Method for preparing high-purity magnesium oxide with high boron salt lake brine
A salt lake brine and magnesium oxide technology, which is applied in the field of comprehensive utilization of salt lake resources, can solve the problems of low purity and poor product performance, and achieve the effects of good filtration performance, low cost and good economic benefits
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment
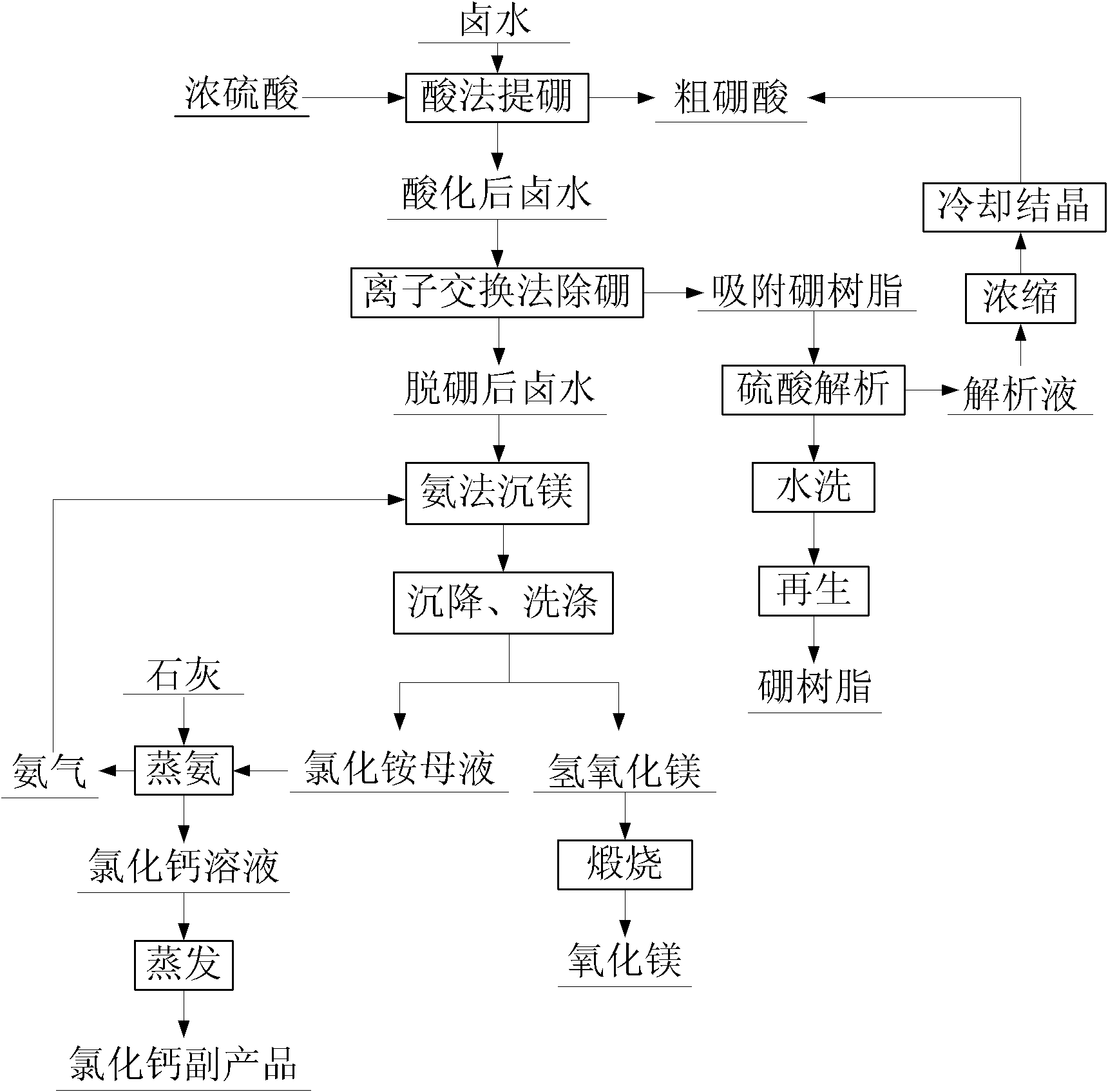
[0034] The salt lake brine is evaporated in the salt field, concentrated and crystallized to produce potassium sulfate, sodium chloride, and potassium chloride. After absorbing and extracting lithium, the old brine containing magnesium and boron is obtained. The composition is shown in Table 1.
[0035] 1) Sulfuric acid extraction of boric acid
[0036] Add 10L of brine into the reaction tank, slowly add 140mL of concentrated sulfuric acid at a rate of 10mL / min under stirring, react for 1 hour after adding sulfuric acid, add 800mL of deionized water, put the brine in a freezing tank to cool for 4 hours, control The cooling temperature was -5°C, and then filtered to obtain crude boric acid and 10.4L brine.
[0037] 2) Deep extraction of boron by resin exchange method
[0038] 1.3 L of XSC-700 boron resin was filled in the exchange column with a diameter of 46 mm and a length of 100 mm, and was rinsed with deionized water. 1) The brine obtained after boron extraction was adjus...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com