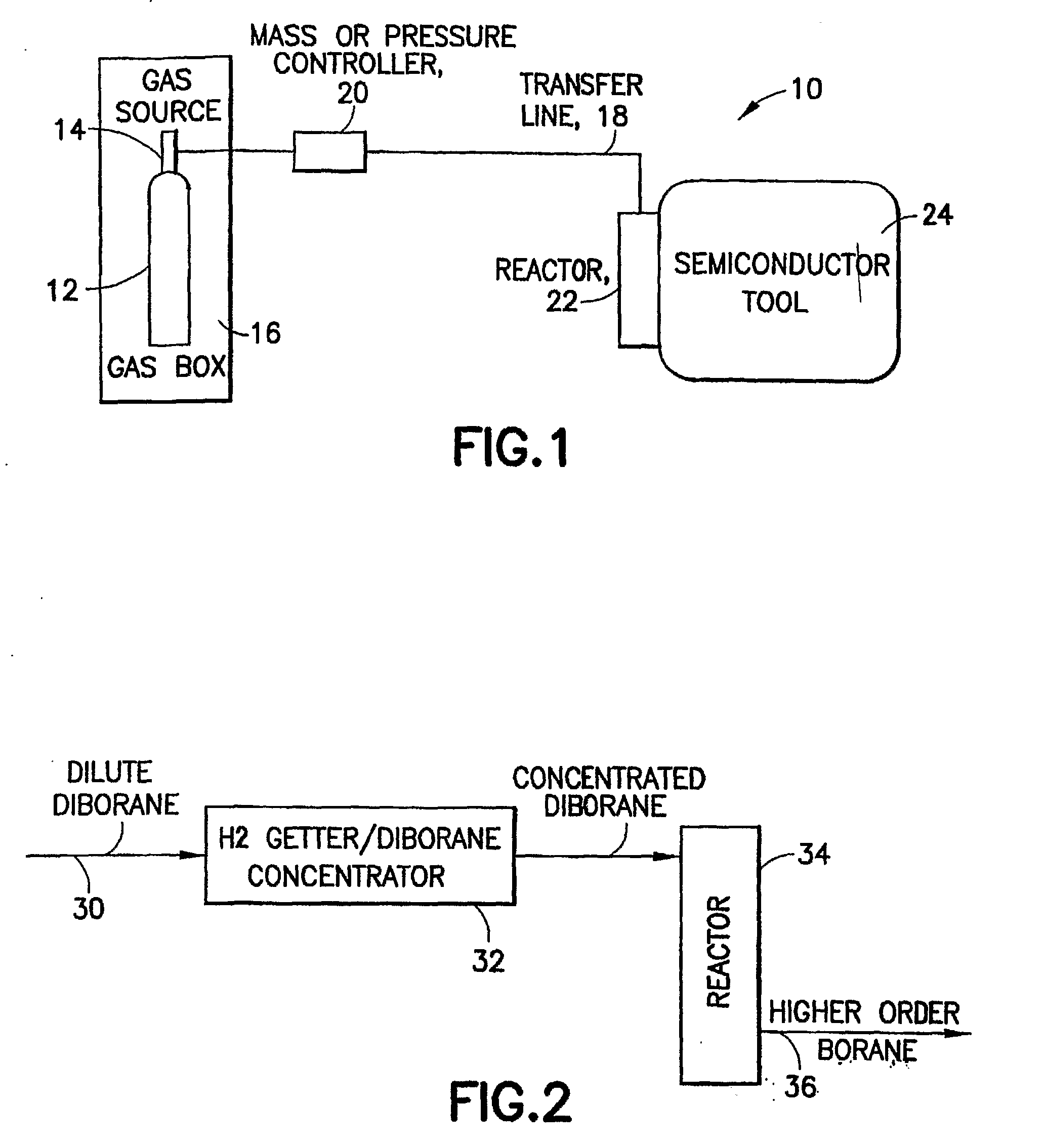
Boron Ion Implantation Using Alternative Fluorinated Boron Precursors, and Formation of Large Boron Hydrides for Implanation
ActiveUS20080248636A1Easy to cutImprove efficiencyOther chemical processesElectric discharge tubesBoron trifluorideIon implantation
Methods of implanting boron-containing ions using fluorinated boron-containing dopant species that are more readily cleaved than boron trifluoride. A method of manufacturing a semiconductor device including implanting boron-containing ions using fluorinated boron-containing dopant species that are more readily cleaved than boron trifluoride. Also disclosed are a system for supplying a boron hydride precursor, and methods of forming a boron hydride precursor and methods for supplying a boron hydride precursor. In one implementation of the invention, the boron hydride precursors are generated for cluster boron implantation, for manufacturing semiconductor products such as integrated circuitry.
Owner:ENTEGRIS INC
Method for Exfoliation of Hexagonal Boron Nitride
ActiveUS20110045223A1Simplify the chemical processEasy post-processingNitrogen compoundsLayered productsBulk crystalOrganic solvent
A new method is disclosed for the exfoliation of hexagonal boron nitride into mono- and few-layered nanosheets (or nanoplatelets, nanomesh, nanoribbons). The method does not necessarily require high temperature or vacuum, but uses commercially available h-BN powders (or those derived from these materials, bulk crystals) and only requires wet chemical processing. The method is facile, cost efficient, and scalable. The resultant exfoliated h-BN is dispersible in an organic solvent or water thus amenable for solution processing for unique microelectronic or composite applications.
Owner:NASA
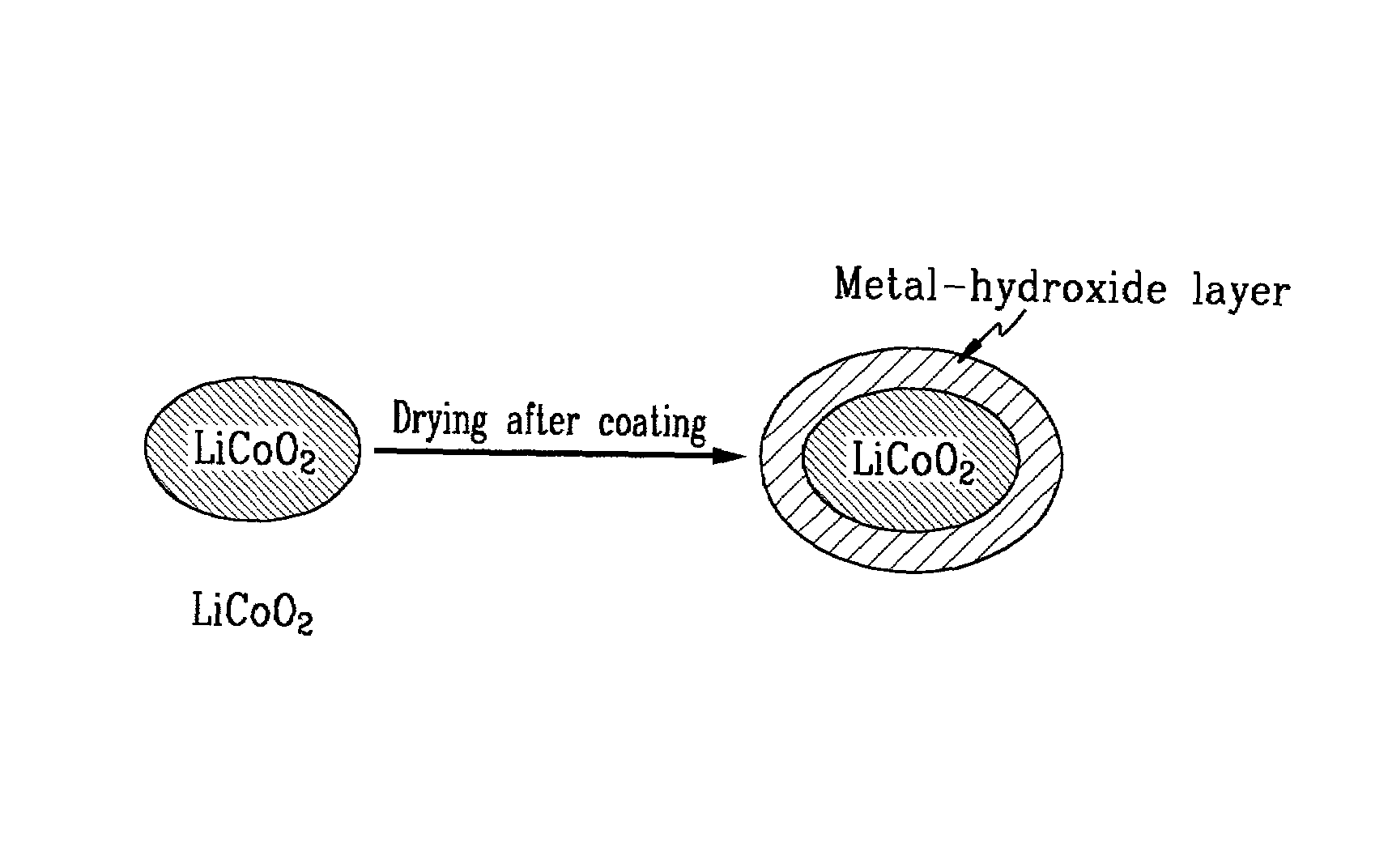
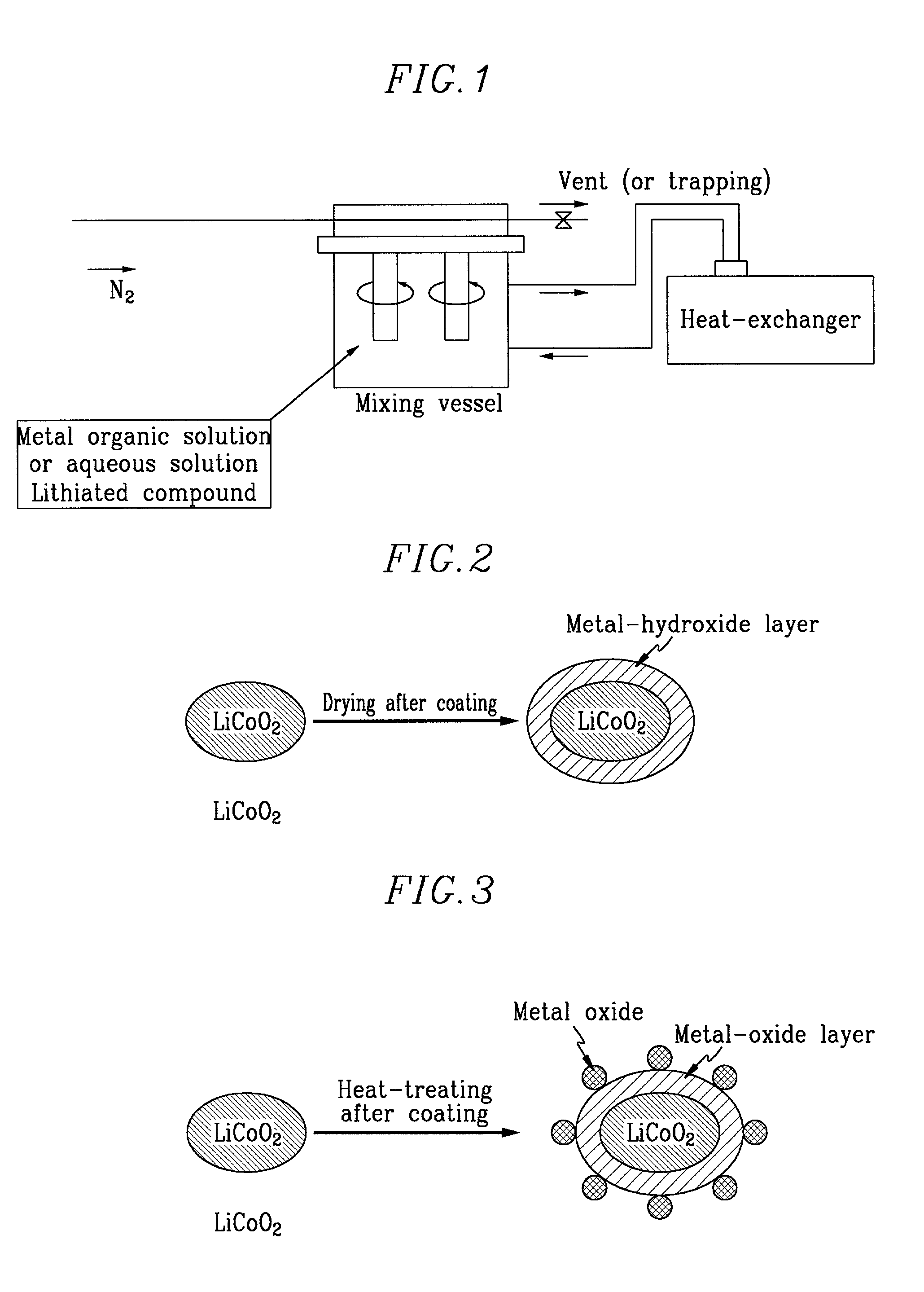
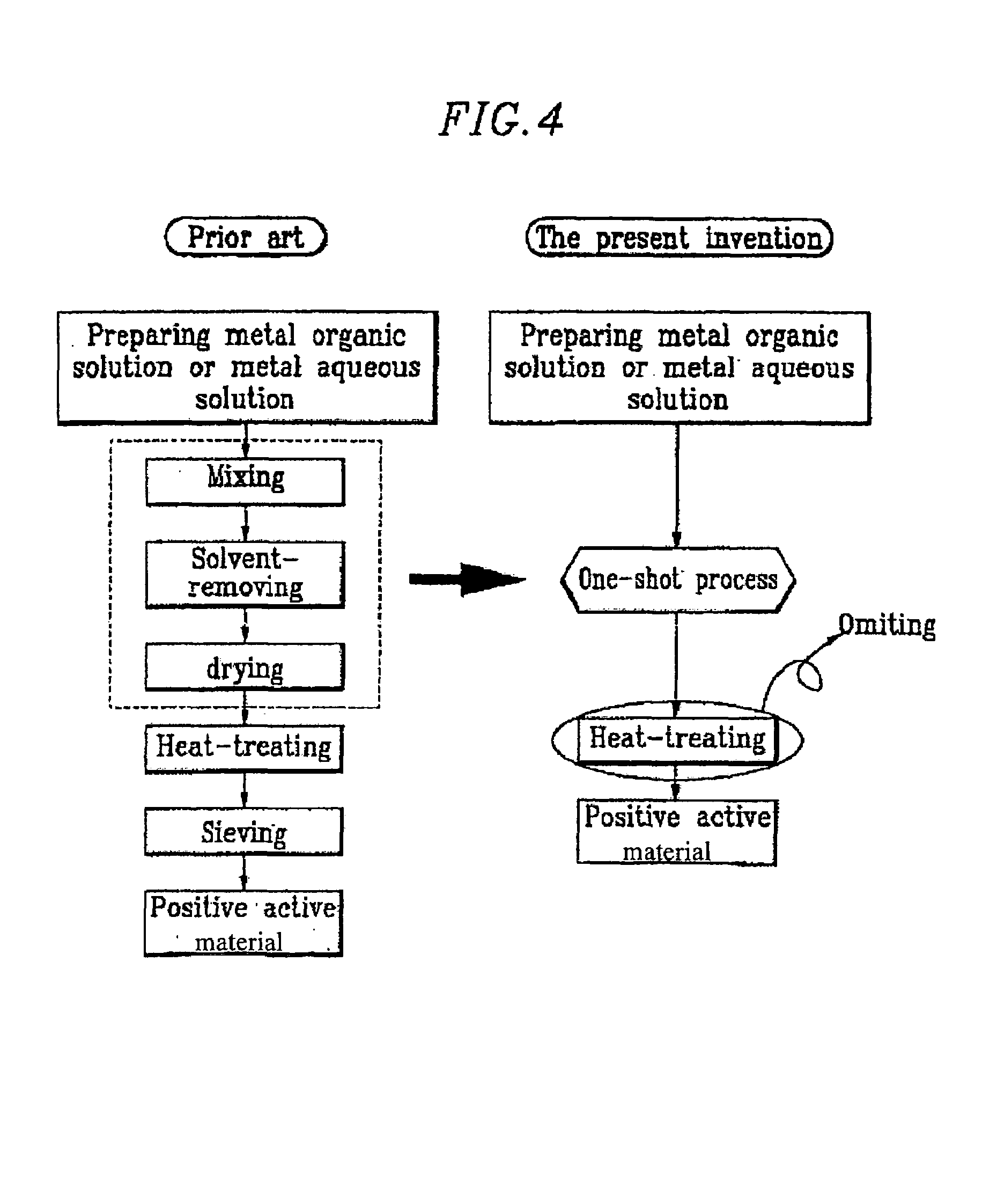
Method of preparing positive active material for rechargeable lithium batteries
InactiveUS6949233B2Improved cycle life characteristicsHigh discharge rateElectrode manufacturing processesPhosphatesPhysical chemistryHeat treated
Owner:SAMSUNG SDI CO LTD
Plasma synthesis of metal oxide nanoparticles
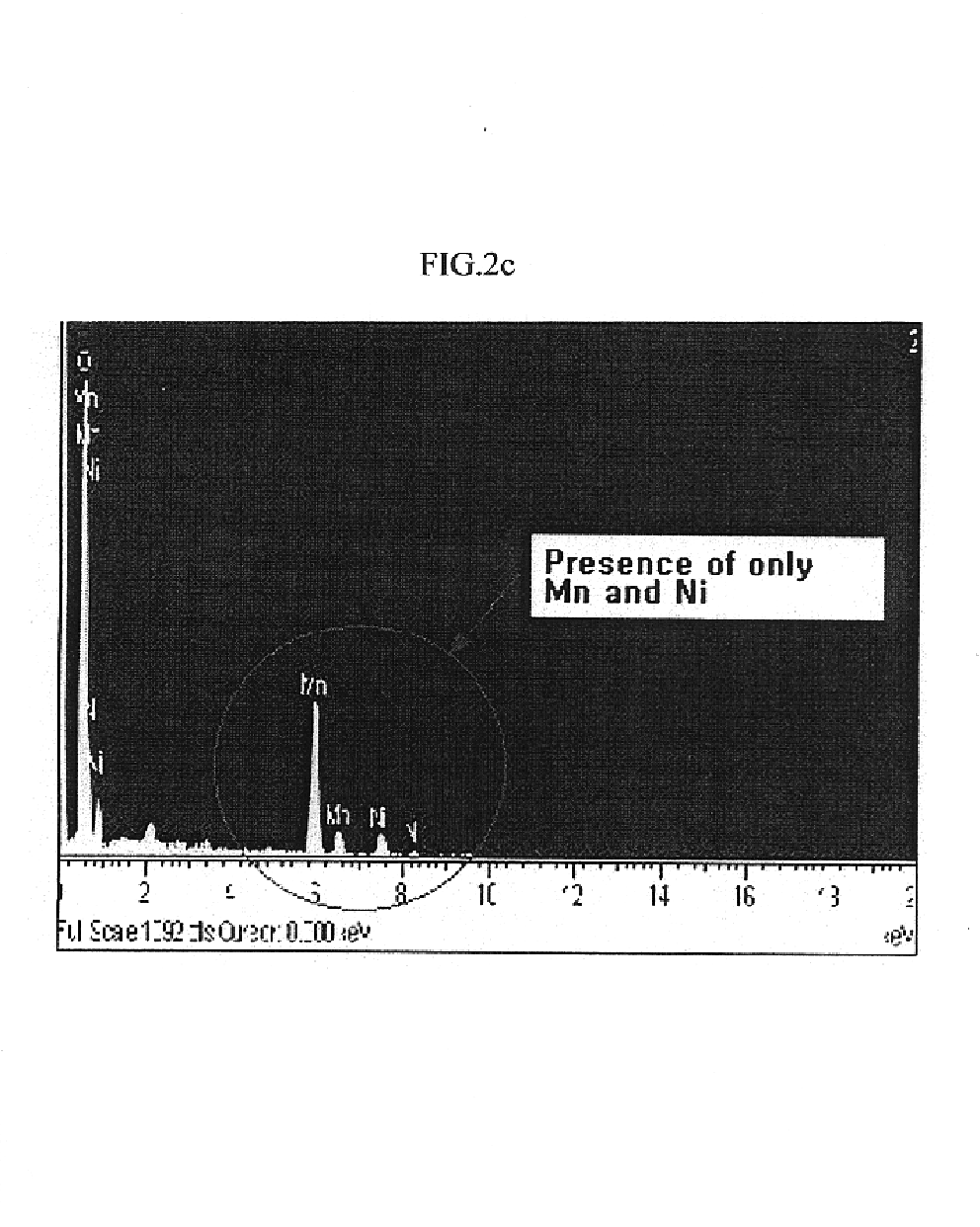
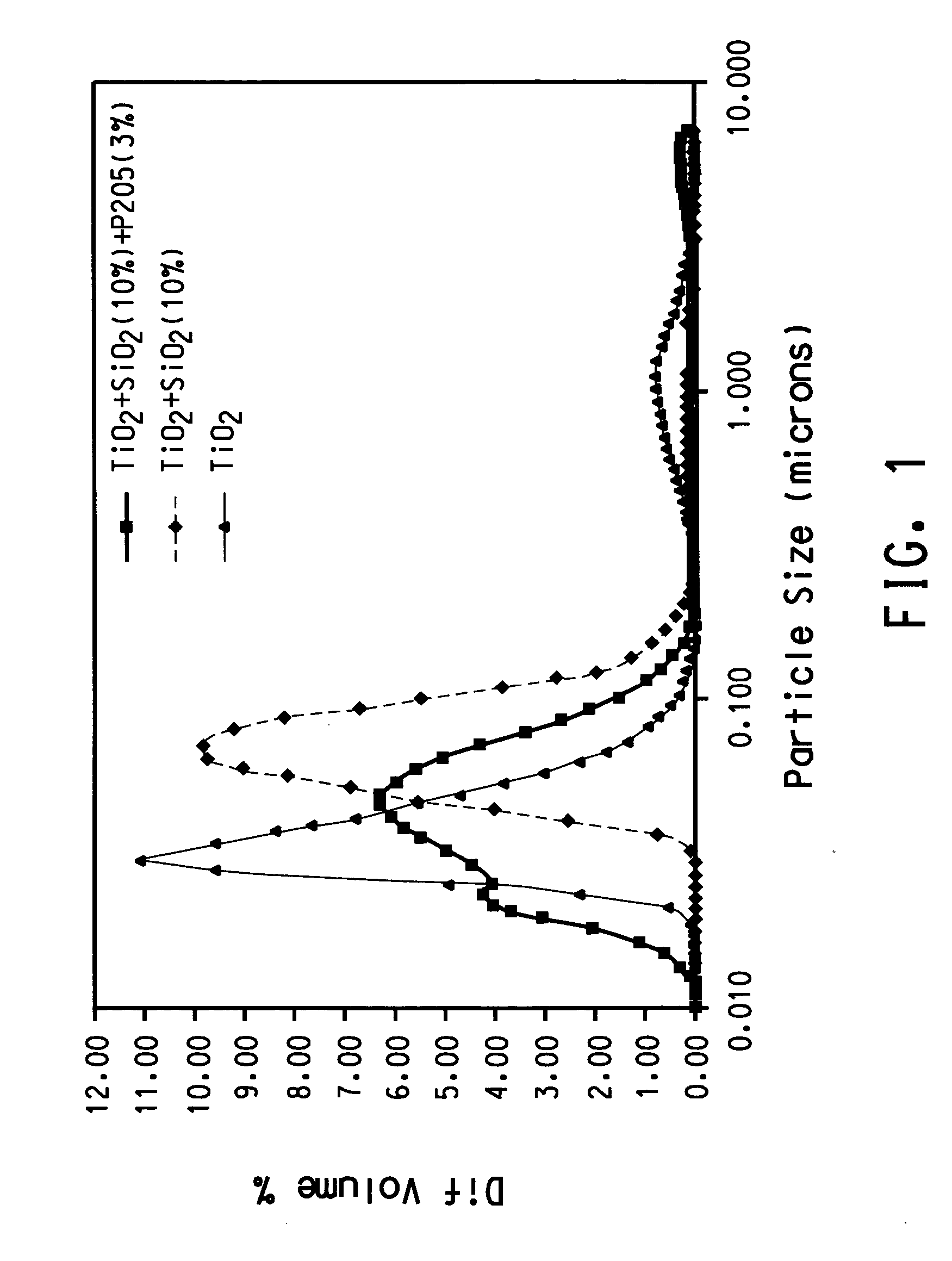
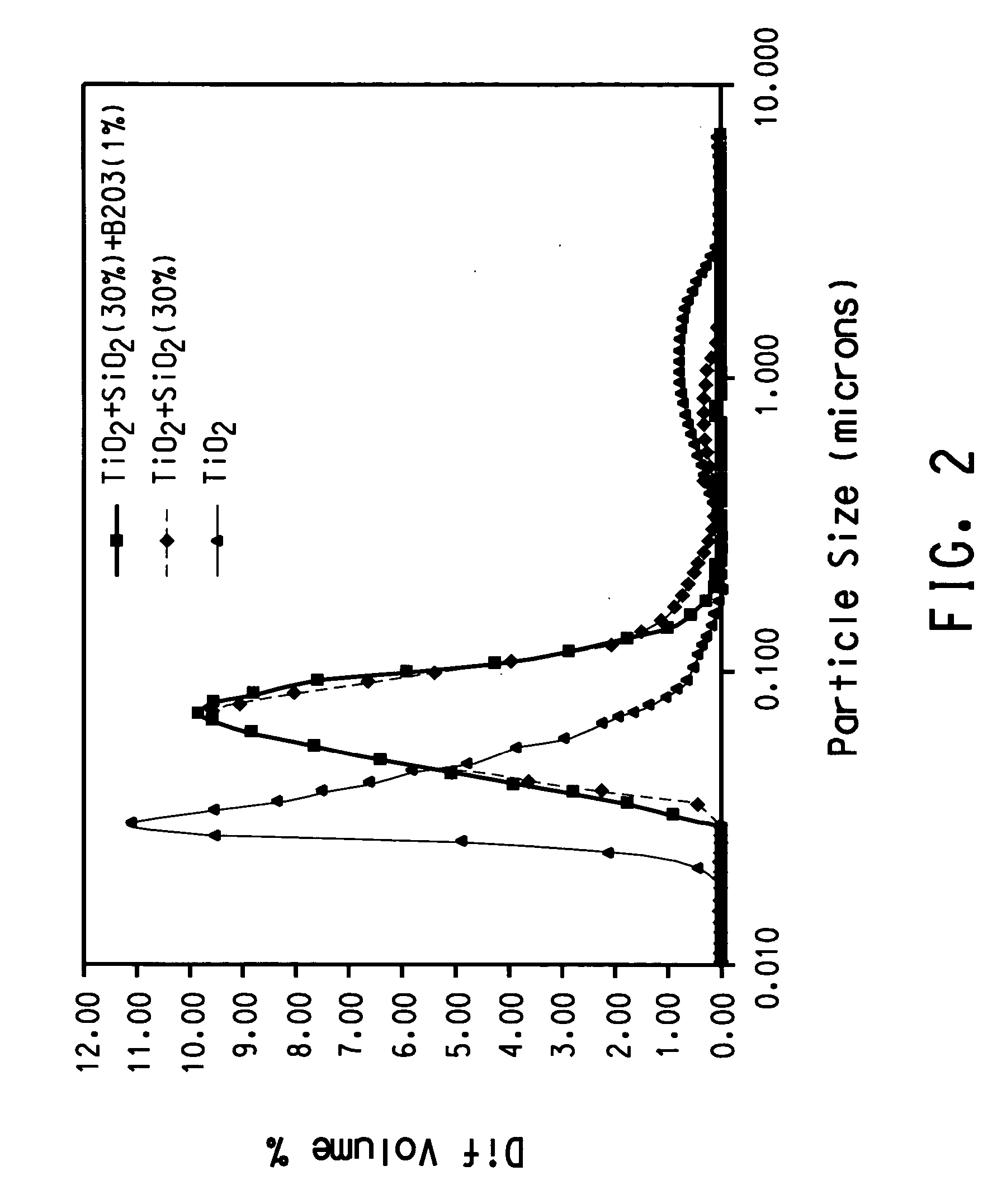
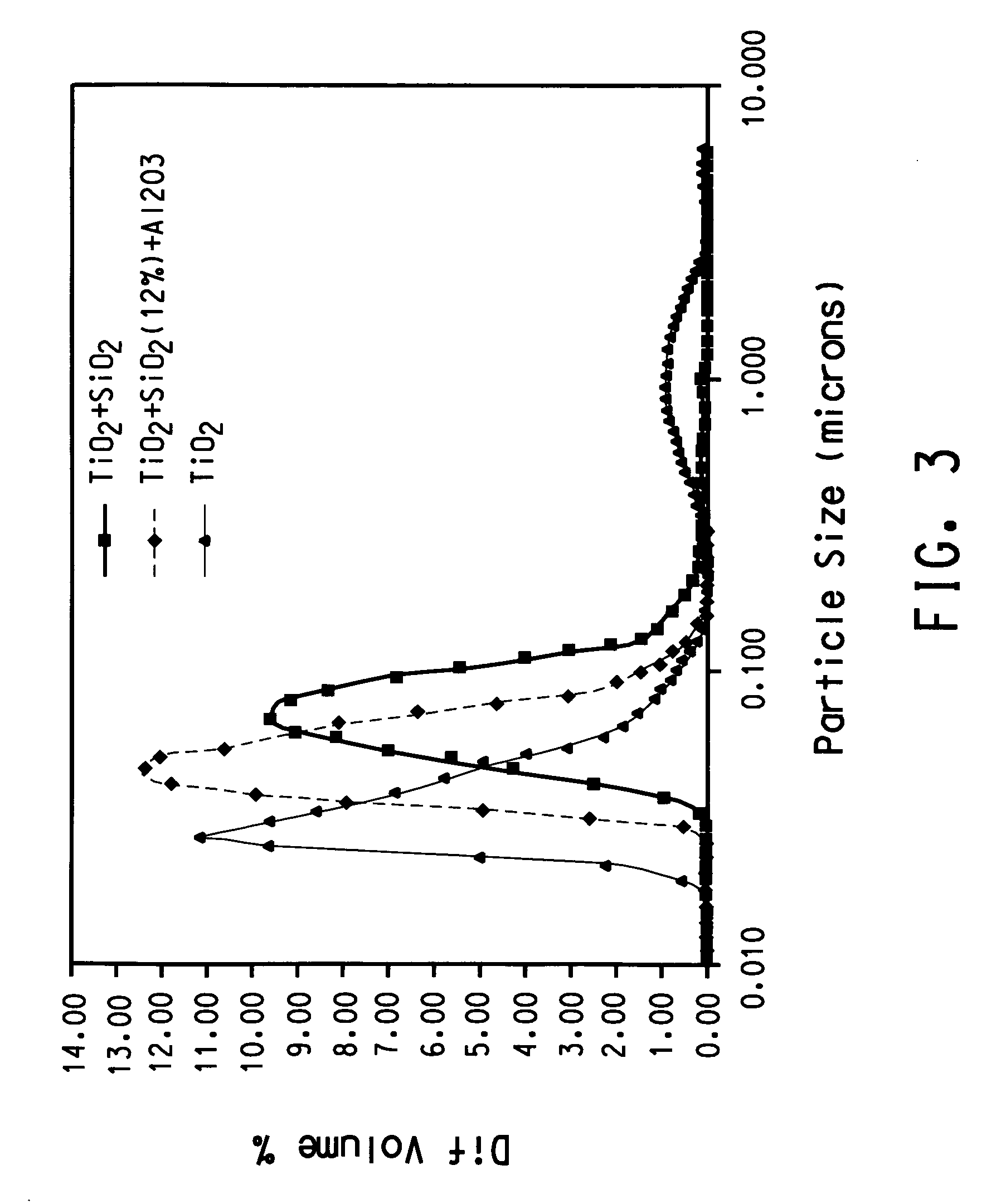
A process for minimizing and even eliminating over-sized particles in a vapor phase synthesis of metal oxide-containing particles comprising reacting oxygen with one of more vapor streams comprising a titanium halide, a silicon halide and a compound selected from the group consisting of phosphorous, germanium, boron, tin, niobium, chromium, silver, gold, palladium aluminum, and mixtures thereof in a plug flow, plasma reactor.
Owner:EI DU PONT DE NEMOURS & CO
Method of increasing retention in papermaking using colloidal borosilicates
InactiveUS6361653B2Additional componentPromote flocculationNatural cellulose pulp/paperSpecial paperSilicateChemistry
The invention comprises a borosilicate retention aid composition and a method for improving the production of paper by addition of the borosilicate. The borosilicate may be utilized in conjunction with a high molecular weight synthetic flocculent and / or starch, with or without the addition of a cationic coagulant. The borosilicate material is preferably a colloidal borosilicate. Methods for the preparation of the borosilicate material are disclosed.
Owner:ECOLAB USA INC
Method of production of B10H102-ammonium salts and methods of production of B18H22
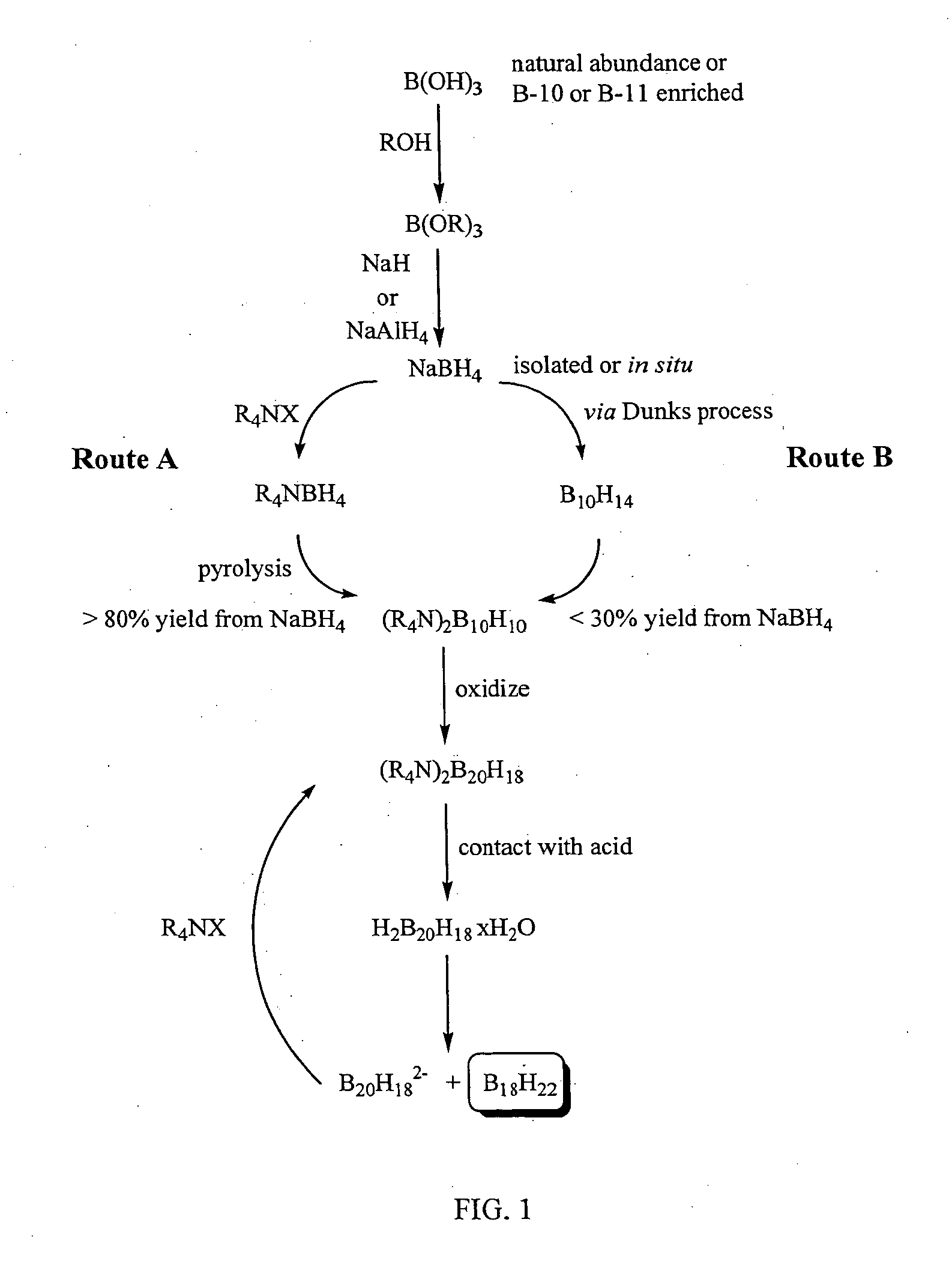
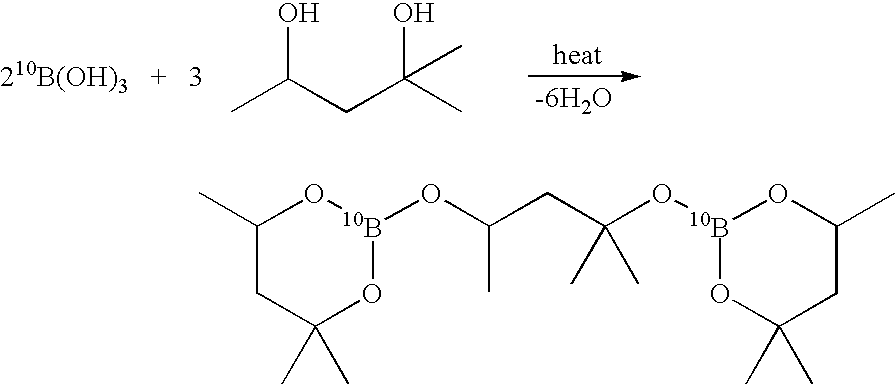
InactiveUS20050169828A1High yieldSemiconductor/solid-state device manufacturingGroup 3/13 element organic compoundsMethods of productionOctadecaborane
The invention provides new methods for synthesis of B9H9−, B10H102−, B11H14−, and B12H122− salts, particularly alkylammonium salts of B9H9−, B10H102−, B11H14−, and B12H122−. More particularly, the invention provides methods of preparing tetraalkylamronium salts of B9H9−, B10H102−, B11H14−, and B12H122− by pyrolysis of tetraalkylammonium borohydrides under controlled conditions. The invention additionally provides methods of preparing, in an atom efficient process, octadecaborane from the tetraalkylammonium salts of the invention. Preferred methods of the invention are suitable for preparation of isotopically enriched boranes, particularly isotopically enriched 10B18H22 and 11B18H22.
Owner:SEMEQUIP
Spherical boron nitride process, system and product of manufacture
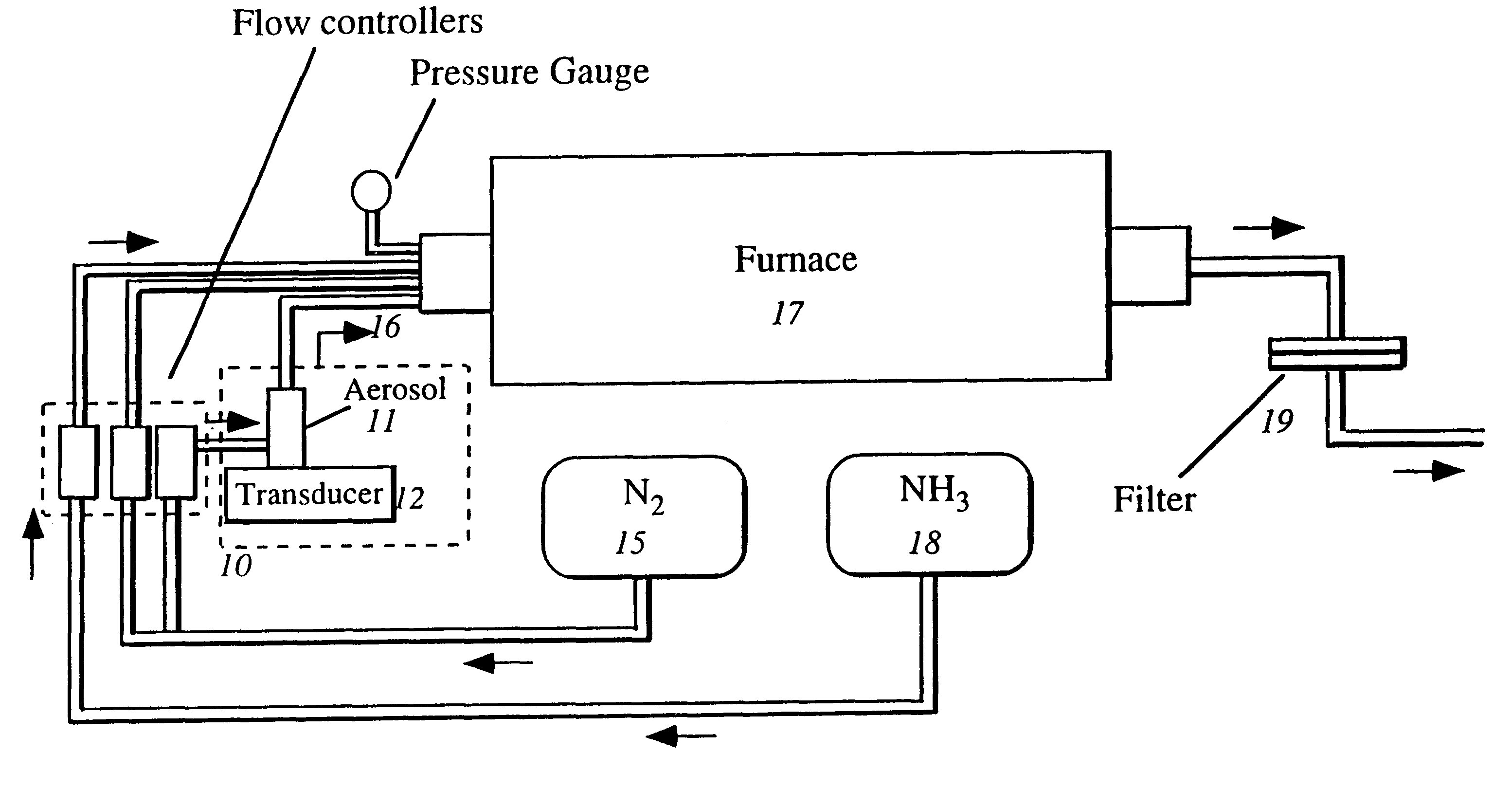
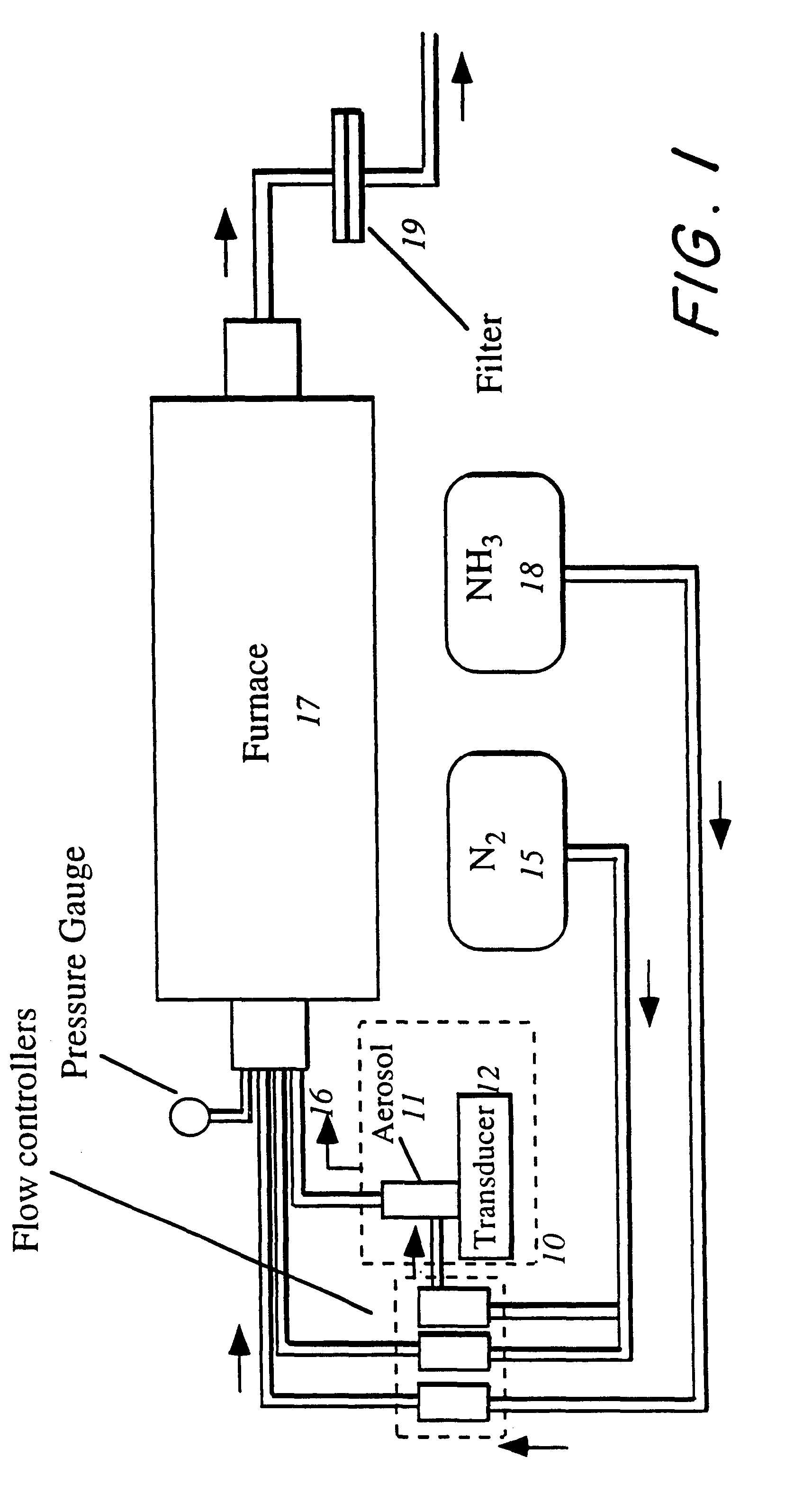
The present invention involves a process and system for producing spherical BNxOy particles that are converted to crystalline BN. The process involves adding a boron compound to an aqueous solution, creating an aerosol spray from the solution in the form of aerosol droplets using an aerosol generator. The aerosol droplets are fed with an inert carrier gas into a heated furnace at a preset flow rate while simultaneously injecting a gaseous nitriding agent into the heated furnace in a direct proportion to the flow rate of the carrier gas containing the aerosol droplets whereby a precursor of spherically shaped BNxOy particles are formed which are further heat treated into particles of spherically shaped BN having a turbostratic or hexagonal structure.
Owner:STC UNM +1
Production method of high-purity lithium hydroxide
ActiveCN106011917AHigh yieldReduce consumptionCellsBoron-oxygen compoundsLithium hydroxideUltrafiltration
The present invention relates to a production method of high-purity lithium hydroxide. A crystallization mother liquor waste water is obtained after production of potassium chloride by using a salt lake bittern as the raw material, a magnesium-lithium ratio of the crystallization mother liquor waste water is 200-500:1, an eligible eluant is obtained through ion sieve adsorption and elution of the crystallization mother liquor waste water, and after treatment of the eluant through the ultrafiltration membrane technology, the sectional type nanofiltration technology, the external regeneration continuous hybridization technology and the reverse osmosis technology, a reverse osmosis concentrated solution is obtained. In the reverse osmosis concentrated solution, the content of magnesium ions is <=300 ppm, the content of lithium ions is 4-6 g / L, the content of sodium ions is 3-5 g / L, the content of calcium ions is <=5 ppm, the content of sulfate ions is 1-30 ppm and the content of boron is <=400 ppm. According to the production method provided by the present invention, the reverse osmosis concentrated solution is used as the raw material, the process comprises ultrahigh pressure reverse osmosis continuous hybridization for boron-removing, continuous hybridization for sulfate radical removing, ion-exchange membrane electrolysis, and crystallizing evaporation, and by using the hybridization boron-removing technology, the ion-exchange membrane electrolysis technology and the crystallizing evaporation technology, the high-purity lithium hydroxide is obtained, with the byproducts being the boric acid and sodium hydroxide. The process is continuous, controllable, high in extraction rate, low in production cost and easy to industrialize.
Owner:启迪清源(上海)新材料科技有限公司
Cathode material for lithium rechargeable batteries
InactiveUS6855461B2Increase capacityImproved cyclabilityAluminium compoundsActive material electrodesManganeseOxygen
A crystal which can be employed as the active material of a lithium-based battery has an empirical formula of Lix1A2Ni1-y-zCoyBzOa, wherein “x1” is greater than about 0.1 and equal to or less than about 1.3, “x2,”“y” and “z” each is greater than about 0.0 and equal to or less than about 0.2, “a” is greater than about 1.5 and less than about 2.1, “A” is at least one element selected from the group consisting of barium, magnesium, calcium and strontium and “B” is at least one element selected from the group consisting of boron, aluminum, gallium, manganese, titanium, vanadium and zirconium. A method includes combining lithium, nickel, cobalt and at least one element “A” selected from the group consisting of barium, magnesium, calcium and strontium, has at least one element “B” selected from the group consisting of boron, aluminum, gallium, manganese, titanium, vanadium and zirconium, in the presence of oxygen, wherein the combined components have the relative ratio of Lix1:Ax2:Ni1-y-z:Coy:Bz, wherein “x1,”“x2,”“y” and “z” have the values given for the empirical formula shown above.
Owner:TIAX LLC
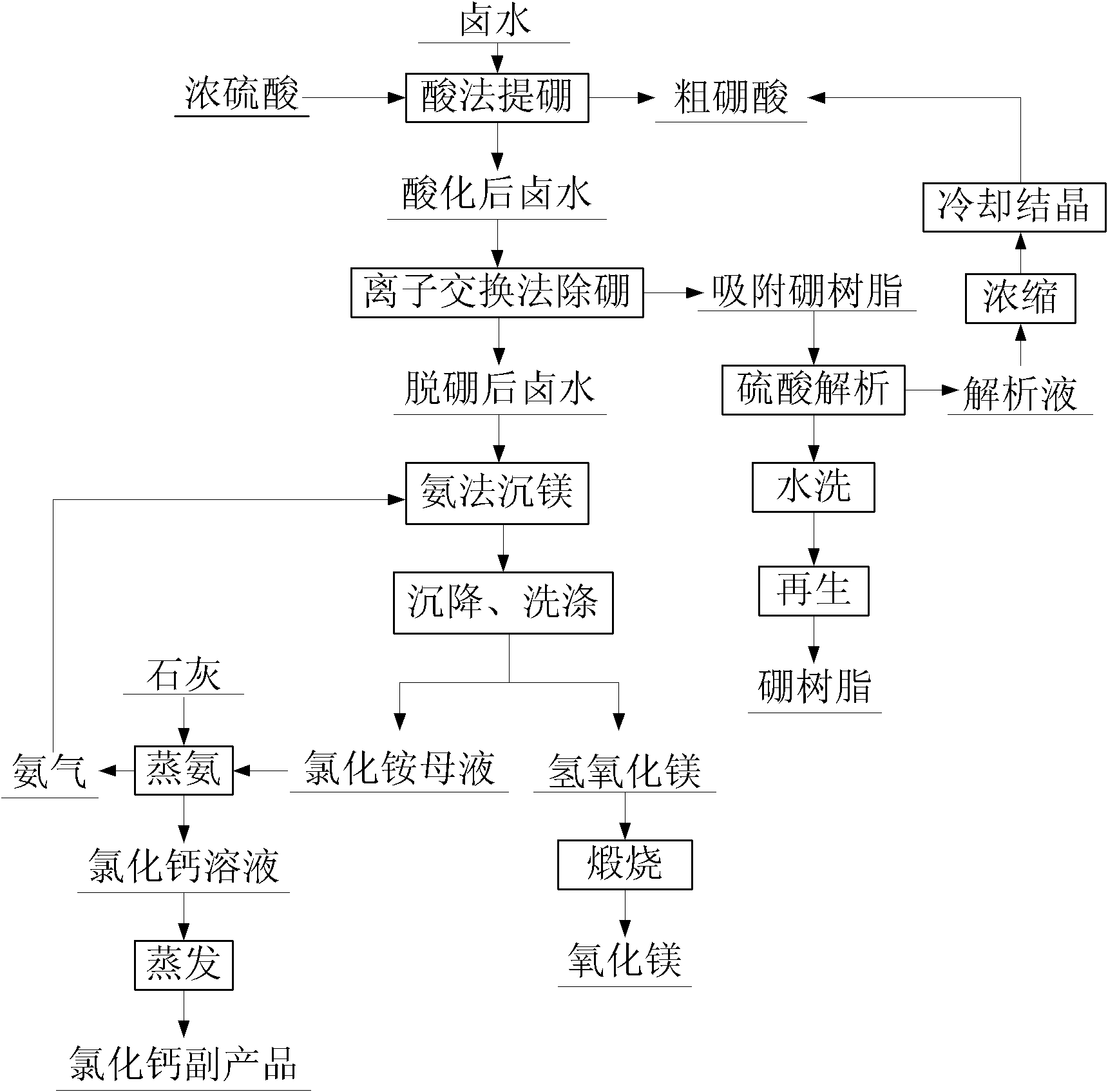
Comprehensive utilization method for bittern
InactiveCN101691239ARealize closed loopSimple process controlCalcium/strontium/barium carbonatesIodineSimple componentChemistry
The invention relates to a comprehensive utilization method for bittern, which belongs to the technical field of salt chemical engineering. The bittern is a liquid mineral product, and is rich in multiple elements such as potassium, sodium, lithium, boron, bromine, iodine and the like; and at present, in the prior domestic bittern development and utilization, some simple components or components with high additional value in the elements are extracted, and the un-extracted components are discharged along with old bittern to be abandoned so as to cause serious waste of resources and pollute the environment. Through reasonable combination of processes of removing H2S from the bittern, settling magnesium, settling calcium and preparing calcium carbonate, preparing potassium-sodium mixed saltthrough primary salt preparation and secondary salt preparation, extracting potassium chloride through flotation, extracting boron, iodine, bromine, rubidium and cesium through acidification, preparing rubidium chloride and cesium chloride, extracting lithium and the like, the method implements step-by-step ordered extraction of main components; the toil yield of several main components reaches over 95 percent; and the method has the advantages of mutually exclusive loss in component extraction, implementation of closed cycle of processes, no mother liquor discharge, simple process control, low cost, high yield, environmental protection and the like.
Owner:DAZHOU HENGCHENG ENERGY GROUP
Method for preparing high-purity lithium carbonate and other available byproducts from salt lake brine
InactiveCN101712481ATake advantage ofCalcium/strontium/barium carbonatesLithium halidesLithium chlorideLithium carbonate
The invention relates to a method for preparing high-purity lithium carbonate and other available byproducts from salt lake brine. The method comprises the following steps: (1) separating potassium and sodium by exposing salt in a salt field, (2) separating boron by an acidification method, (3) separating magnesium by a sedimentation method, (4) separating calcium by the sedimentation method, (5) preparing lithium chloride, and (6) preparing the lithium carbonate. The preparation method of the invention can purify the potassium, sodium, boron, magnesium and calcium ions respectively besides obtaining the lithium carbonate product of which the purity is over 99.5 percent so as to fully utilize the salt lake brine resource.
Owner:DALIAN LICHANG NEW MATERIAL CO LTD
Organoboron route and process for preparation of boron nitride
InactiveUS20020155052A1Simple preparation processEasy to purifyMaterial nanotechnologyPolycrystalline material growthCrystal structureWhiskers
An organoboron route and process for preparation of boron nitride utilizing aerosol assisted vapor phase synthesis (AAVS) wherein organoboron precursors are nitrided in one or two heating steps, and wherein a boron oxide nitride intermediary composition is formed after the first heating step and may be further nitrided to form resultant spheroidal boron nitride powders including spheroidal particles that are smooth, bladed, have protruding whiskers, and are of turbostratic or hexagonal crystalline structure.
Owner:NEW MEXICO UNIV OF
Method for combined extracting boron, magnesium and lithium from salt lake bittern
InactiveCN101024502AEfficient use ofIncrease profitBoron-oxygen compoundsNitrogen and non-metal compoundsResource utilizationPotassium
The invention relates to salt lake resource developing and comprehensive utilization. It uses boron, magnesium and lithium bittern as raw material, adopting combination distilling technology to make boric acid, magnesium hydroxide, lithium carbonate, and ammonium chloride. The recycling ratio of boron, magnesium and lithium reach 87%, 95%, and 92%. It has the advantages of simple technology, little device investment, high utilization coefficient of resource, high yield, good product quality, low producing cost and no pollution.
Owner:WESTERN MINING GROUP +1
Positive-electrode material for lithium secondary battery, secondary battery employing the same, and process for producing positive-electrode material for lithium secondary battery
InactiveUS20050106463A1Reduce capacityImpairs battery performanceAntimony compoundsSecondary cellsBoronComposite oxide
A subject for the invention is to provide a positive-electrode material, which has high capacity and high output and is inhibited from suffering a decrease in output with repetitions of charge and use. The invention provides a positive-electrode material for lithium secondary battery, which comprises a secondary particle of a lithium / transition metal composite oxide containing boron and / or bismuth, and wherein the atomic ratio of the sum of boron and bismuth to the sum of the metallic elements other than lithium, boron, and bismuth in a surface part of the secondary particle is from 5 times to 70 times the atomic ratio in the whole secondary particle.
Owner:MITSUBISHI CHEM CORP
Positive active material for rechargeable lithium battery and method of preparing same
InactiveUS7138209B2Improve cycle lifeImprove power densityFinal product manufactureIron compoundsLithium batteryHydroxide
Owner:SAMSUNG SDI CO LTD
Method of increasing drainage in papermaking using colloidal borosilicates
InactiveUS6361652B2Additional componentPromote flocculationNatural cellulose pulp/paperSpecial paperColloidMaterials science
The invention comprises a borosilicate retention aid composition and a method for improving the production of paper by addition of the borosilicate. The borosilicate may be utilized in conjunction with a high molecular weight synthetic flocculant and / or starch, with or without the addition of a cationic coagulant. The borosilicate material is preferably a colloidal borosilicate. Methods for the preparation of the borosilicate material are disclosed.
Owner:ECOLAB USA INC
Method for preparing high-purity magnesium oxide with high boron salt lake brine
InactiveCN102491379ALarge particlesReduce extraction costsBoron-oxygen compoundsMagnesiaReaction temperatureIon-exchange resin
Provided is a method for preparing high-purity magnesium oxide with high boron salt lake brine. Salt lake brine is evaporated through a salt pan, concentrated to crystallize potassium sulfate, sodium chloride and potassium chloride and is drawn with lithium in adsorption mode so as to obtain master sauce brine containing magnesium and boron. Concentrated sulfuric acid is added into master sauce brine for reacting, and coarse boracic acid and acidized brine are obtained after cooling and filtering. Potential of hydrogen (pH) value of acidized brine is adjusted to be 5.5-6.5, and acidized brine passes through ion exchange resin adsorbing boron. When boron concentration in effluent liquid is higher than 5 mg / L, brine is not injected, boron-removed brine is obtained, then boron-removed brine and ammonium chloride solution are filled with ammonia for stirring and to produce magnesium sedimentation reaction, reaction temperature ranges from 60 DEG C to 80 DEG C, pH ranges from 7.5 to 8.0, the reaction is stopped when concentration of free ammonia reaches 1.8-2.2 mol / L, and magnesium hydroxide and magnesium sedimentation mother solution are obtained. Magnesium oxide is obtained by calcining magnesium hydroxide, content of magnesium oxide is larger than 99.8%, and magnesium extraction ratio is larger than 90%. Sedimentation mother solution adopts lime to steam ammonia, and generated ammonia circulates to magnesium sedimentation reaction. Mother solution after ammonia steaming is evaporated, concentrated and crystallized to obtain calcium chloride. Ion exchange resin adsorbing boron is washed, analyzed and regeneratively cycled for use. Boron-containing analysis solution is concentrated and cooled to pick up coarse boracic acid, and coarse boracic acid is recrystallized to obtain refined boracic acid with purity larger than 99%. High-purity magnesium oxide prepared by the method is high in purity, good in economic benefit, free of environment pollution, strong in operability and favorable for industrial production.
Owner:CENT SOUTH UNIV
Spherical boron nitride nanoparticles and synthetic method thereof
InactiveUS20110033707A1Increase productionLow oxygenMaterial nanotechnologyNitrogen compoundsNanoparticleBoron nitride
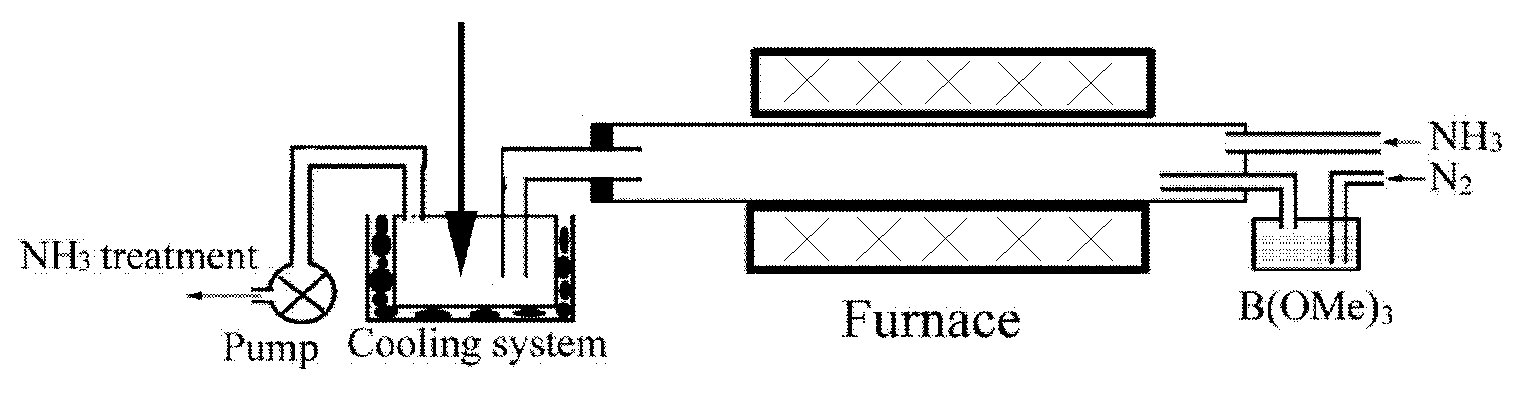
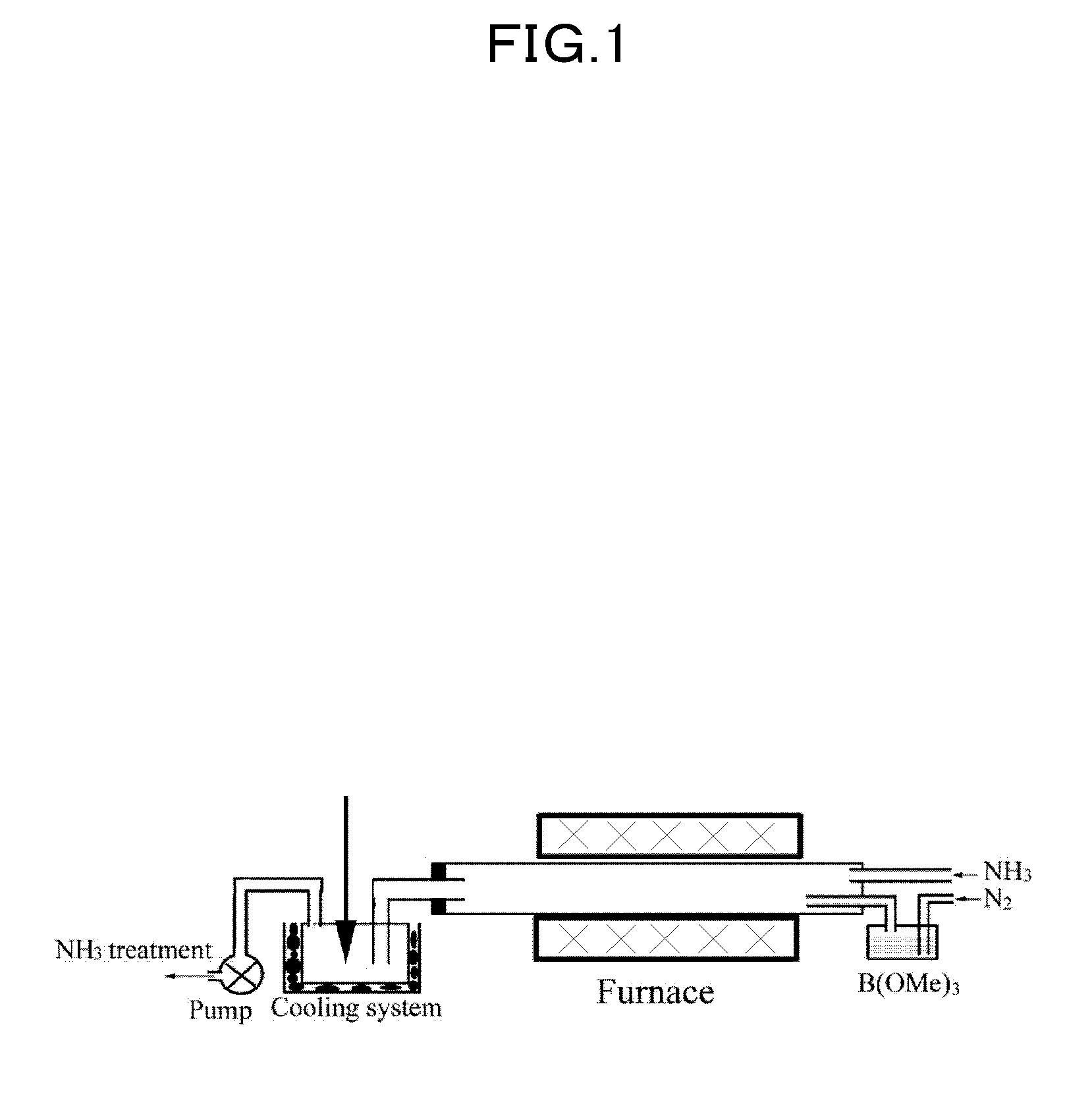
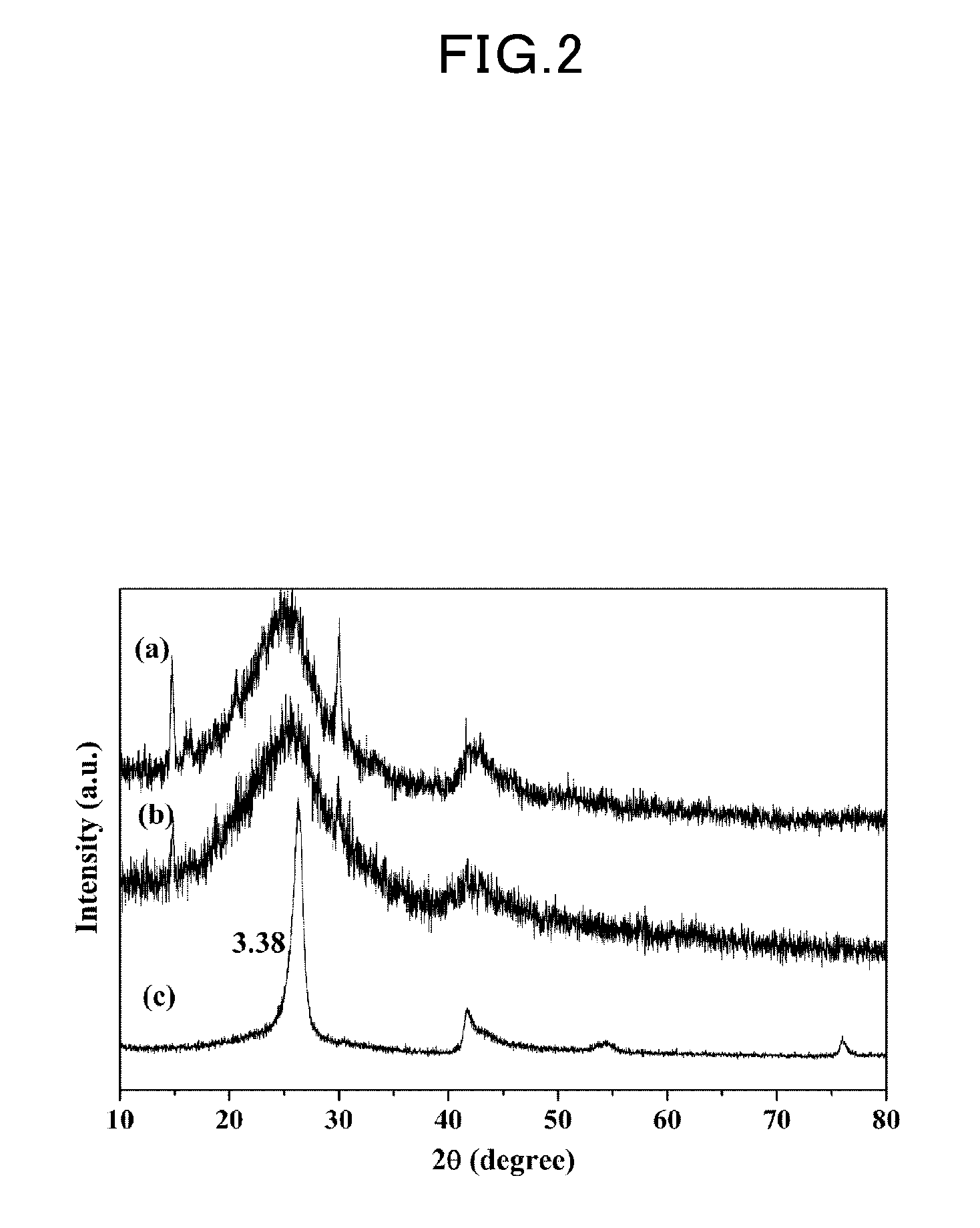
Spherical boron nitride nanoparticles having an average particle diameter is less than 50 nm is obtained by a method of synthesizing spherical boron nitride nanoparticles including the following steps; heating a mixture of boric acid ester and nitrogen gas in ammonia gas and argon gas to form reaction product; crystallizing reaction product to form precursor of spherical boron nitride nanoparticles; and, heating the precursor in inert gas.
Owner:NAT INST FOR MATERIALS SCI
Extraction system for extracting boric acid from magnesium-containing brine and extraction method thereof
InactiveCN108017067AReduce solubilityHigh extractionLiquid solutions solvent extractionBoron-oxygen compoundsSolubilityAlcohol
The invention discloses an extraction system for extracting boric acid from magnesium-containing brine and an extraction method thereof. The system comprises an extractant and a diluent which are mixed with one another, wherein the structure of the extractant is as shown in the formula 1, in the formula 1, R1 is the alkyl group selected from C4-C8, and R2 is the alkyl group selected from C6-C10. According to the system, solubility of the extraction system in water is very low, the boric acid can be effectively extracted from the magnesium-containing brine, and extraction saturated capacity ofthe extraction system is high, emulsification does not easily occur, and the extraction system is more suitable for industrial production. Compared with a conventional alcohol extractant in the priorart, not only is the conflict between extraction performance of a monohydric alcohol extractant and the problem of solution loss caused by solubility solved, but also the problem of polyhydric alcoholextractant loss in an aqueous phase is solved. The invention further provides an extraction method for extracting the boric acid from the magnesium-containing brine based on the extraction system.
Owner:QINGHAI INST OF SALT LAKES OF CHINESE ACAD OF SCI
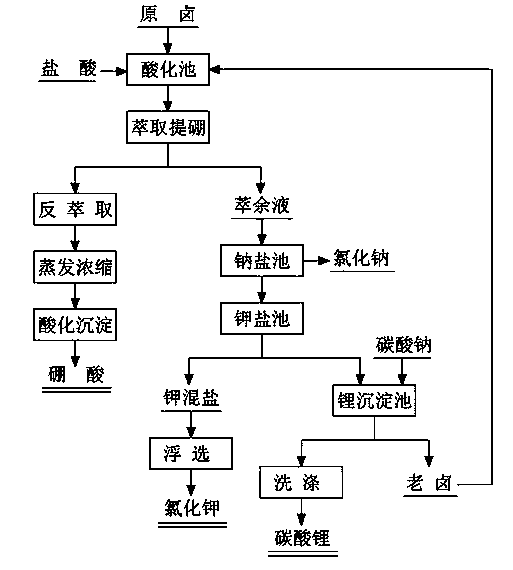
Method for comprehensively utilizing potassium, boron and lithium in carbonate type salt lake brine
ActiveCN103508462AGood for concentration and enrichmentSolve the difficulty of comprehensive utilization of lithiumBoron-oxygen compoundsAlkali metal chloridesChloride potassiumLithium carbonate
The invention provides a method for comprehensively utilizing potassium, boron and lithium in carbonate type salt lake brine. The brine type is converted from the carbonate type into the chloride type through introducing the acidification technology, so that the brine composition is simplified, and further the technical problem of combined extraction of potassium, boron and lithium from the carbonate type salt lake brine is solved. According to the method, the acidification technology is adopted to regulate the pH value of the brine, and the solvent extraction method is adopted to extract boric acid from the acidified brine; raffinate enters a sodium salt pond, then is subjected to solarization and evaporation to separate out sodium salt, and gets into a potassium salt pond to separate out potassium mixed salt; the potassium mixed salt is purified to prepare potassium chloride by adopting the flotation method; the precipitation method is adopted to extract lithium carbonate from the potassium separated mother liquor with enrichment of lithium; old brine subjected to lithium extraction is returned to the acidification pond for recycling.
Owner:ZHENGZHOU MINERALS COMPOSITIVE UTILIZATION RES INST CHINESE GEOLOGICAL ACAD
Sorbents for removing mercury from emissions produced during fuel combusion
InactiveUS20130157845A1Good predictor of thermal stabilitySuitable thermal stabilityUrea derivatives preparationNitrogen compoundsActivated carbonHalogen
Activated carbon is rendered more thermally stable by exposure to a non-halogenated additive, and optionally to a halogen and / or a halogen-containing compound. Such treated carbon is suitable for use in mitigating the content of hazardous substances in flue gases, especially flue gases having a temperature within the range of from about 100° C. to about 420° C.
Owner:ALBEMARLE AMENDMENTS LLC
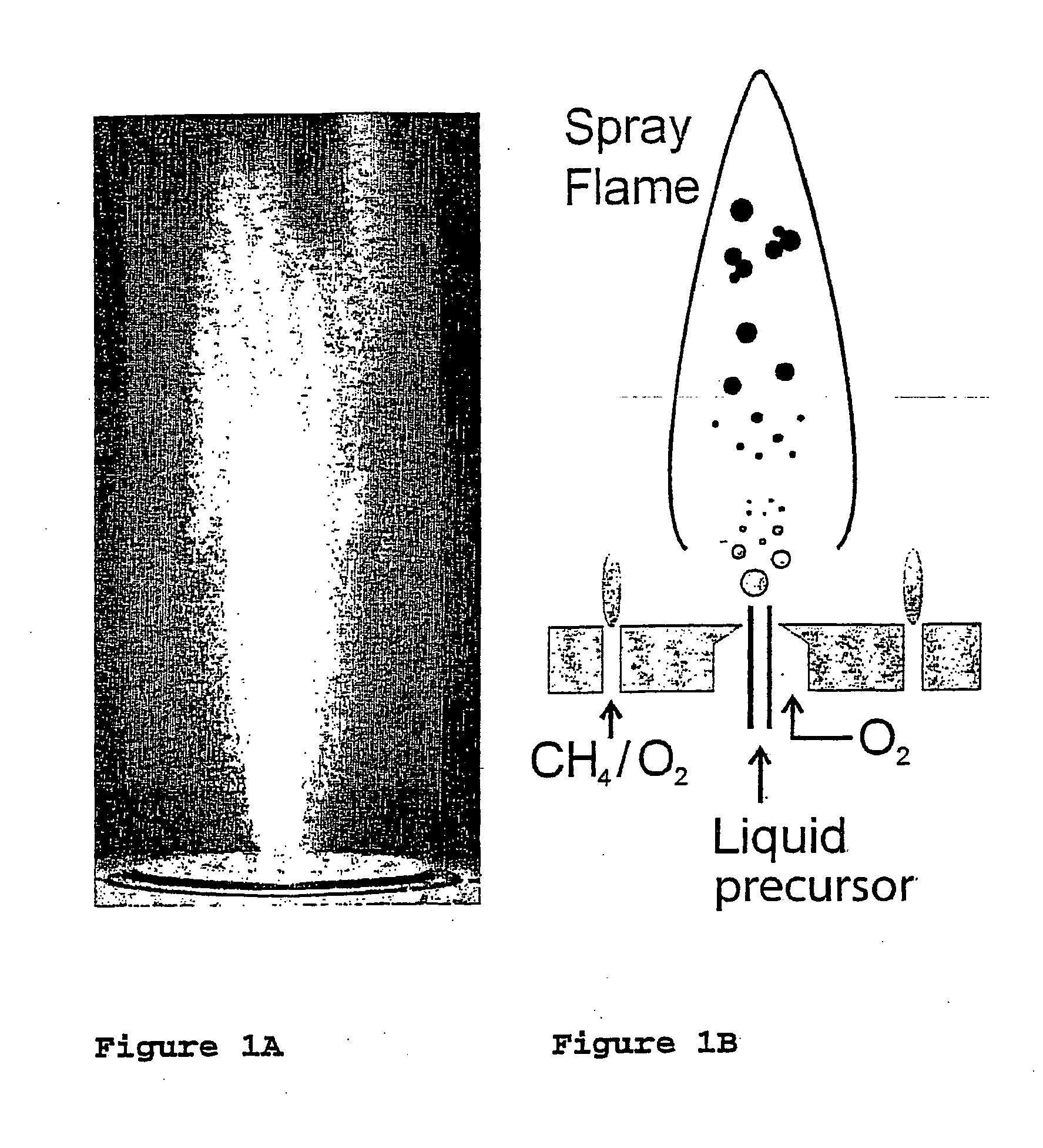
Flame synthesis of metal salt nanoparticles, in particular calcium and phosphate comprising nanoparticles
InactiveUS20070196259A1Increase chanceCalcium/strontium/barium carbonatesMaterial nanotechnologyBiocompatibility TestingMetallacarboxylic acid
Described is a method for the production of metal salts, wherein the cationic metal is preferably selected from Group I to IV metals and mixtures thereof and the anionic group is selected from phosphates, silicates, sulfates, carbonates, hydroxides, fluorides and mixtures thereof, and wherein said method comprises forming a mixture of at least one metal source that is a metal carboxylate with a mean carbon value per carboxylate group of at least 3 and at least one anion source into droplets and oxiding said droplets in a high temperature environment, preferably a flame. This method is especially suited for the production of calcium phosphate biomaterials such as hydroxyapatite (HAp,Cal0(P04)6(OH)2) and tricalcium phosphate (TCP,Ca3(P04)2) that exhibit excellent biocompatibility and osteoconductivity and therefore are widely used for reparation of bony or periodontal defects, coating of metallic implants and bone space fillers.
Owner:EIDENGOSSICHE TECHN HOCHSCHULE ZURICH
Cathode material for a lithium secondary battery and method for manufacturing same
InactiveUS6497854B2Large capacityImprove cycle performanceConductive materialCobalt compoundsElectrical batteryThermal stability
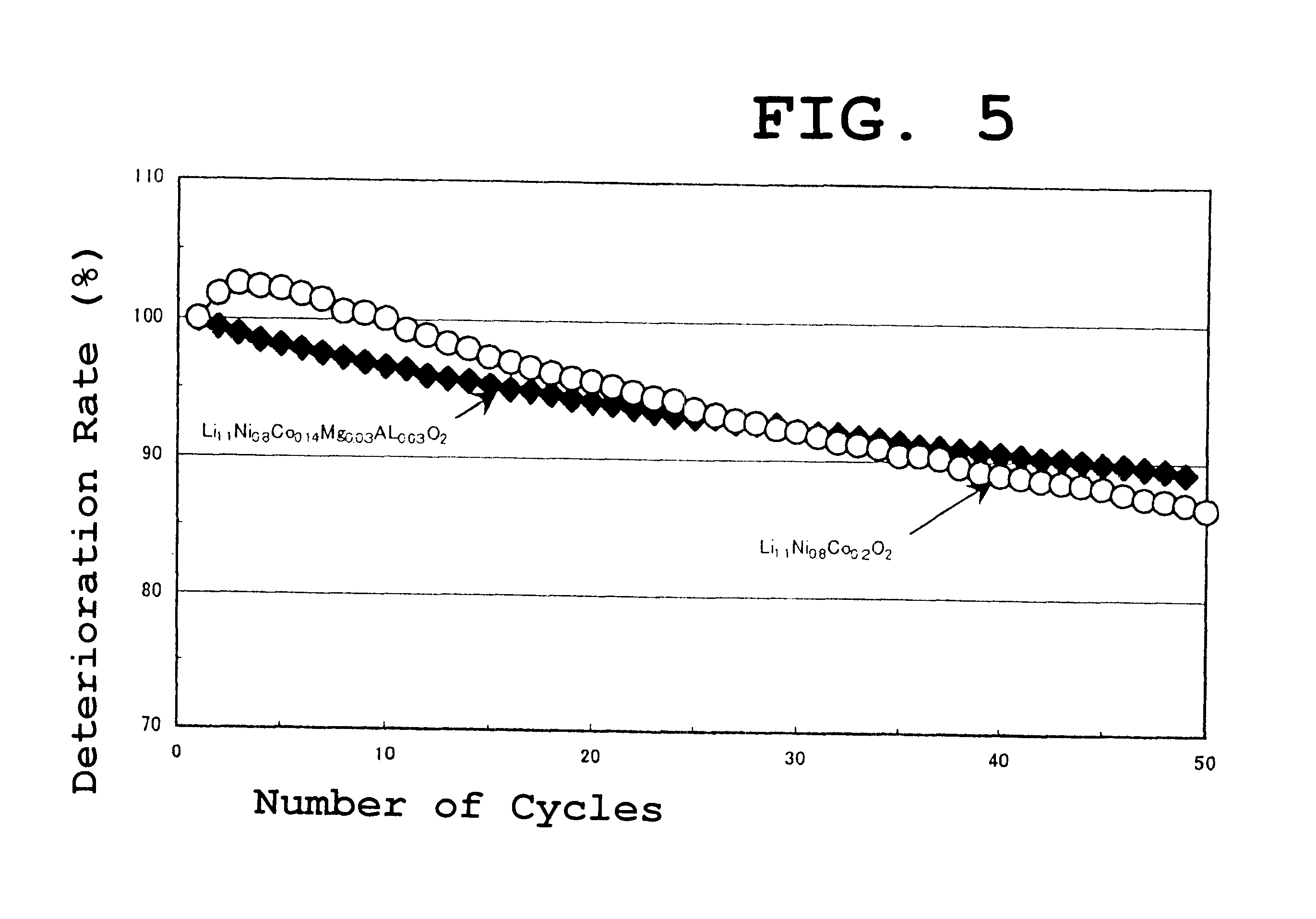
A cathode material for a lithium secondary battery having a high capacity, an excellent cycle property, and an excellent thermal stability. The cathode material for the lithium secondary battery is a layered compound having a general formula: LixNi1-a-b-c-dCOaM1bM2cM3dO2, wherein M1, M2, M3 are selected from Ti, Mg, B and Al and wherein the characters x, a, b, c and d respectively satisfy 1.0<=x<=1.2; 0.3<=a<=0.3; 0.005<=b<=0.1; 0.005<=c<=0.1; 0.005<=d<=0.1; and 0.115<=a+b+c+d<=0.4
Owner:JX NIPPON MINING & METALS CORP
Method for exfoliation of hexagonal boron nitride
ActiveUS8303922B2Simple processEasy to processNitrogen compoundsLayered productsBulk crystalOrganic solvent
A new method is disclosed for the exfoliation of hexagonal boron nitride into mono- and few-layered nanosheets (or nanoplatelets, nanomesh, nanoribbons). The method does not necessarily require high temperature or vacuum, but uses commercially available h-BN powders (or those derived from these materials, bulk crystals) and only requires wet chemical processing. The method is facile, cost efficient, and scalable. The resultant exfoliated h-BN is dispersible in an organic solvent or water thus amenable for solution processing for unique microelectronic or composite applications.
Owner:NASA
Method of controlled alcoholysis and regeneration of a borohydride
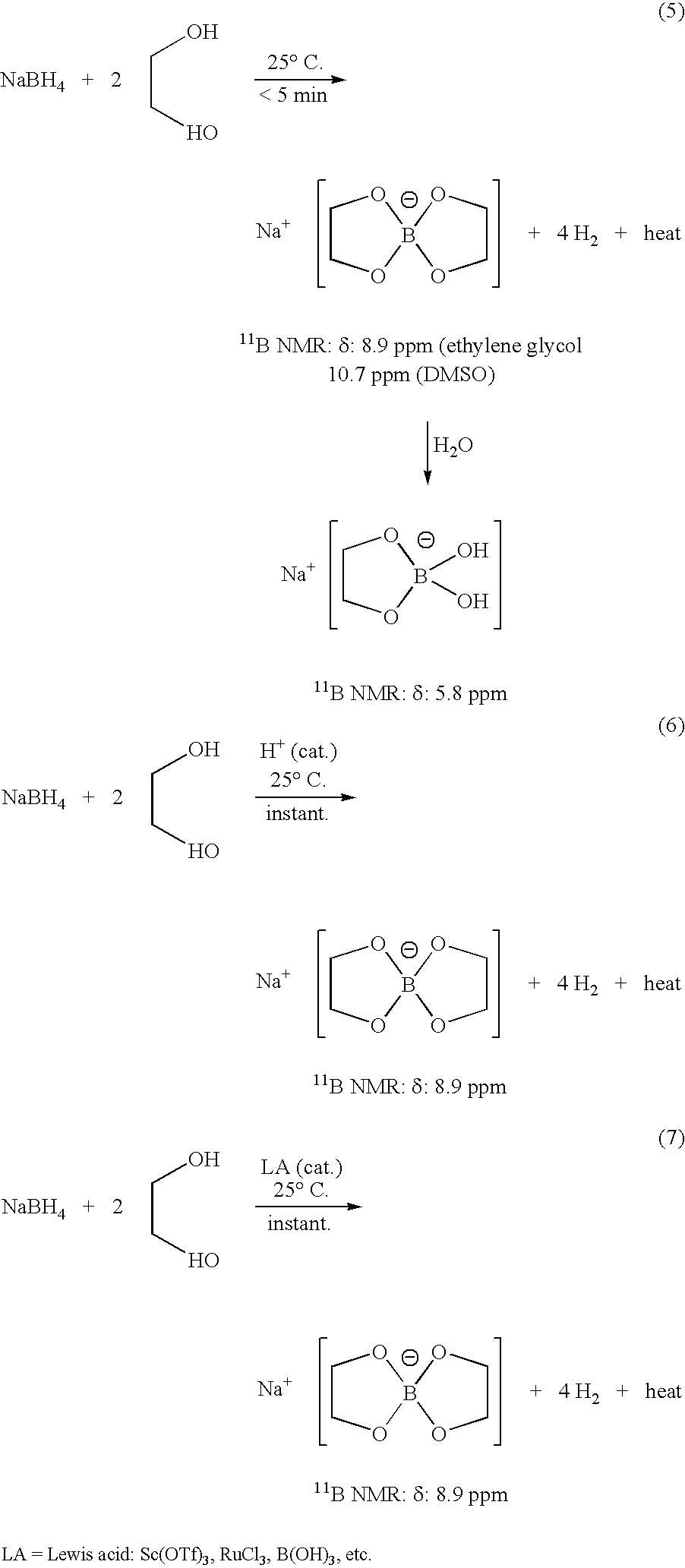
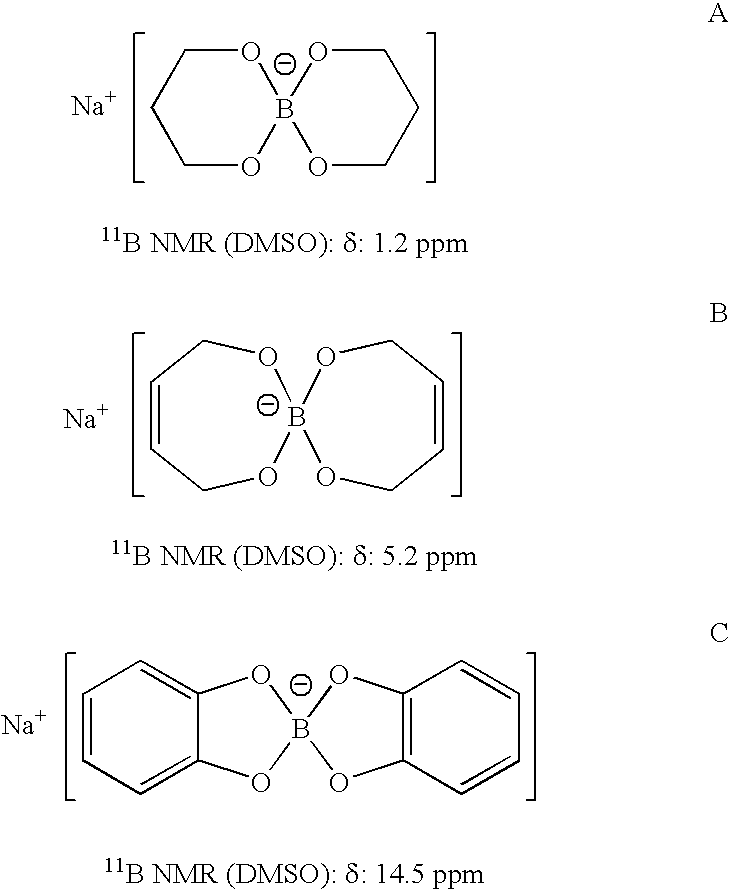
InactiveUS20050255024A1Efficient procedureEffective protocolMonoborane/diborane hydridesBoron/boridesSodium borohydrideBoric acid
Methods of controlled hydrolysis / alcoholysis and regeneration of a borohydride are disclosed. Examples of the present invention show that hydrolysis of sodium borohydride or lithium borohydride with dilute acid provides simultaneous generation of H2 and boric acid for recycling. Other examples of the present invention show methods for regenerating a borohydride by reacting an aluminum hydride to a borate compound to provide a regenerated borohydride.
Owner:PURDUE RES FOUND INC
Method for removing metallic impurities in boric acid by complex crystallizing method
InactiveCN101575100ANo pollution in the processSimple processBoron-oxygen compoundsChemical productsSolvent
The invention discloses a method for removing metallic impurities in boric acid by complex crystallizing method, belonging to the technical field of producing refine chemical products. The method comprises the following steps: taking industrial boric acid as raw material, mixing with solvent, dissolving at a certain temperature, and adding auxiliary agent and complexing agent, after agitating and reacting for certain time, hot filtering, cooling and re-crystallizing, centrifugally separating, washing, drying to obtain the crystal product, which is highly pure boric acid, wherein purity of the product is 99.99%. The invention has evident advantages on the aspect of raw material cost, product performance, technical flow, friendly environment and economical benefit, is suitable for industrial production, and can satisfy the special requirements on boric acid in high technology fields such as optical material, special glass, communication optical fiber and crystal material.
Owner:DALIAN UNIV OF TECH
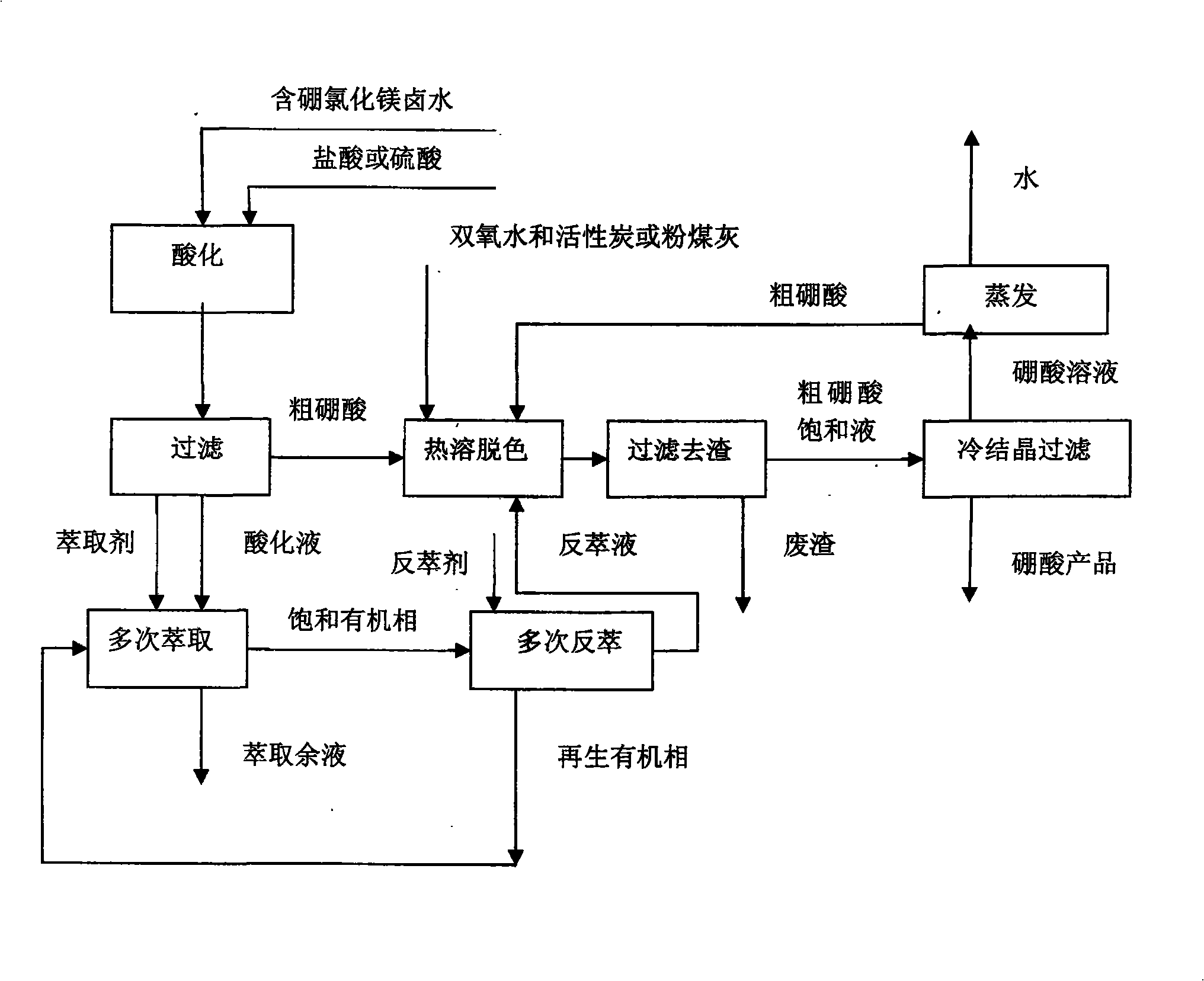
Method for extracting boric acid from boron-containing magnesium chloride solution by using an acidification-extraction method
The invention belongs to the field of inorganic chemical technology, and particularly relates to a method for extracting boric acid from boron-containing magnesium chloride solution by using an acidification-extraction method. The method is characterized in that: (a) the process flows comprise acidification, filtering, and multiple extraction, and also have four processes, namely thermosol colorization, slag removal by filtration, cold crystallization filtration, and evaporation, and then a boric acid product is obtained after the crystallization filtration; and (b) the process conditions comprises that hydrochloric acid or sulfuric acid is added into boron-containing magnesium chloride brine in acidification, thereby leading the pH-value of brine to be 0-3; the acidification temperature is room temperature; the acidification time is 10-60 minutes; the mass percent of crude boric acid: industrial hydrogen peroxide: active carbon or fly ash in thermosol colorization is 100:0.01-5:0.5-5; and the heating temperature in thermosol colorization is 80-95 DEG C. The method is fully applicable to the industrial production need of the existing salt lake brine boron extract, greatly lowers the production cost and equipment investment, and lays down solid technology basis for the large-scale industrial salt lake brine extract.
Owner:青海中信国安科技发展有限公司
Popular searches
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com