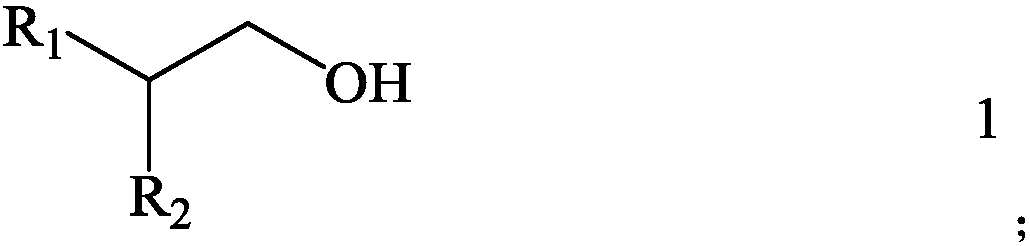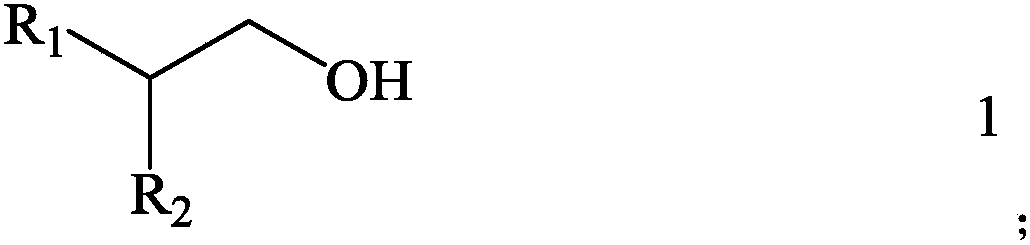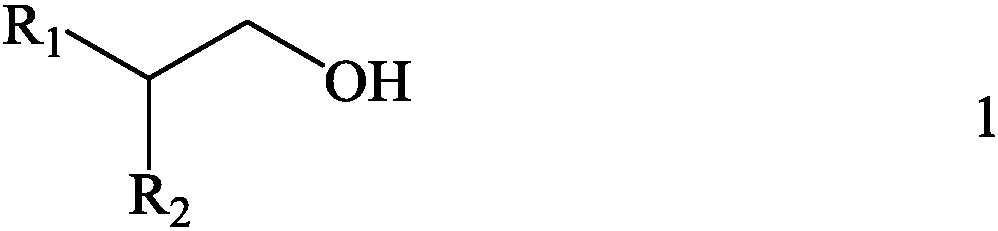Extraction system for extracting boric acid from magnesium-containing brine and extraction method thereof
An extraction and system technology, applied in the field of boric acid preparation, can solve the problems of high solvent loss, high cost, and economic inapplicability of the extractant, and achieve the effect of overcoming the problem of dissolution loss, solving the problem of loss, and optimizing the extraction effect.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0053] First of all, the old magnesium salt lakes after the potassium of salt lake brine is used as the extraction water phase, and the pH is 1.5, which contains 14.99g / L boric acid.
[0054] The main composition of the old magnesium salt lake is shown in Table 1.
[0055] Table 1 The main component of the old braised magnesium salt lake
[0056]
[0057] Then, with sulfide kerosene as a diluent, 2-butadyl-1-positive glycol (as shown in the following type 1-1) as the extracting agent, the extraction system composed of extracting agent and diluent The concentration of the extraction agent in the organic phase is 1.5mol / L, that is, the volume percentage of the extract agent in the corresponding extraction organic phase is 33.4 %.
[0058]
[0059] Again, the control extraction is 1, and the first -level extraction is performed at a room temperature at 25 ° C to obtain the organic phase and the extraction solution; Essence
[0060] Finally, the water is used as an anti -extract, ...
Embodiment 2
[0062] First of all, the old magnesium salt lake old halogen after the potassium of salt lake brine is used as the extraction water phase. The pH is 1.5, which contains 14.99g / L boric acid; the composition of the old salt lake old halogen is the same as the embodiment 1.
[0063] Then, with 1,2-dichloroethane as a diluent, the boron extracting agent with 2-hexyl-1-decirol (as shown in the following type 1-2), the extract system composed of extract agent and diluent is used as the Extracting organic phase, the concentration of extracting agent in organic phase is 1.5mol / L, that is, the volume percentage of the volume of the extract agent in the corresponding extraction organic phase is 43.3 %.
[0064]
[0065] Again, the control extraction is 1, and the first -level extraction is performed at a room temperature at 25 ° C to obtain the organic phase and the extraction solution; Essence
[0066] Finally, the water is used as an anti -extract, and the anti -extract is compared to 1...
Embodiment 3
[0068] First of all, the old magnesium salt lake old halogen after the potassium of salt lake brine is used as the extraction water phase. The pH is 1.5, which contains 14.99g / L boric acid; the composition of the old salt lake old halogen is the same as the embodiment 1.
[0069] Then, with kerosene as a diluent, 2-orthopedic -1-dulate (as shown in the following type 1-3) as the extracting agent, the extracting system composed of extracting agent and diluent is used as the extraction organic phase The concentration of the extraction agent in the organic phase is 1.5mol / L, that is, the volume percentage of the volume of the extraction agent in the organic phase of the organic phase is 53.2 %.
[0070]
[0071] Again, the control extraction is 1, and the first -level extraction is performed at a room temperature at 25 ° C to obtain the organic phase and the extraction solution; Essence
[0072] Finally, the water is used as an anti -extract, and the anti -extract is compared to 1....
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com