Patents
Literature
60results about "Calcium/strontium/barium halides" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
Method for producing nitride semiconductor, crystal growth rate increasing agent, single crystal nitride, wafer and device
InactiveUS20100104495A1Improve performanceIncrease probabilityPolycrystalline material growthFrom normal temperature solutionsNitrogenCrystal structure
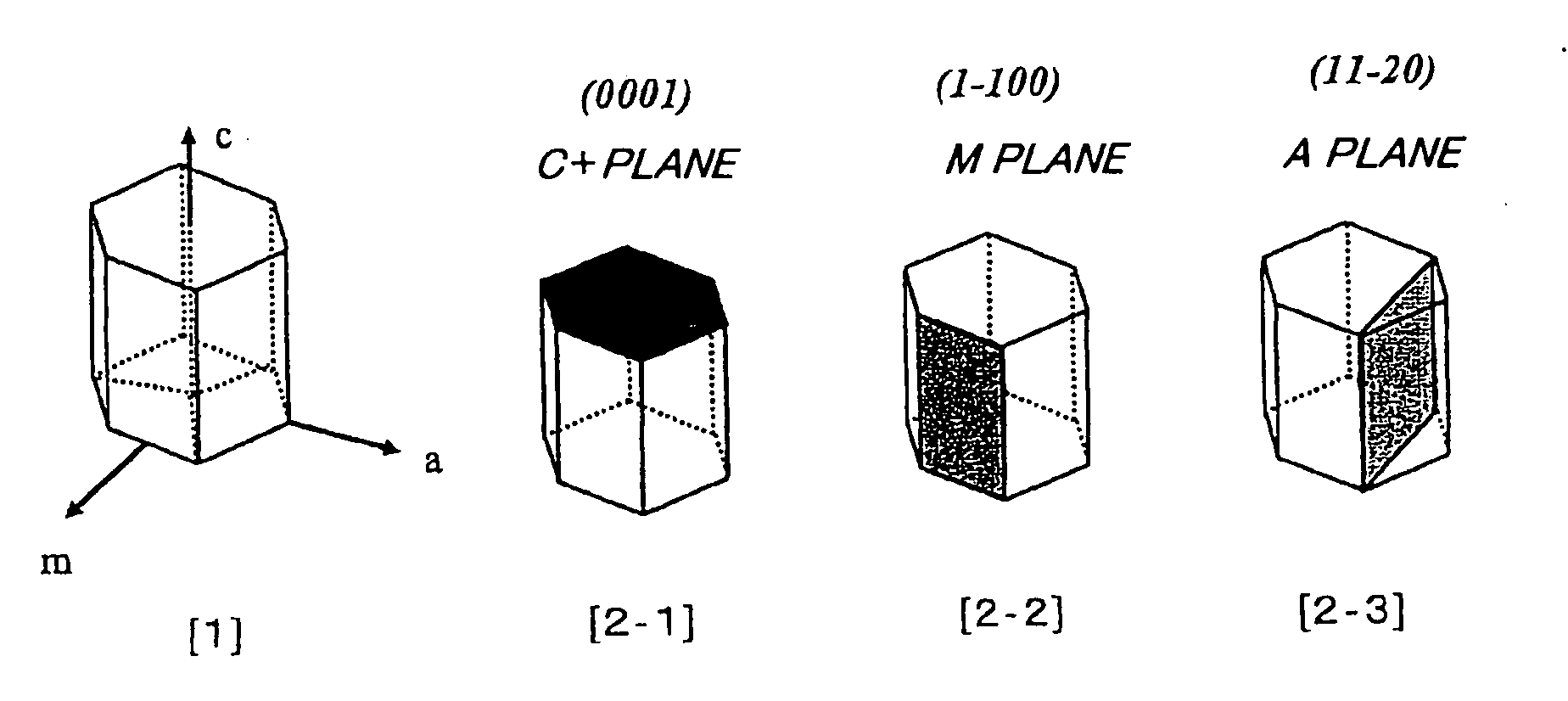
A method for producing a nitride semiconductor, comprising controlling temperature and pressure in a autoclave containing a seed having a hexagonal crystal structure, a nitrogen element-containing solvent, a raw material substance containing a metal element of Group 13 of the Periodic Table, and a mineralizer so as to put said solvent into a supercritical state and / or a subcritical state and thereby ammonothermally grow a nitride semiconductor crystal on the surface of said seed, wherein the crystal growth rate in the m-axis direction on said seed is 1.5 times or more the crystal growth rate in the c-axis direction on said seed. By the method, a nitride semiconductor having a large-diameter C plane or a nitride semiconductor thick in the m-axis direction can be efficiently and simply produced.
Owner:MITSUBISHI CHEM CORP +1
Processes for preparing highly pure lithium carbonate and other highly pure lithium containing compounds
ActiveUS20110200508A1Electrolysis componentsLithium organic compoundsLithium carbonateLithium hydroxide
Owner:TERRALITHIUM LLC
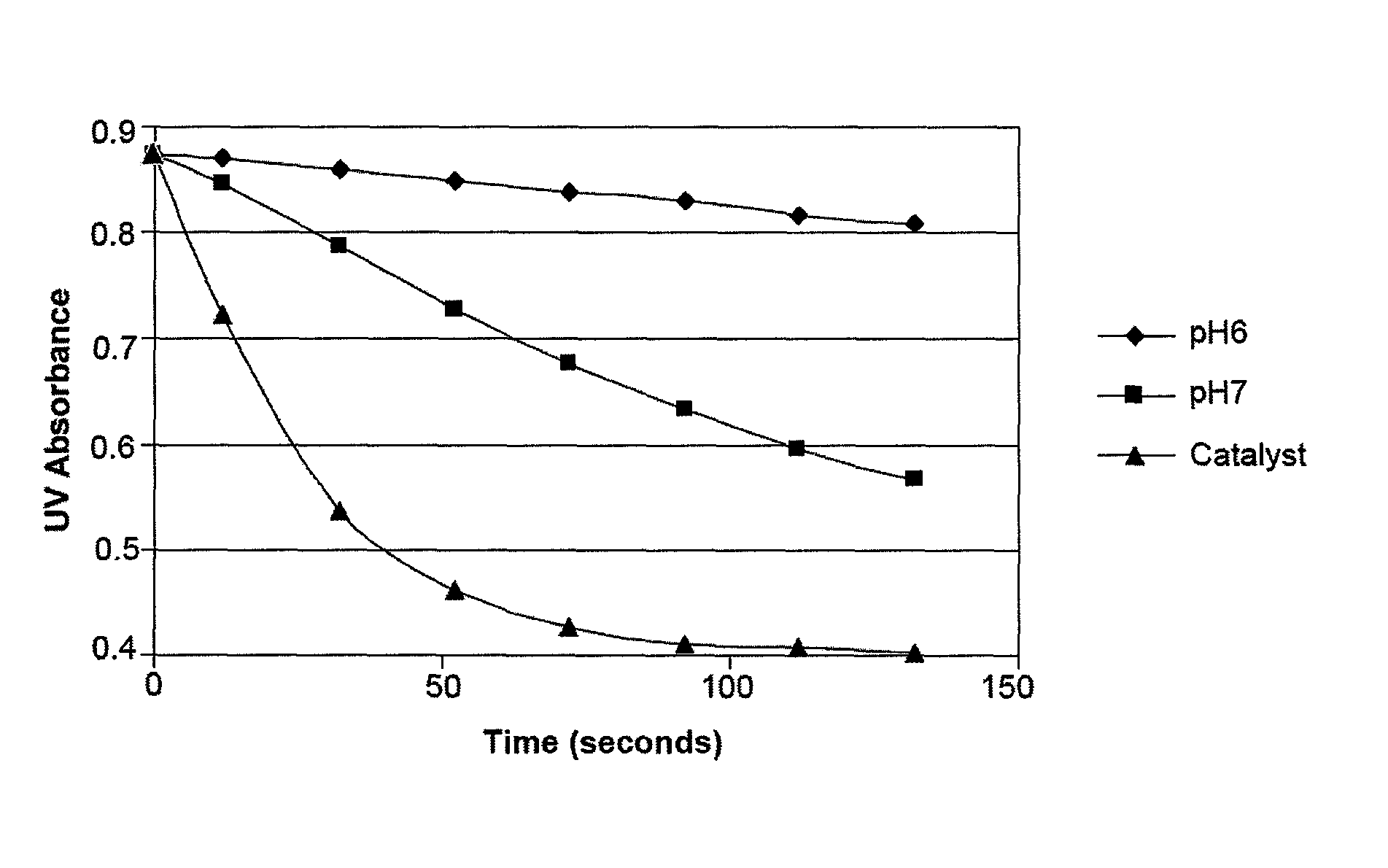
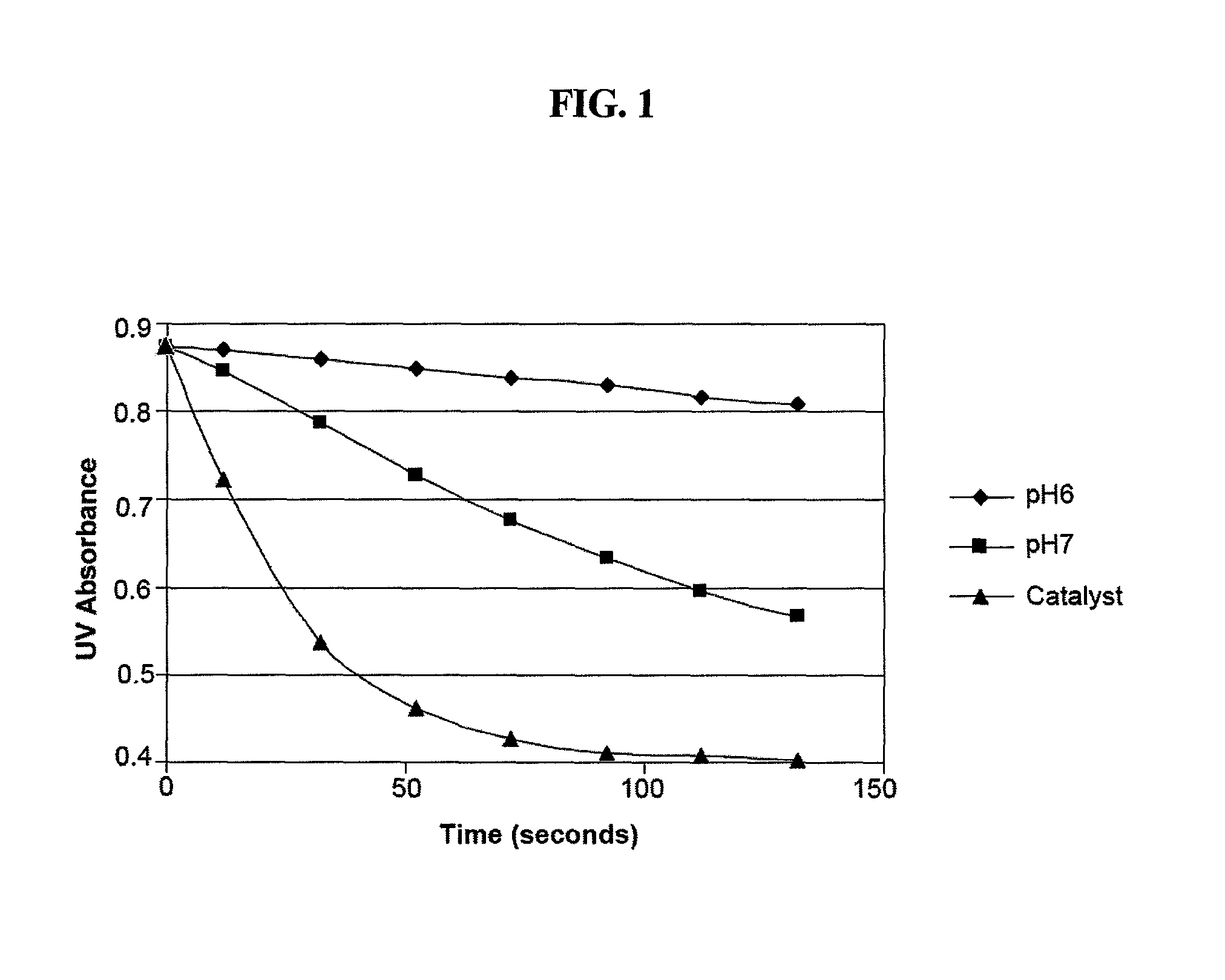
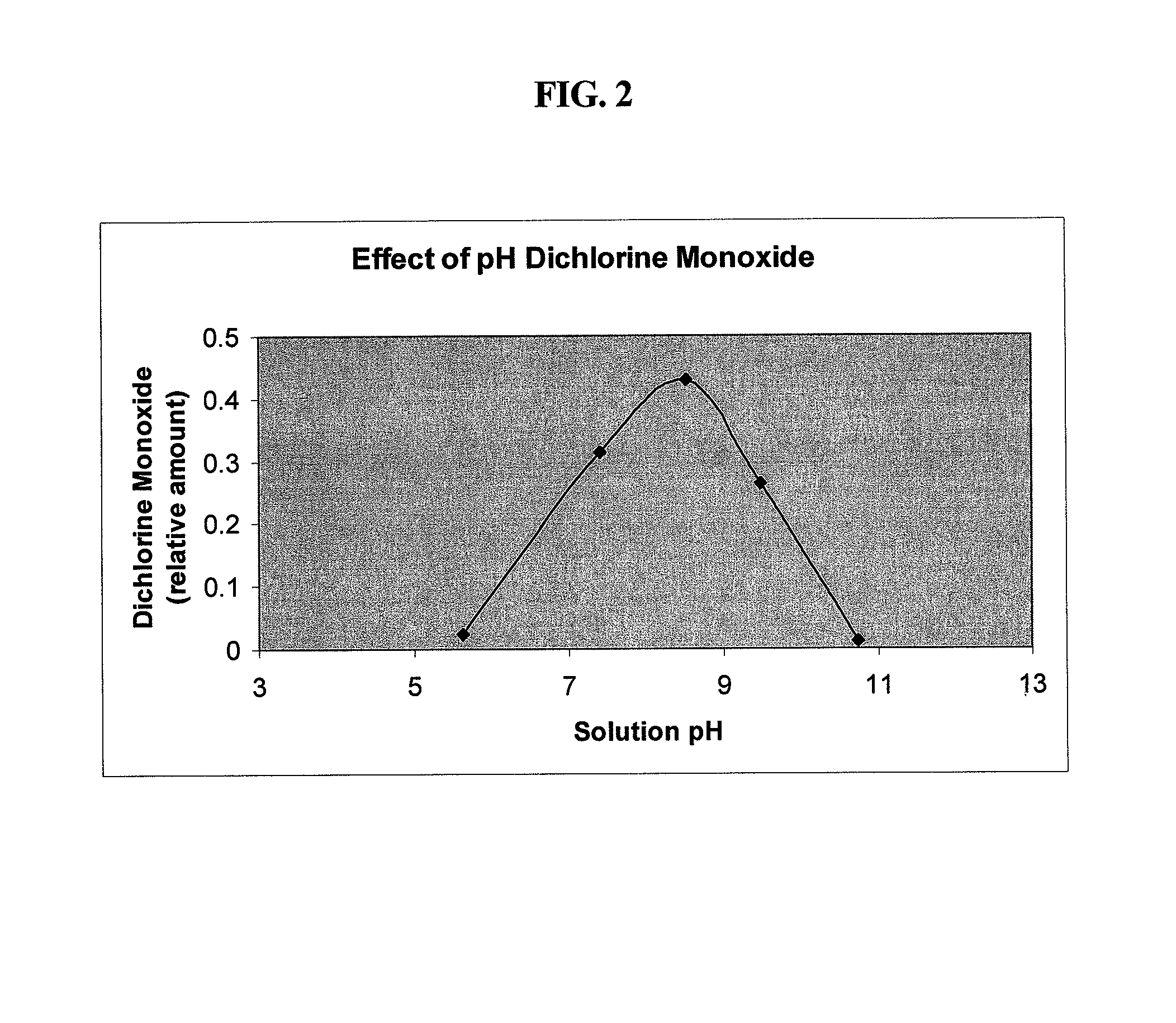
Antimicrobial solutions containing dichlorine monoxide and methods of making and using the same
Methods and products are provided for treating a wound or infection in a mammal or disinfecting a surface with a hypochlorous acid solution that has been activated by a catalyst. Additionally provided is a process for preparing an antimicrobial product that produces an activated hypochlorous acid solution for use as an antimicrobial.
Owner:MICROSAFE GRP DMCC
Viscoelastic surfactant based cleaning compositions
ActiveUS20140148371A1Improve performanceAdditional componentInorganic/elemental detergent compounding agentsOrganic detergent compounding agentsViscoelasticitySURFACTANT BLEND
Alkaline or neutral viscoelastic cleaning compositions are disclosed which use non polymer thickening agents. According to the invention, cleaning compositions have been developed using viscoelastic surfactants in a neutral, acidic or alkaline cleaning formulations. These provide the dual benefit of thickening as well as an additional cleaning, thereby improving performance. Applicants have also identified several pseudo linking agents which when, used with viscoelastic surfactants provide viscoelasticity in alkaline cleaning compositions.
Owner:ECOLAB USA INC
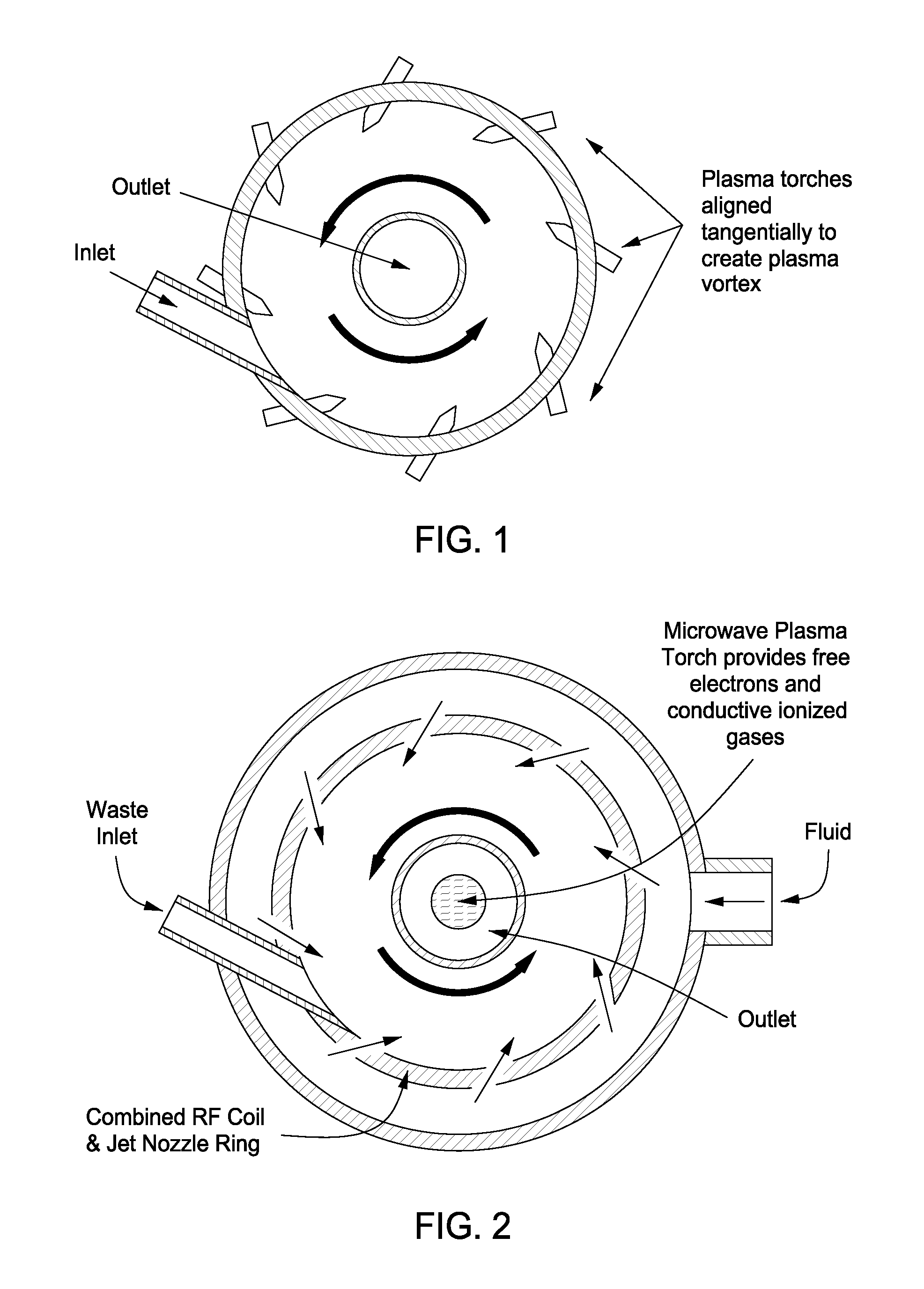
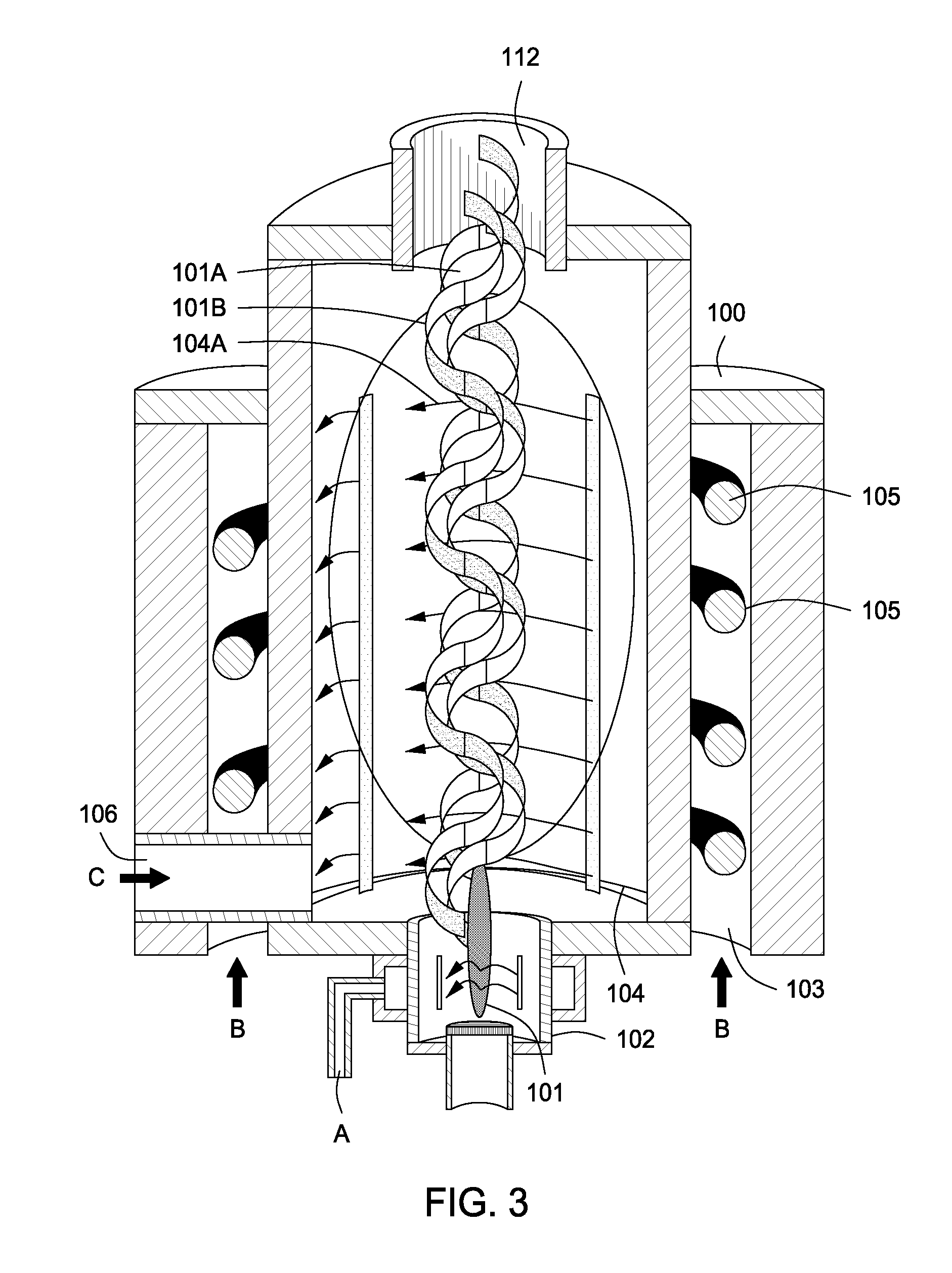
Plasma whirl reactor apparatus and methods of use
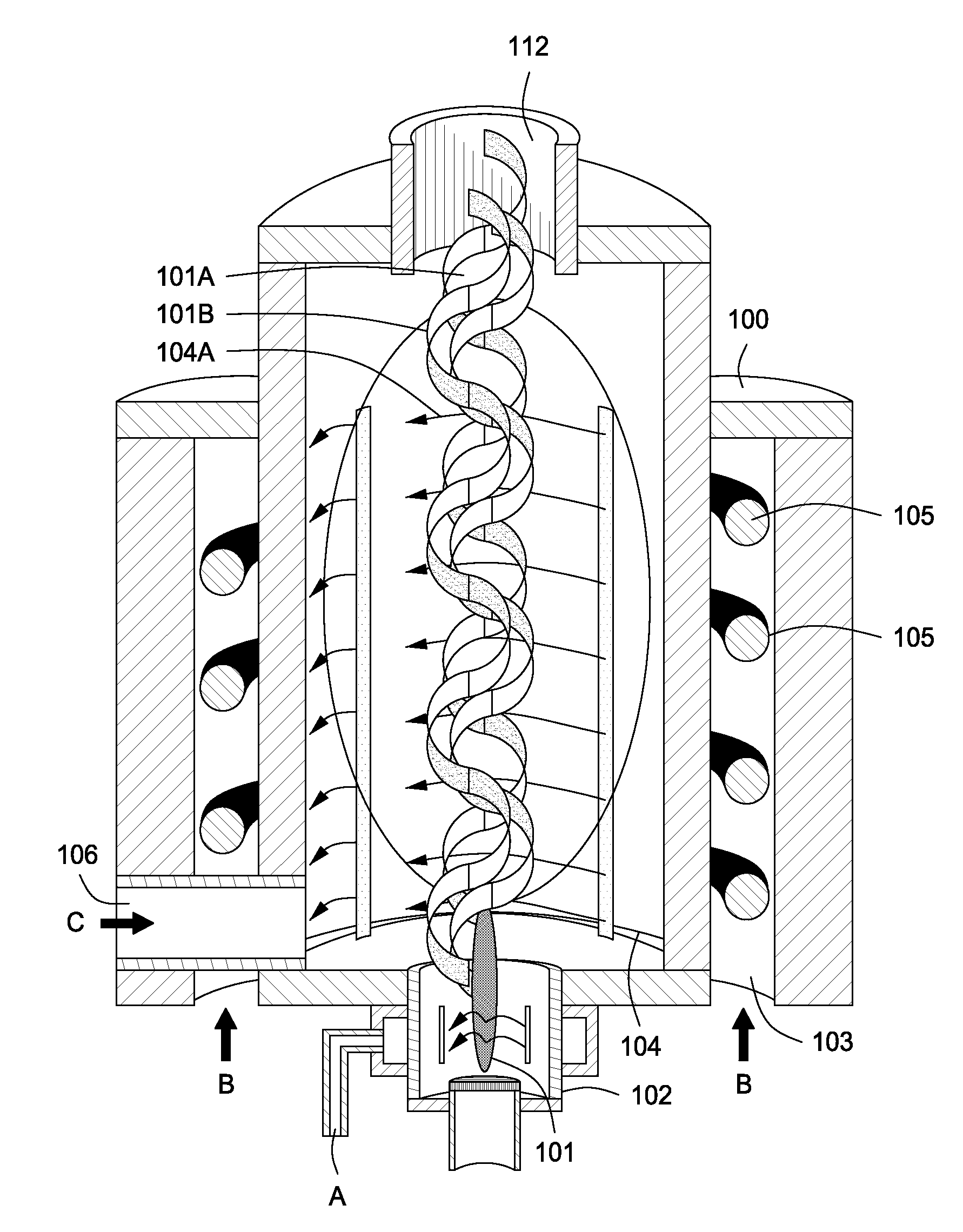
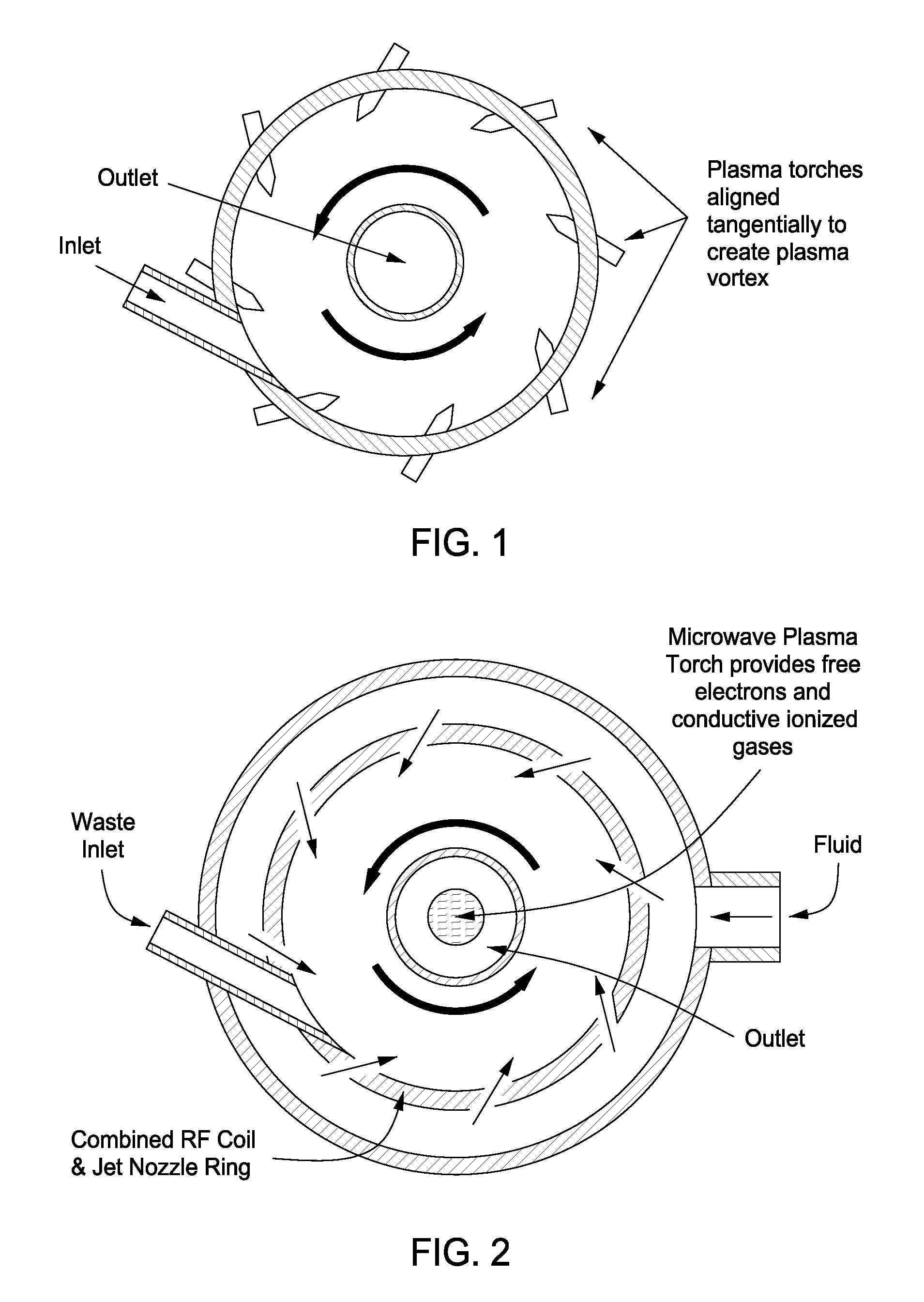
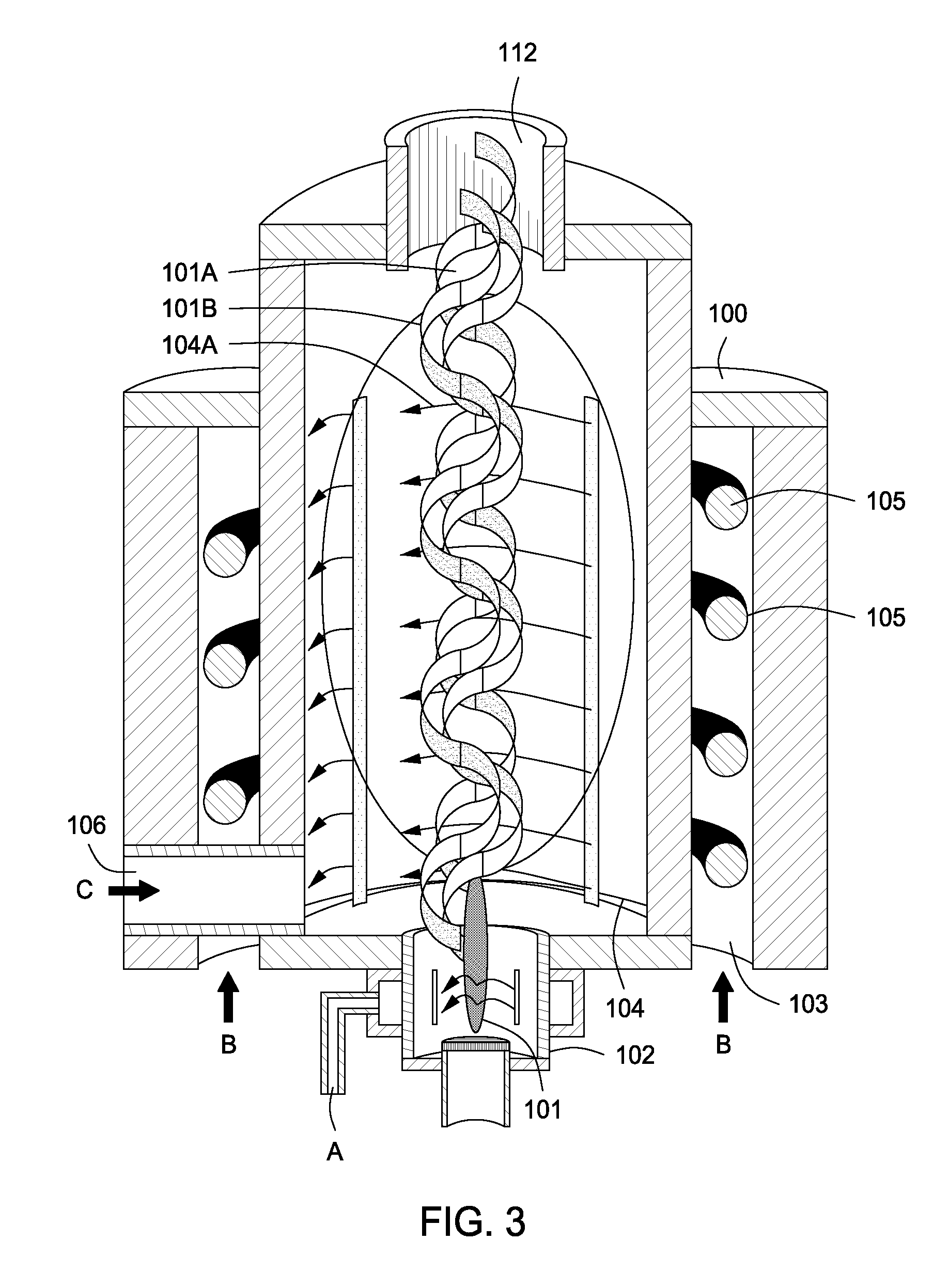
InactiveUS20100044483A1Calcium/strontium/barium carbonatesAluminium compoundsSingle processAngular momentum
An apparatus for synergistically combining a plasma with a comminution means such as a fluid kinetic energy mill (jet mill), preferably in a single reactor and / or in a single process step is provided by the present invention. Within the apparatus of the invention potential energy is converted into kinetic energy and subsequently into angular momentum by means of wave energy, for comminuting, reacting and separation of feed materials. Methods of use of the apparatus in the practice of various processes are also provided by the present invention.
Owner:FORET PLASMA LABS
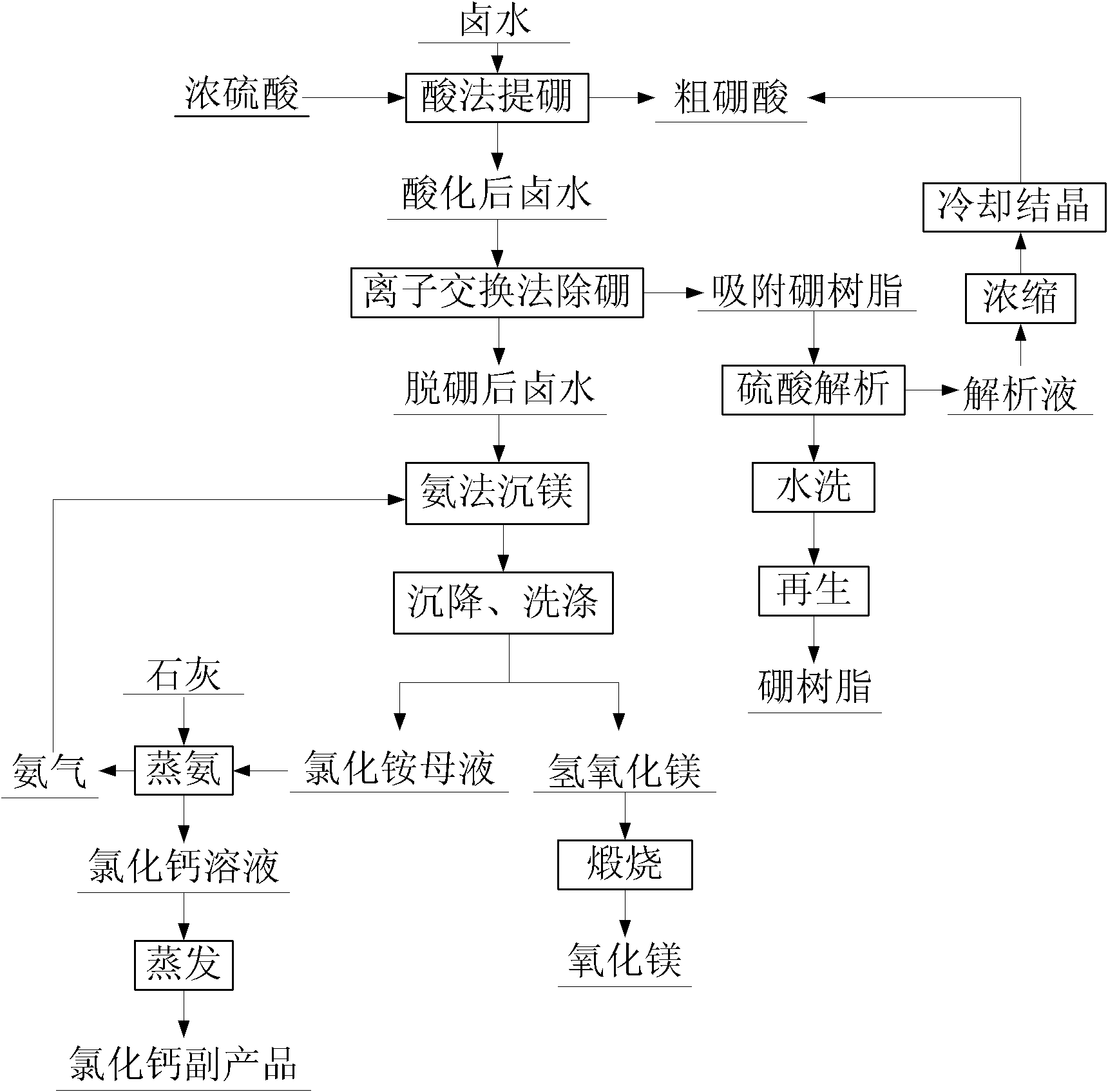
Method for preparing high-purity magnesium oxide with high boron salt lake brine
InactiveCN102491379ALarge particlesReduce extraction costsBoron-oxygen compoundsMagnesiaReaction temperatureIon-exchange resin
Provided is a method for preparing high-purity magnesium oxide with high boron salt lake brine. Salt lake brine is evaporated through a salt pan, concentrated to crystallize potassium sulfate, sodium chloride and potassium chloride and is drawn with lithium in adsorption mode so as to obtain master sauce brine containing magnesium and boron. Concentrated sulfuric acid is added into master sauce brine for reacting, and coarse boracic acid and acidized brine are obtained after cooling and filtering. Potential of hydrogen (pH) value of acidized brine is adjusted to be 5.5-6.5, and acidized brine passes through ion exchange resin adsorbing boron. When boron concentration in effluent liquid is higher than 5 mg / L, brine is not injected, boron-removed brine is obtained, then boron-removed brine and ammonium chloride solution are filled with ammonia for stirring and to produce magnesium sedimentation reaction, reaction temperature ranges from 60 DEG C to 80 DEG C, pH ranges from 7.5 to 8.0, the reaction is stopped when concentration of free ammonia reaches 1.8-2.2 mol / L, and magnesium hydroxide and magnesium sedimentation mother solution are obtained. Magnesium oxide is obtained by calcining magnesium hydroxide, content of magnesium oxide is larger than 99.8%, and magnesium extraction ratio is larger than 90%. Sedimentation mother solution adopts lime to steam ammonia, and generated ammonia circulates to magnesium sedimentation reaction. Mother solution after ammonia steaming is evaporated, concentrated and crystallized to obtain calcium chloride. Ion exchange resin adsorbing boron is washed, analyzed and regeneratively cycled for use. Boron-containing analysis solution is concentrated and cooled to pick up coarse boracic acid, and coarse boracic acid is recrystallized to obtain refined boracic acid with purity larger than 99%. High-purity magnesium oxide prepared by the method is high in purity, good in economic benefit, free of environment pollution, strong in operability and favorable for industrial production.
Owner:CENT SOUTH UNIV
Process for removal of pollutants
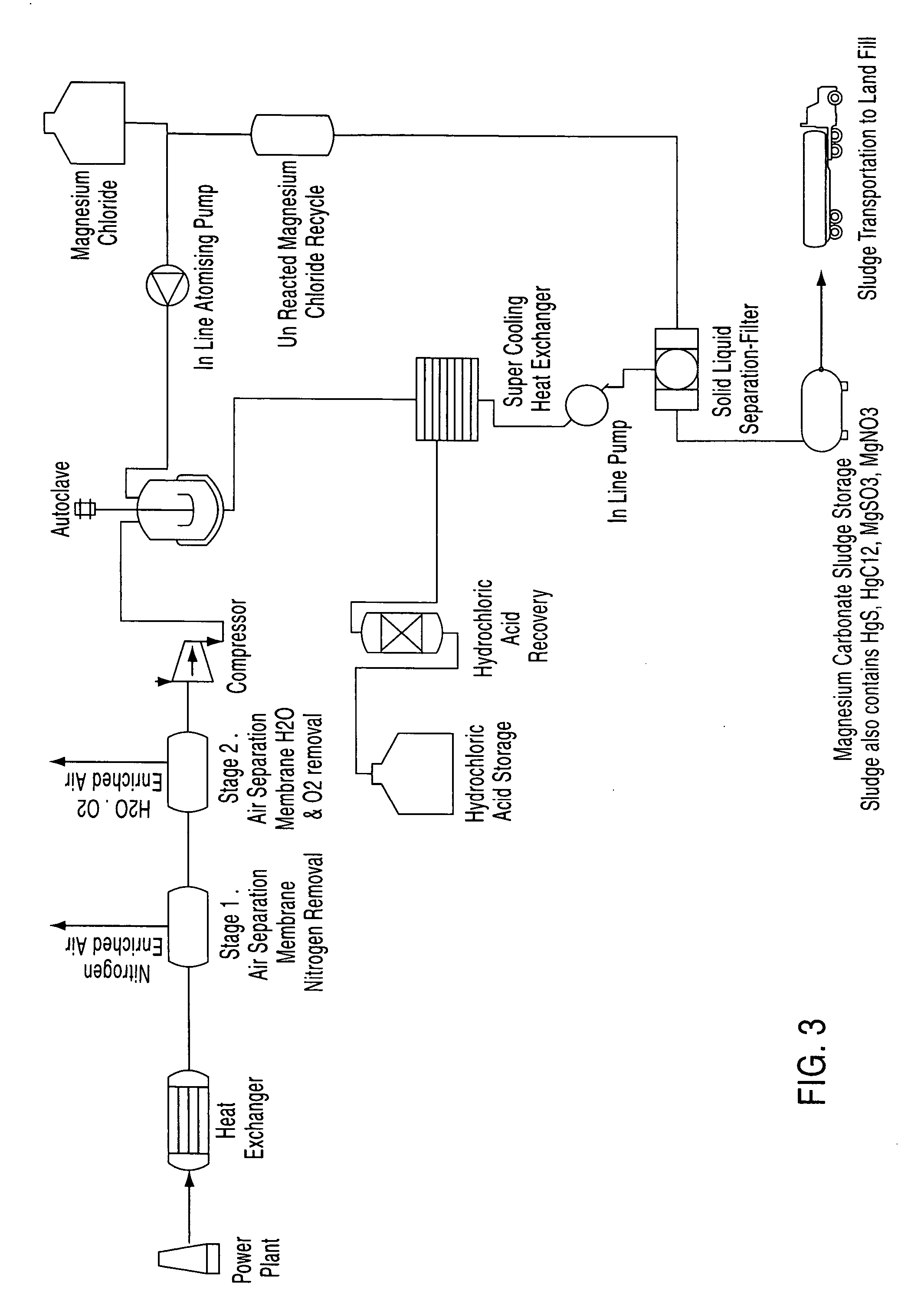
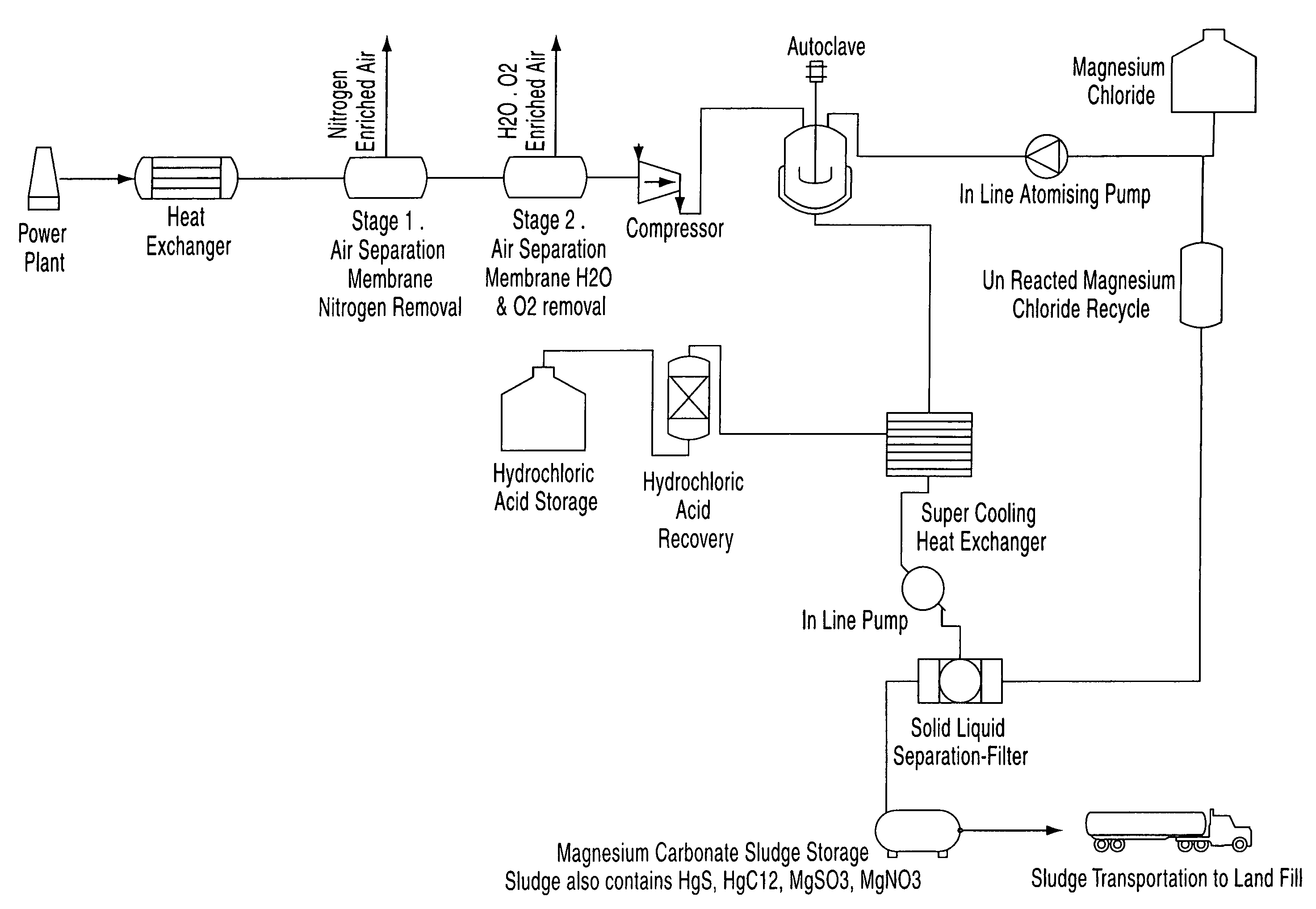
A process for the removal of pollutants from a combustion process and, more particularly, a process for removing pollutants such as carbon dioxide, mercury, sulphur dioxide, nitrogen compounds and oxygen compounds from a combustion process. The process includes the removal of pollutants from a combustion process that produces an emission comprising: cooling the emission to a temperature of about 200° C.; removing nitrogen, water and oxygen from the emission to produce a gas containing a concentration of pollutants; contacting the gas with an aqueous magnesium chloride solution, wherein a slurry mixture is formed; and cooling the gas and the slurry mixture, wherein hydrochloric acid vapour and a sludge are formed.
Owner:CLEAN WORLD STRATEGIES CORP
Apparatus and method for making metal chloride salt product
InactiveUS7217402B1Low densityRare earth metal chloridesChloride preparationMetal chlorideDistillation
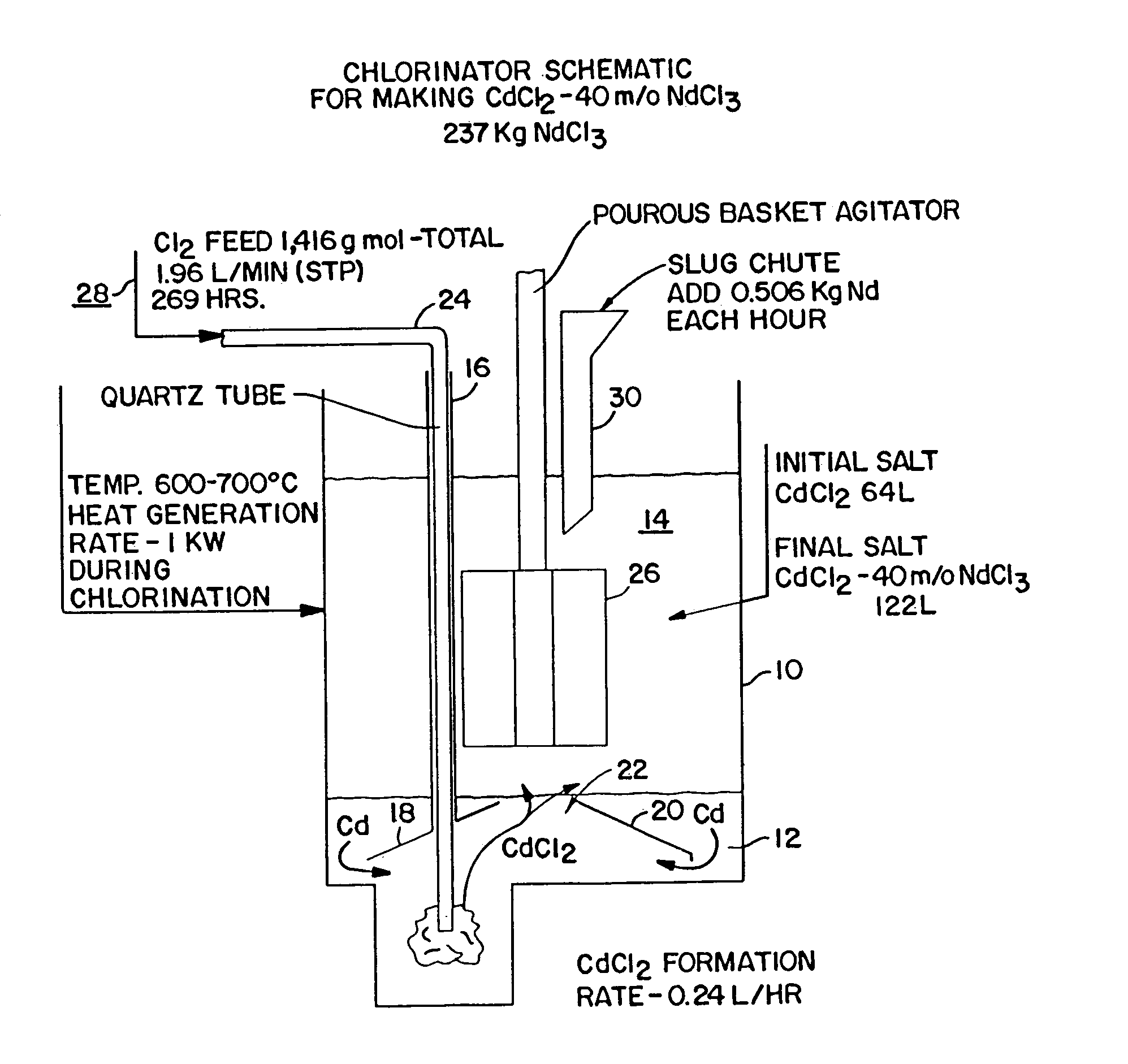
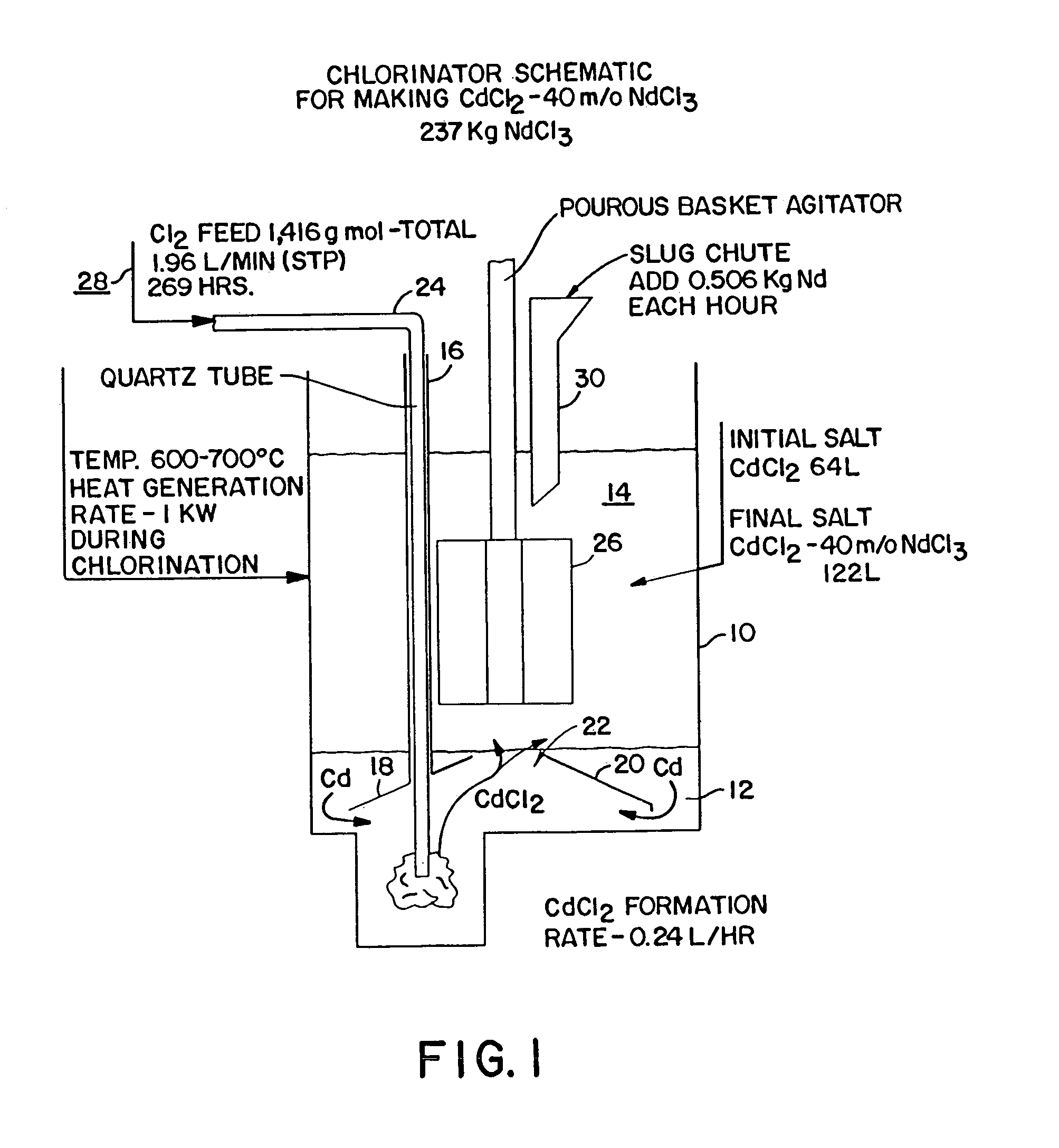
A method of producing metal chlorides is disclosed in which chlorine gas is introduced into liquid Cd. CdCl2 salt is floating on the liquid Cd and as more liquid CdCl2 is formed it separates from the liquid Cd metal and dissolves in the salt. The salt with the CdCl2 dissolved therein contacts a metal which reacts with CdCl2 to form a metal chloride, forming a mixture of metal chloride and CdCl2. After separation of bulk Cd from the salt, by gravitational means, the metal chloride is obtained by distillation which removes CdCl2 and any Cd dissolved in the metal chloride.
Owner:THE UNITED STATES AS REPRESENTED BY THE DEPARTMENT OF ENERGY
Plasma whirl reactor apparatus and methods of use
InactiveUS20100044477A1Calcium/strontium/barium carbonatesAluminium compoundsSingle processAngular momentum
An apparatus for synergistically combining a plasma with a comminution means such as a fluid kinetic energy mill (jet mill), preferably in a single reactor and / or in a single process step is provided by the present invention. Within the apparatus of the invention potential energy is converted into kinetic energy and subsequently into angular momentum by means of wave energy, for comminuting, reacting and separation of feed materials. Methods of use of the apparatus in the practice of various processes are also provided by the present invention.
Owner:FORET PLASMA LABS
Process for the formulation of potassium chloride from a carnallite source
A process for formulating high purity potassium chloride from a carnallite source. The process takes advantage of solubility differences and saturation levels in a multiple salt system generated upon dissolution of carnallite. In the system, the sodium chloride is kept in solution and the magnesium chloride present in the system is controlled to be in a concentration range of between 12% and 25% by weight. This avoids co-precipitation of sodium chloride with the potassium chloride during crystallization and therefore prevents the sodium chloride from contaminating the potassium chloride. The result is high grade potassium chloride.
Owner:KARNALYTE RESOURCES
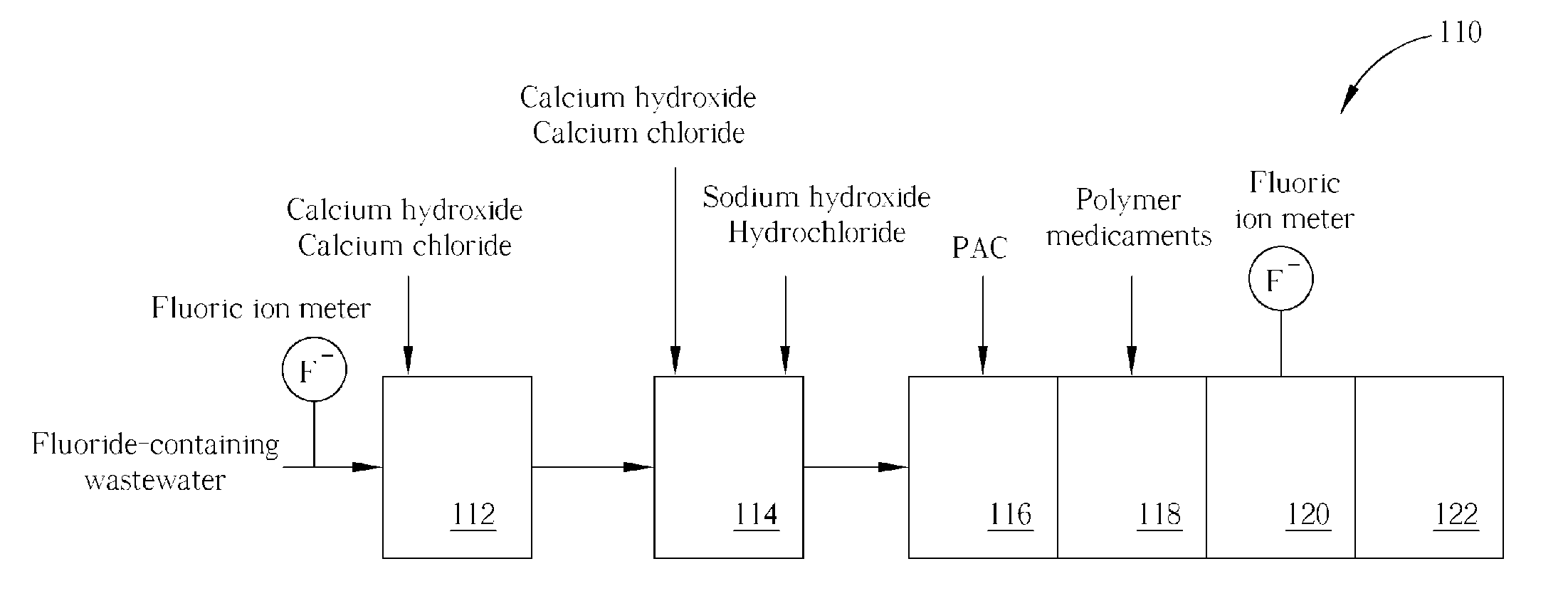
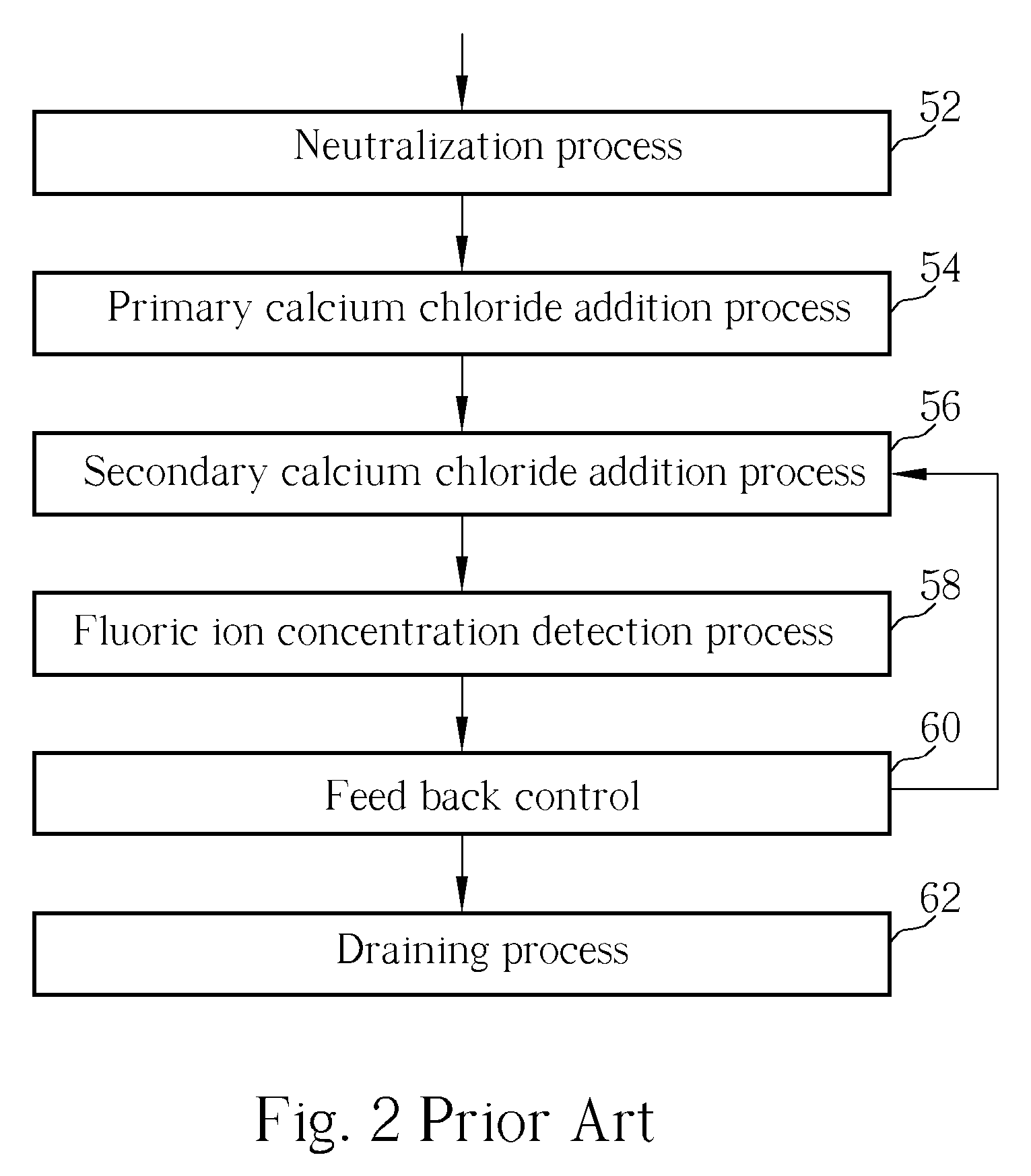
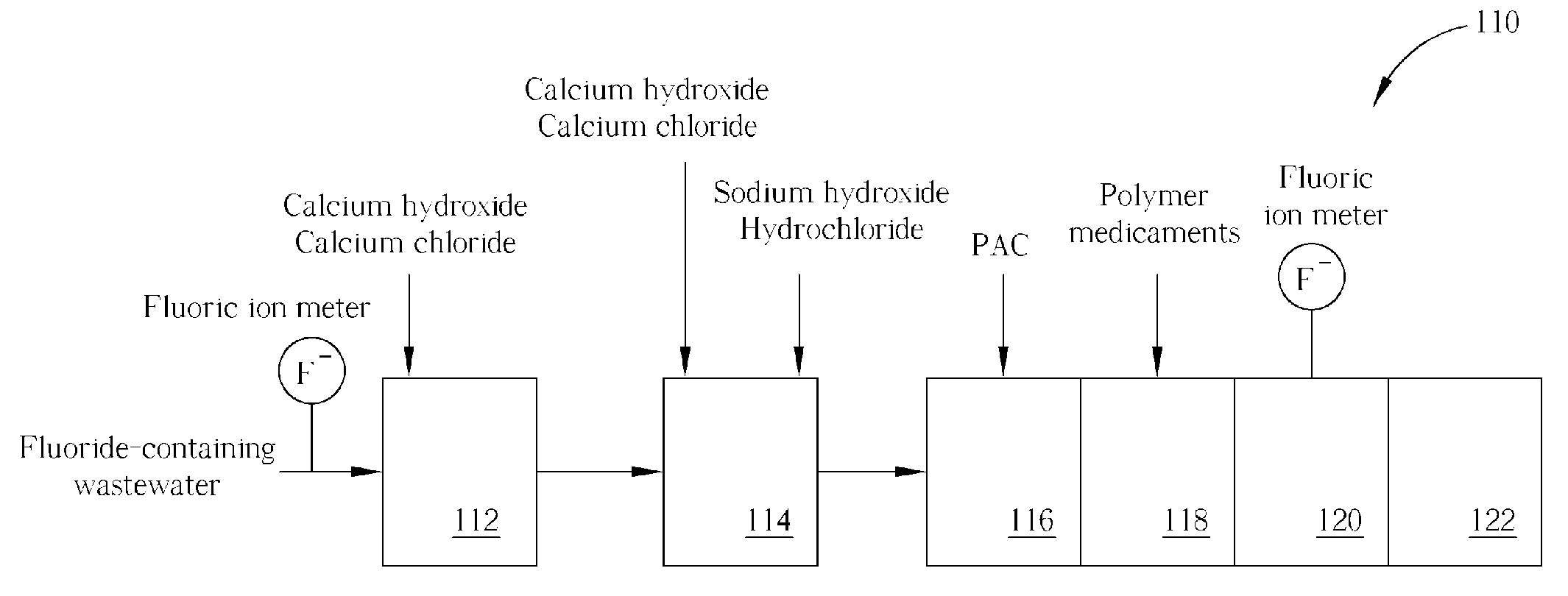
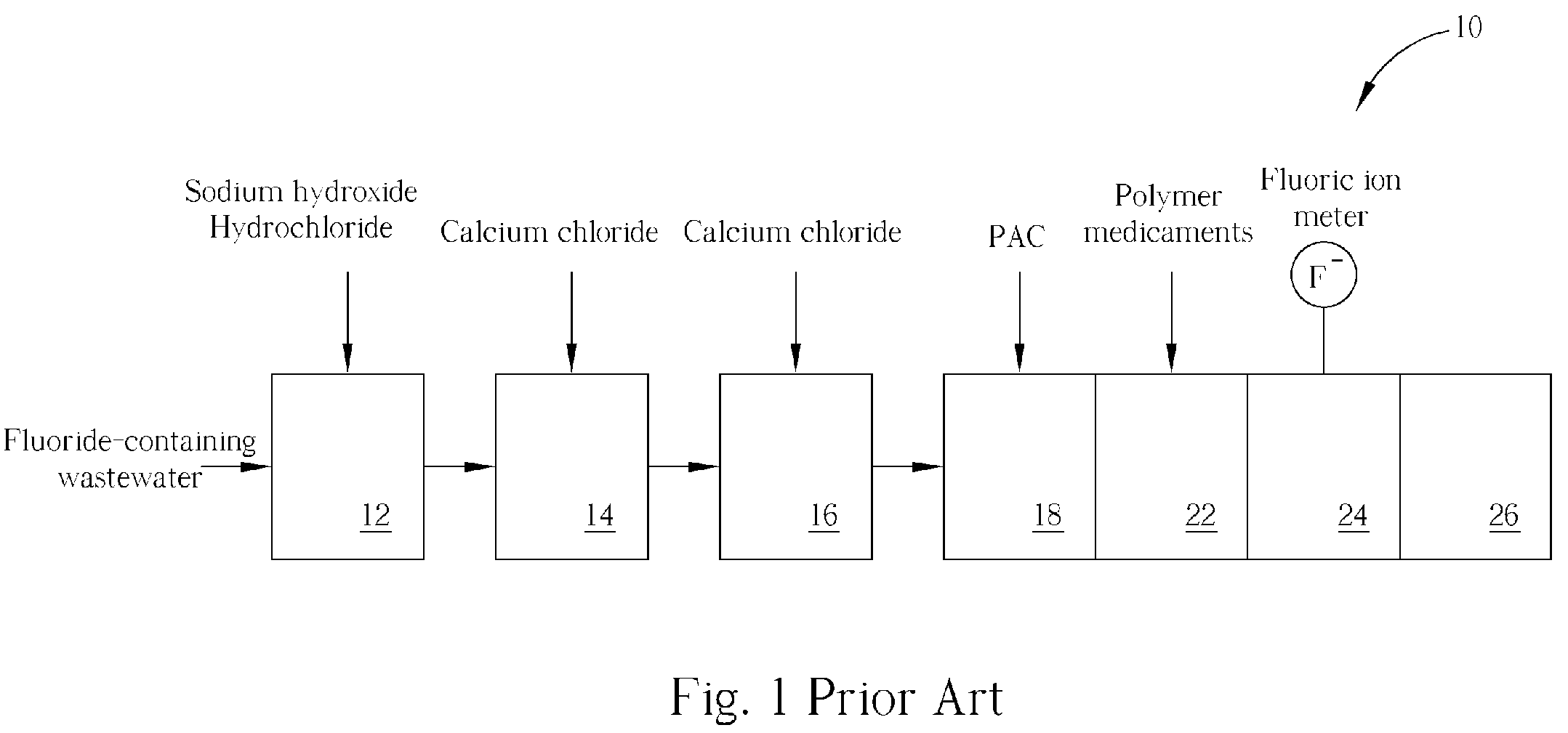
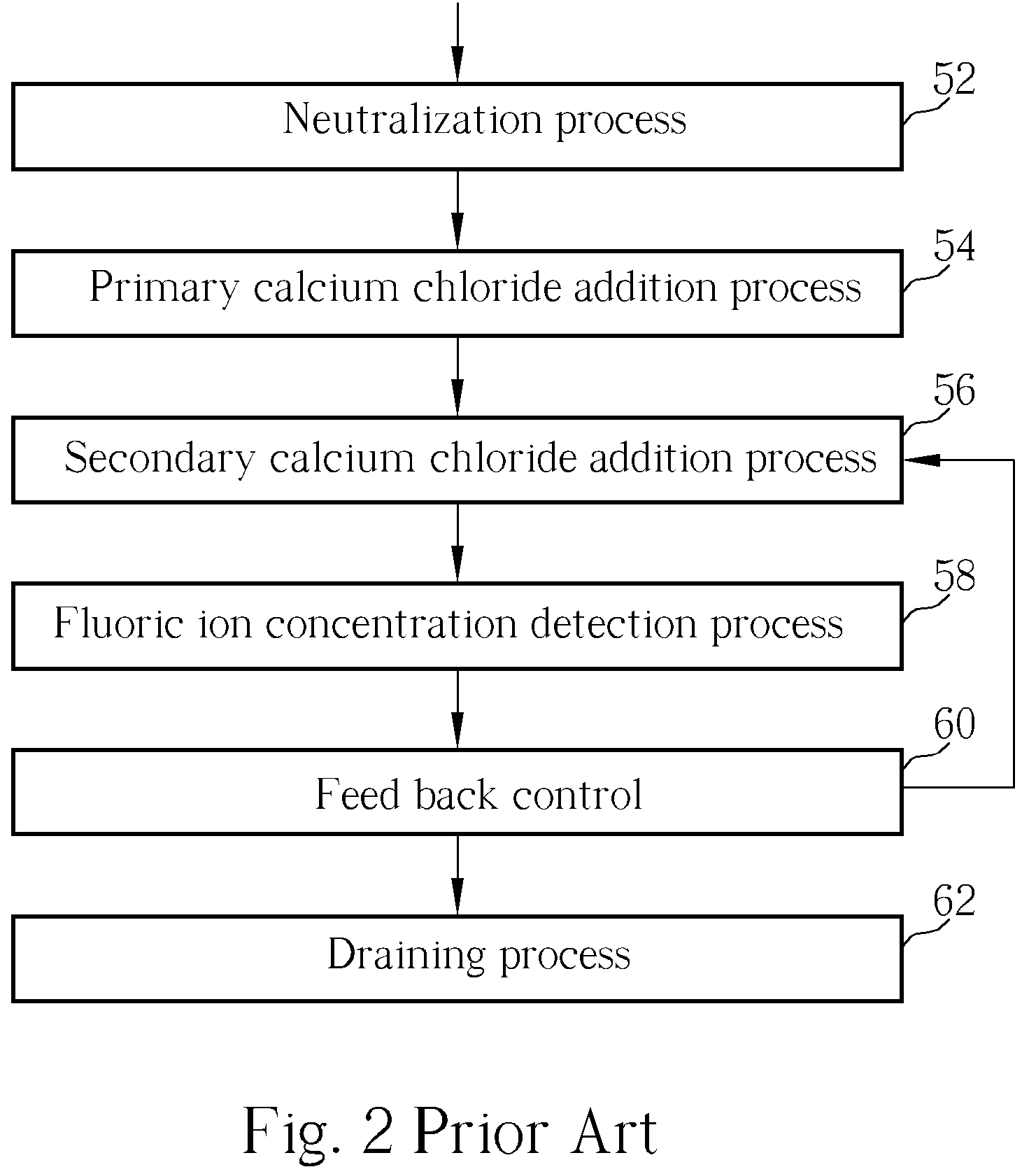
Method of fluoride-containing wastewater treatment
First, a primary fluoric ion concentration detection process is performed, and a primary calcium salt addition process is performed to add calcium salt in a first reaction tank, wherein the dosage of the calcium salt in the primary calcium salt addition process is determined according to the detected fluoric ion concentration. Thereupon, a secondary calcium salt addition process is performed to add calcium salt into the second reaction tank. Following that, a solid-liquid separation process is performed to separate calcium fluoride from the wastewater, and a secondary fluoric ion concentration detection process is performed upon the wastewater after the calcium fluoride is separated. Finally, the dosage of the calcium salt in the secondary calcium salt addition process is determined in a feed back control manner according to the detected fluoric ion concentration.
Owner:POWERCHIP SEMICON MFG CORP
Process for the formulation of potassium chloride from a carnallite source
ActiveUS20110123420A1Speed up the processSolvent extractionCrystallization separationSolubilitySaturated Level
A process for formulating high purity potassium chloride from a carnallite source. The process takes advantage of solubility differences and saturation levels in a multiple salt system generated upon dissolution of carnallite. In the system, the sodium chloride is kept in solution and the magnesium chloride present in the system is controlled to be in a concentration range of between 12% and 25% by weight. This avoids co-precipitation of sodium chloride with the potassium chloride during crystallization and therefore prevents the sodium chloride from contaminating the potassium chloride. The result is high grade potassium chloride.
Owner:KARNALYTE RESOURCES
Treatment of solid containing material derived from effluent
InactiveUS6425973B1Reducing and eliminating cost and environmental impactSimpler and cheapCalcium/strontium/barium carbonatesMagnesium halidesParticulatesSludge
A method of treating solid containing material derived from effluent or sludge from a plant for de-inking paper, the material containing calcium in the form of one or more insoluble calcium compounds, the method including the steps of treating the material with an acid to cause dissolution of the calcium thereby forming a calcium ion-containing solution in which insoluble solids are suspended, separating the solution from the insoluble solids and incinerating the separated solids. The solution containing calcium ions may be treated by adding one or more reagents to form a calcium compound precipitate, eg calcium carbonate. The particulate solids produced following the incineration step and following the precipitate formation may be employed as pigments or fillers in paper making or paper coating.
Owner:IMERYS MINERALS
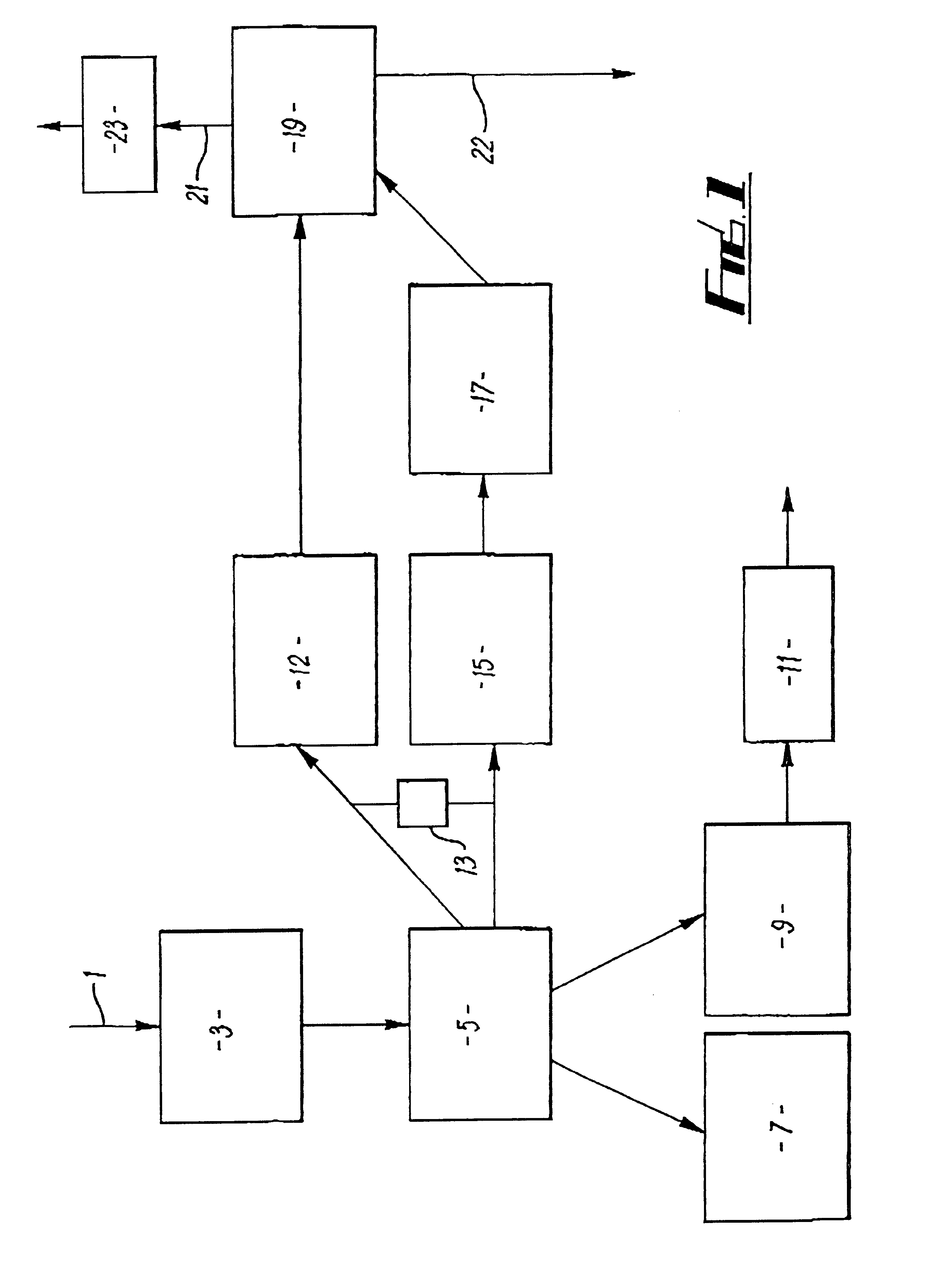
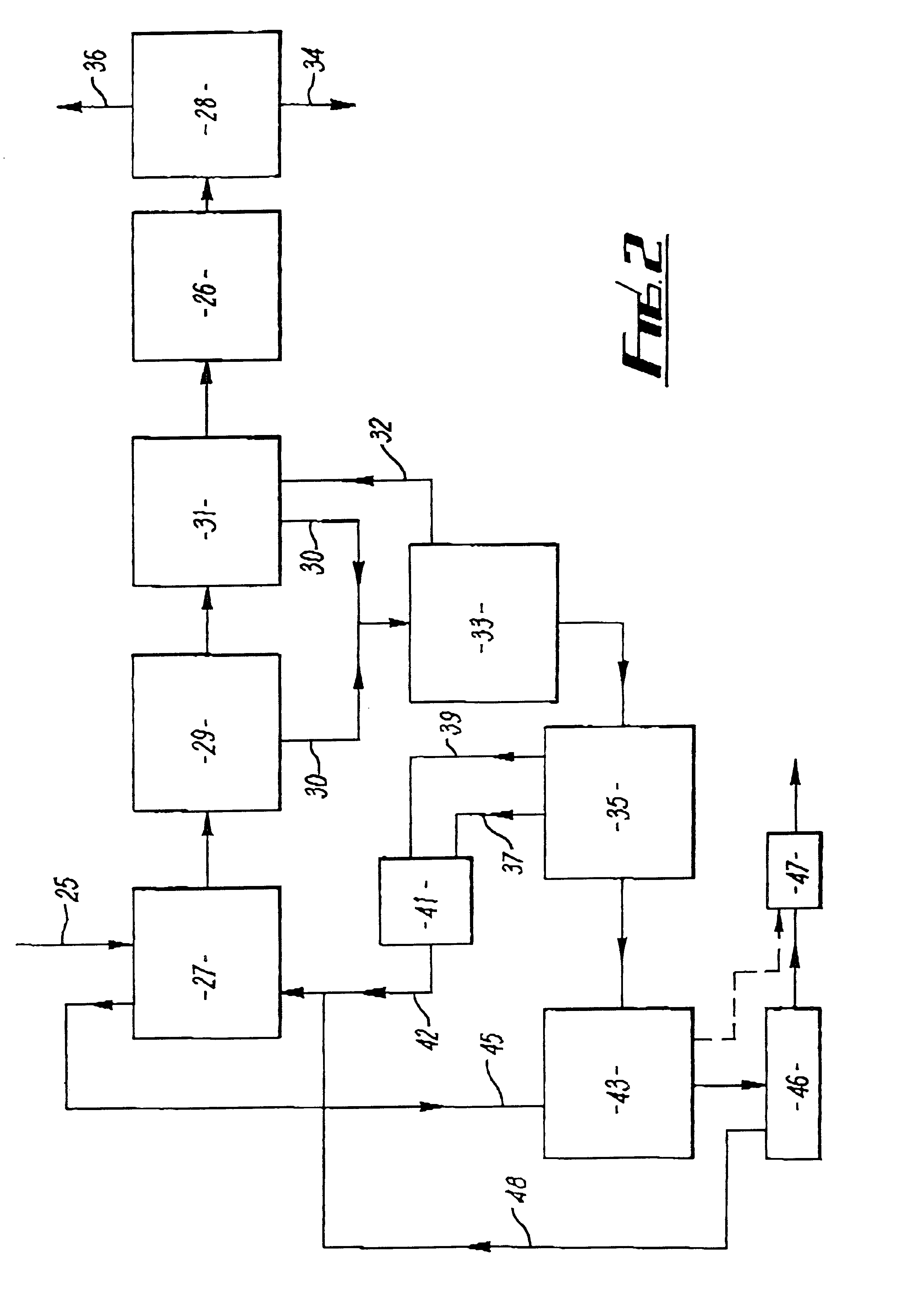
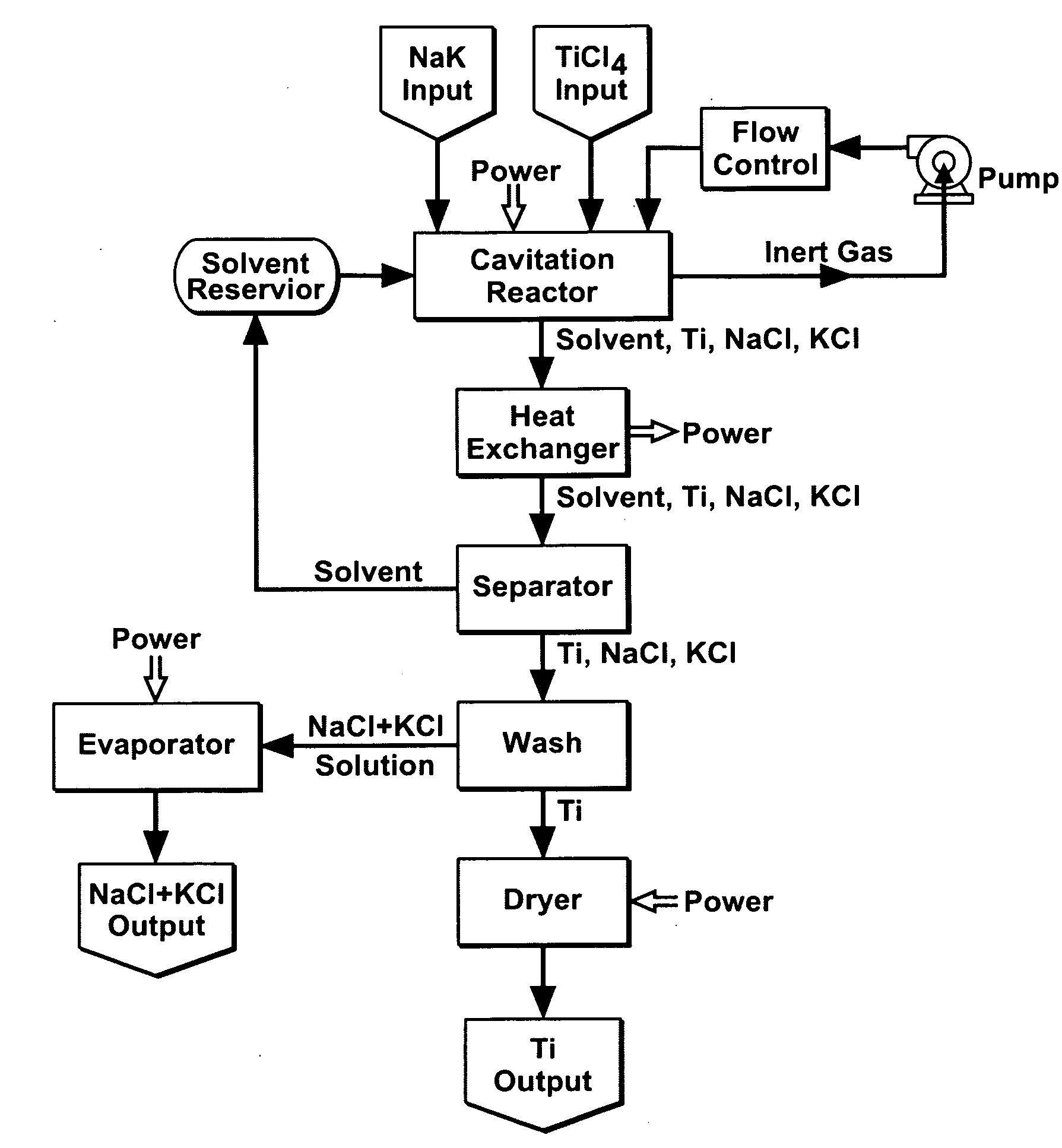
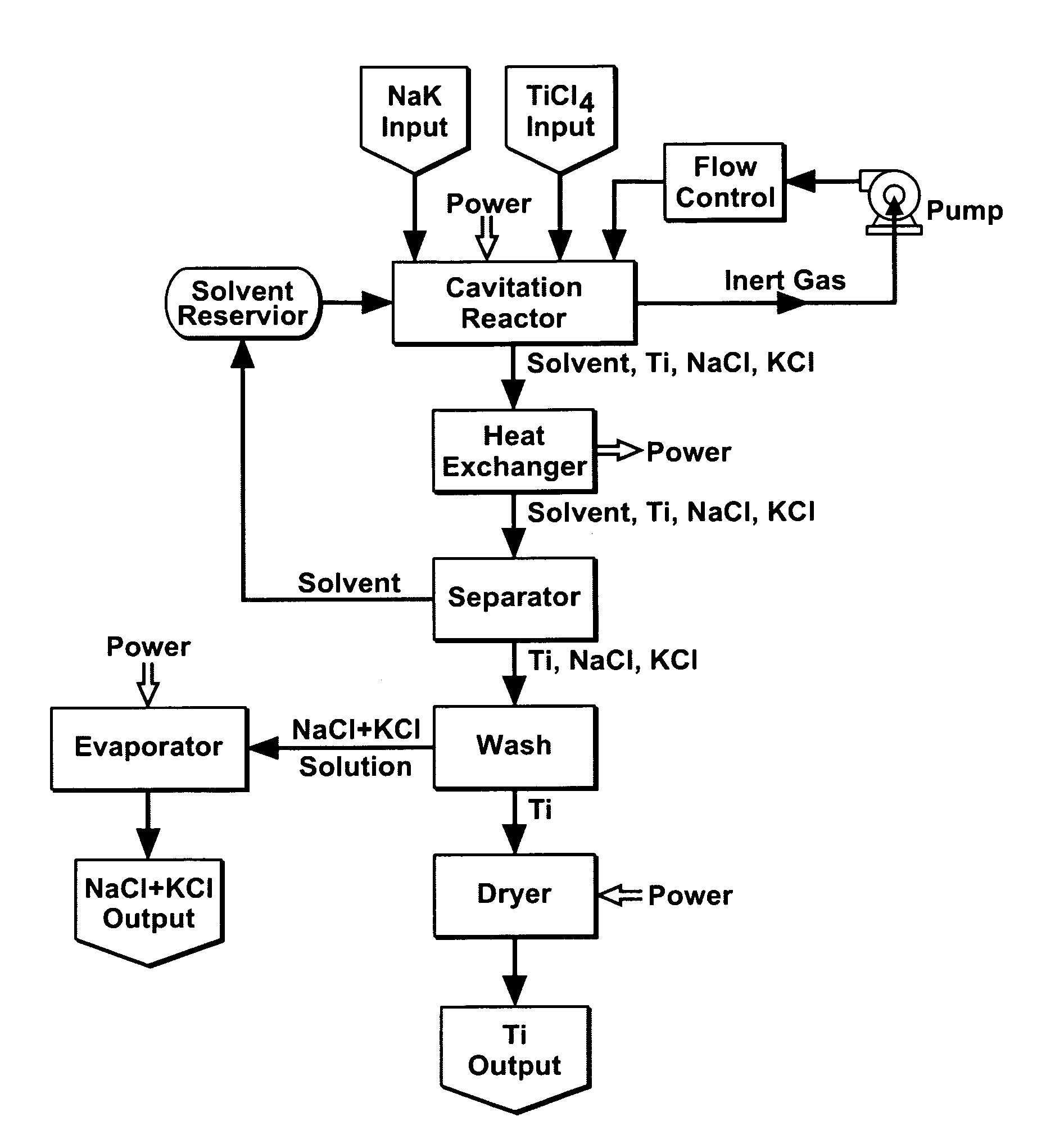
Cavitation process for products from precursor halides
ActiveUS20080295645A1Promote lowerLow vapor pressureMagnesium halidesZirconium compoundsAlkaline earth metalLiquid medium
A precursor halide compound is reduced to a predetermined product at substantially ambient conditions. The halide is added to an anhydrous liquid reaction medium containing one or more alkali metals or alkaline earth metals as reductants. The metal reductants are dispersed as very small globules in the liquid by cavitation of the liquid, such as by application of high intensity ultrasonic vibrations or high-shear mixing to the reaction vessel. Continued cavitation of the liquid medium affects low temperature reduction of the precursor halide(s) to produce a metal, metal alloy, metal compound, ceramic material, metal matrix-ceramic composite material, or the like. The practice may be applied, for example, to titanium tetrachloride, alone or with other chlorides, to produce titanium metal, titanium alloys (for example Ti-6Al-4V), and titanium compounds (TiSi2).
Owner:GM GLOBAL TECH OPERATIONS LLC
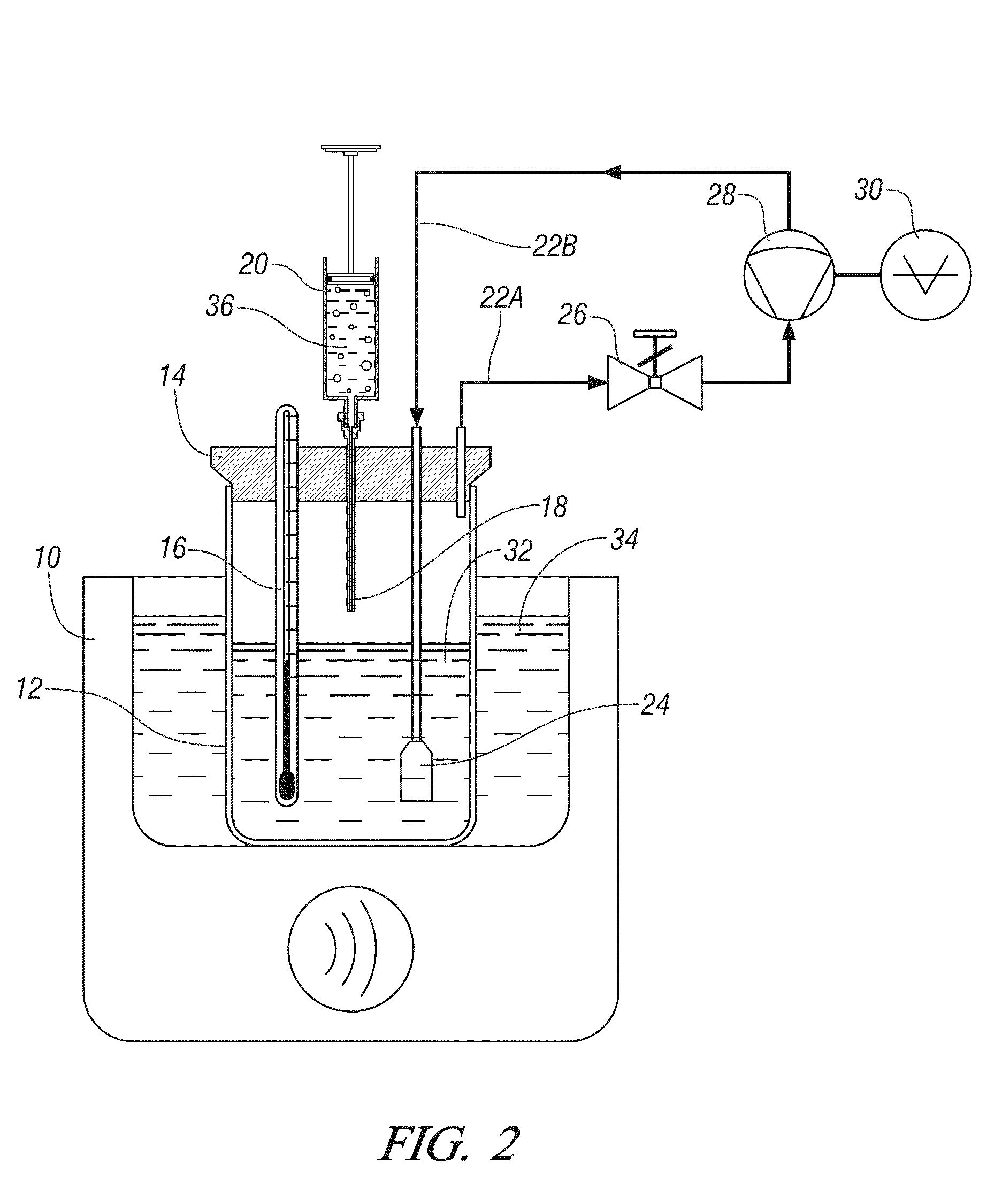
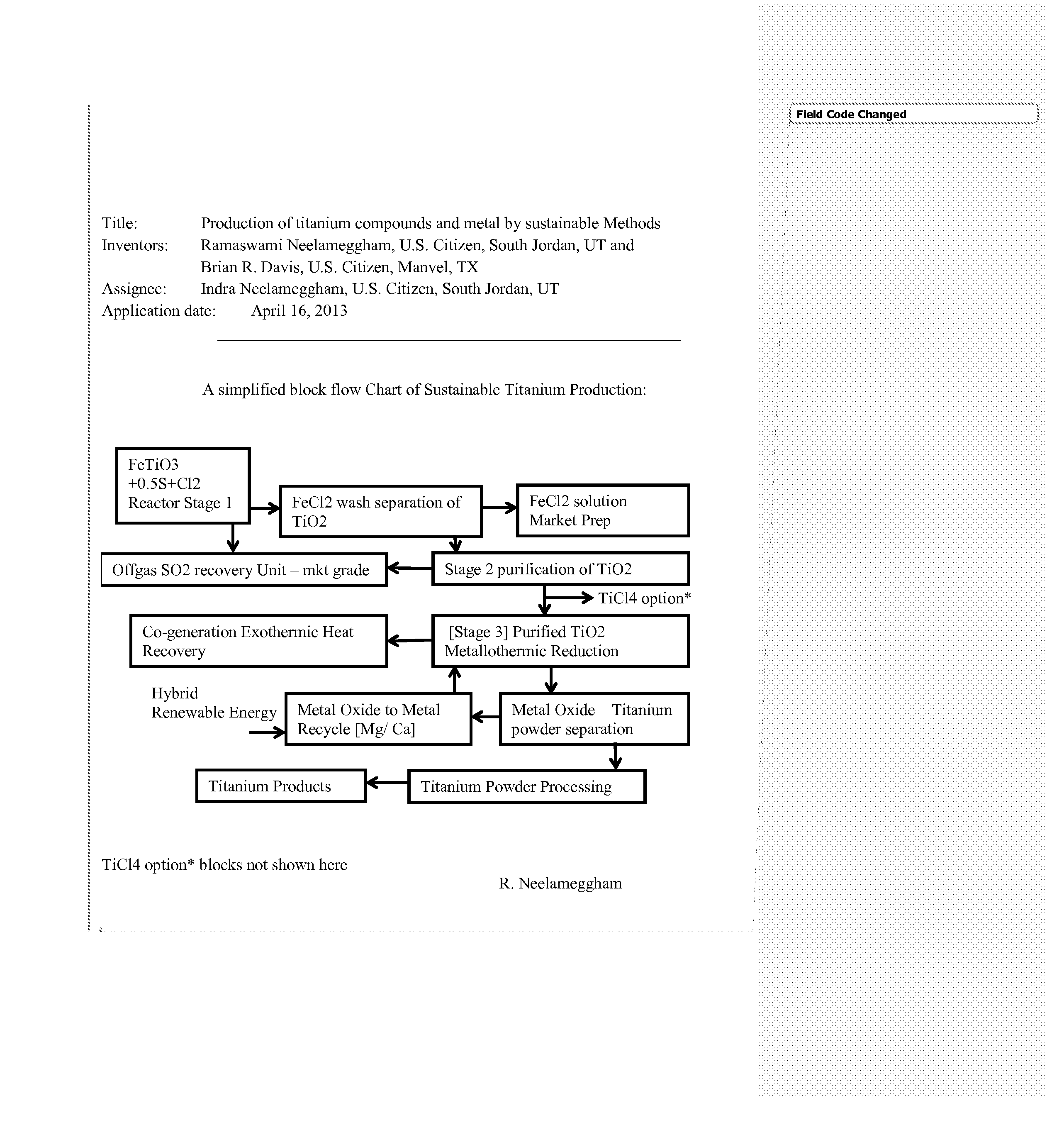
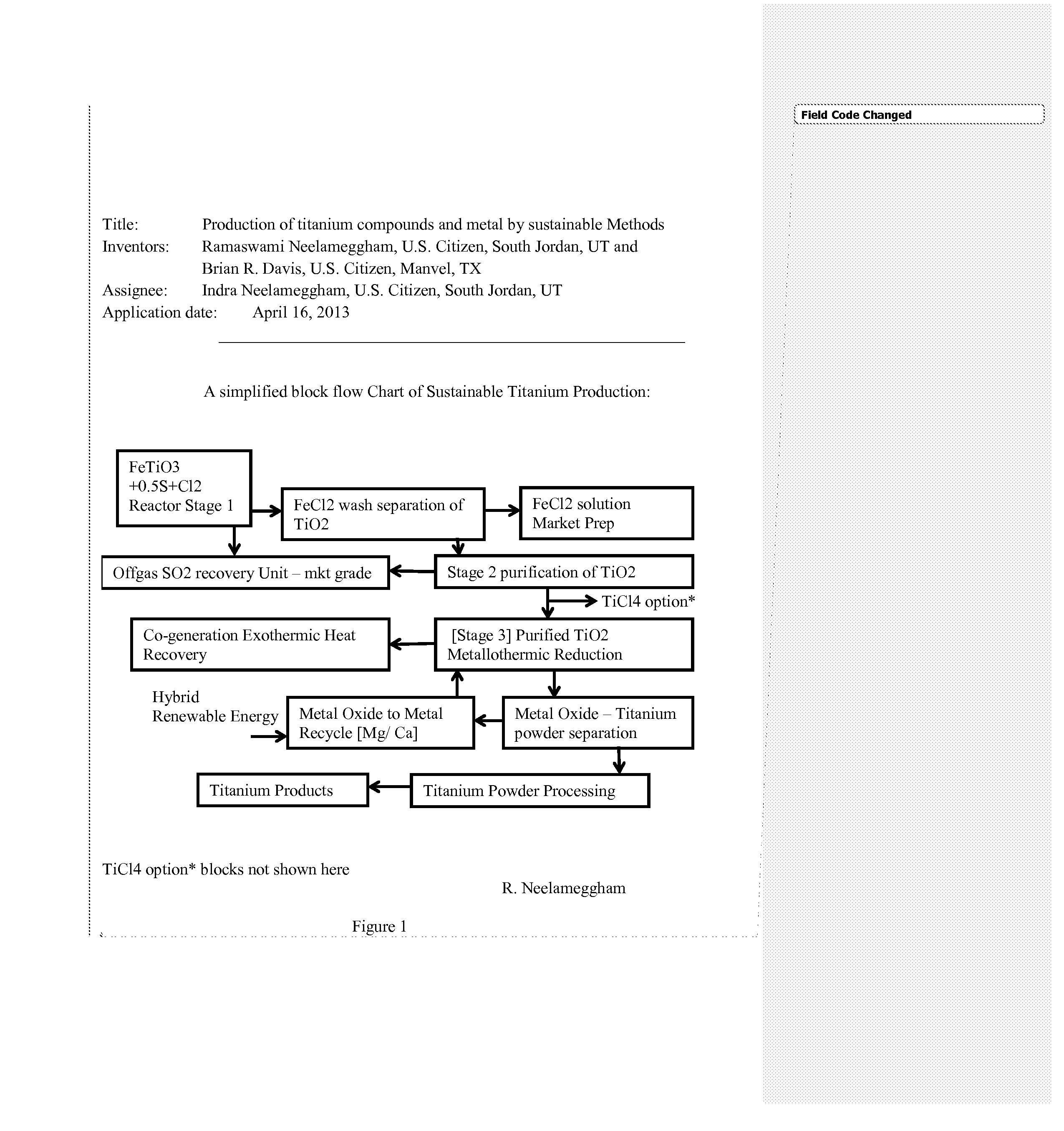
Production of titanium compounds and metal by sustainable Methods
InactiveUS20140308197A1Low carbon dioxide footprintLow costMagnesium chloridesBlast furnace detailsTitanium metalSustainable process
A unique production of titanium compounds and metal by sustainable methods using iron-titanium oxide starting material such as ilmenite, leucoxene, or rutile is described. Here the iron-titanium oxide compound is prepared by converting the iron portion of the compound to ferrous chloride at low temperatures by using close to stoichiometric amounts of sulfur and chlorine required for all the iron oxides and the other non-titanium oxides. The ferrous chloride thus formed is removed recovering a marketable product of ferrous chloride and the ‘sustainable’ titanium oxide starting material by additional process steps. This can be converted to ‘sustainable’ titanium metal, or titanium tetra-chloride by process shown herein for further conversions to titanium dioxide pigment by present chloride process or supplied to existing titanium sponge producers, benefitting them in having a ‘sustainable process’.
Owner:NEELAMEGGHAM INDRA
Persistent and fast acting antiseptics and disinfectants based on calcium fluoride
Antiseptic compounds that act as persistent and fast acting antiseptics and disinfectants. The base of these antiseptic actions is CaF2 as the persistent part, preventing the colonization of tissue and nonliving surfaces with microorganisms through the targeted on-demand release of fluorine ions. For fighting heavy contamination and invasion of transient microbes through new application of the solution, fast acting alcohols and toxic solutions have been added in small percentage. They act fast and evaporate fast, leaving the natural protection of skin undamaged and coated with a persistent antiseptic.
Owner:VOEGELI +2
Precursor of halide-type photostimulable phosphor, halide-type photostimulable phosphor, radiation image conversion panel, and process for producing them
InactiveCN101218319AImprove moisture resistanceIncrease brightnessX-ray/infra-red processesX/gamma/cosmic radiation measurmentHalogenImaging quality
This invention relates to a halide-type photostimulable phosphor having improved moisture resistance and brightness, and a process for producing the same. The halide-type photostimulable phosphor is characterized in that, among elements constituting the outermost surface and inside of the phosphor, there is a difference in composition ratio of a halogen element between the outermost surface and the inside of the phosphor. A radiation image conversion panel, which has been improved, for example, in moisture resistance, brightness and image quality by using the phosphor, and a process for producing the same are also provided.
Owner:KONICA MINOLTA MEDICAL & GRAPHICS INC
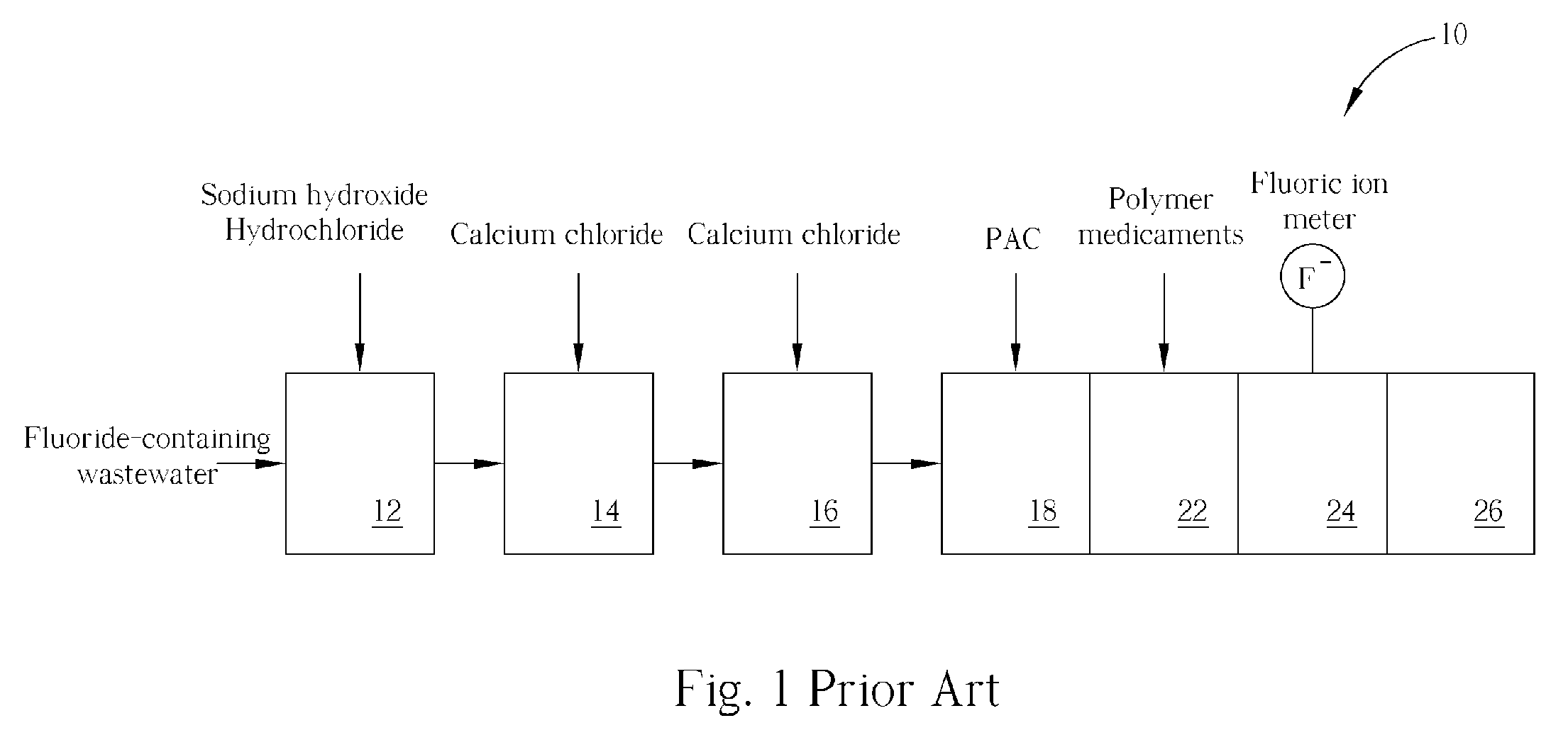
Method of fluoride-containing wastewater treatment
First, a primary fluoric ion concentration detection process is performed, and a primary calcium salt addition process is performed to add calcium salt in a first reaction tank, wherein the dosage of the calcium salt in the primary calcium salt addition process is determined according to the detected fluoric ion concentration. Thereupon, a secondary calcium salt addition process is performed to add calcium salt into the second reaction tank. Following that, a solid-liquid separation process is performed to separate calcium fluoride from the wastewater, and a secondary fluoric ion concentration detection process is performed upon the wastewater after the calcium fluoride is separated. Finally, the dosage of the calcium salt in the secondary calcium salt addition process is determined in a feed back control manner according to the detected fluoric ion concentration.
Owner:POWERCHIP SEMICON MFG CORP
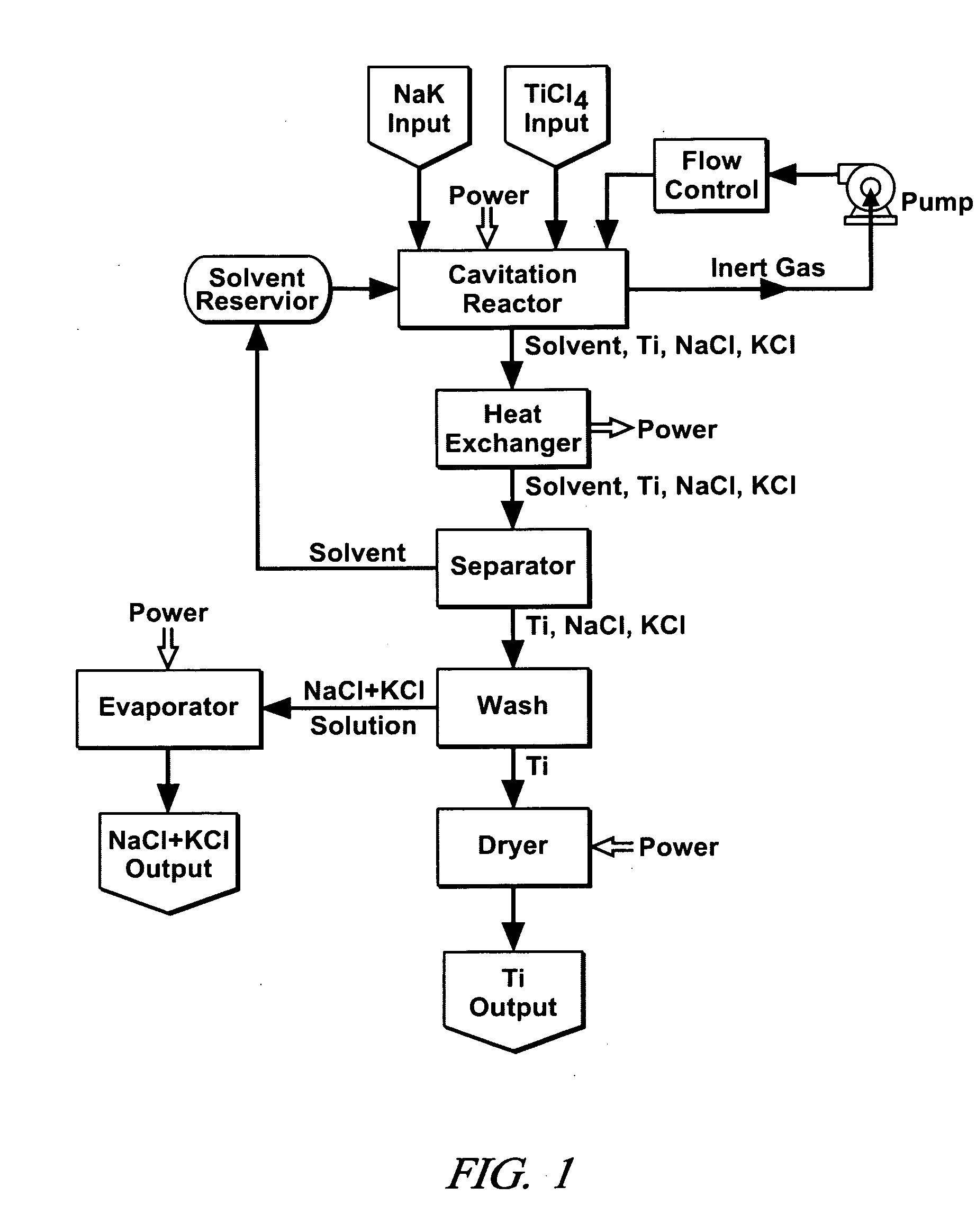
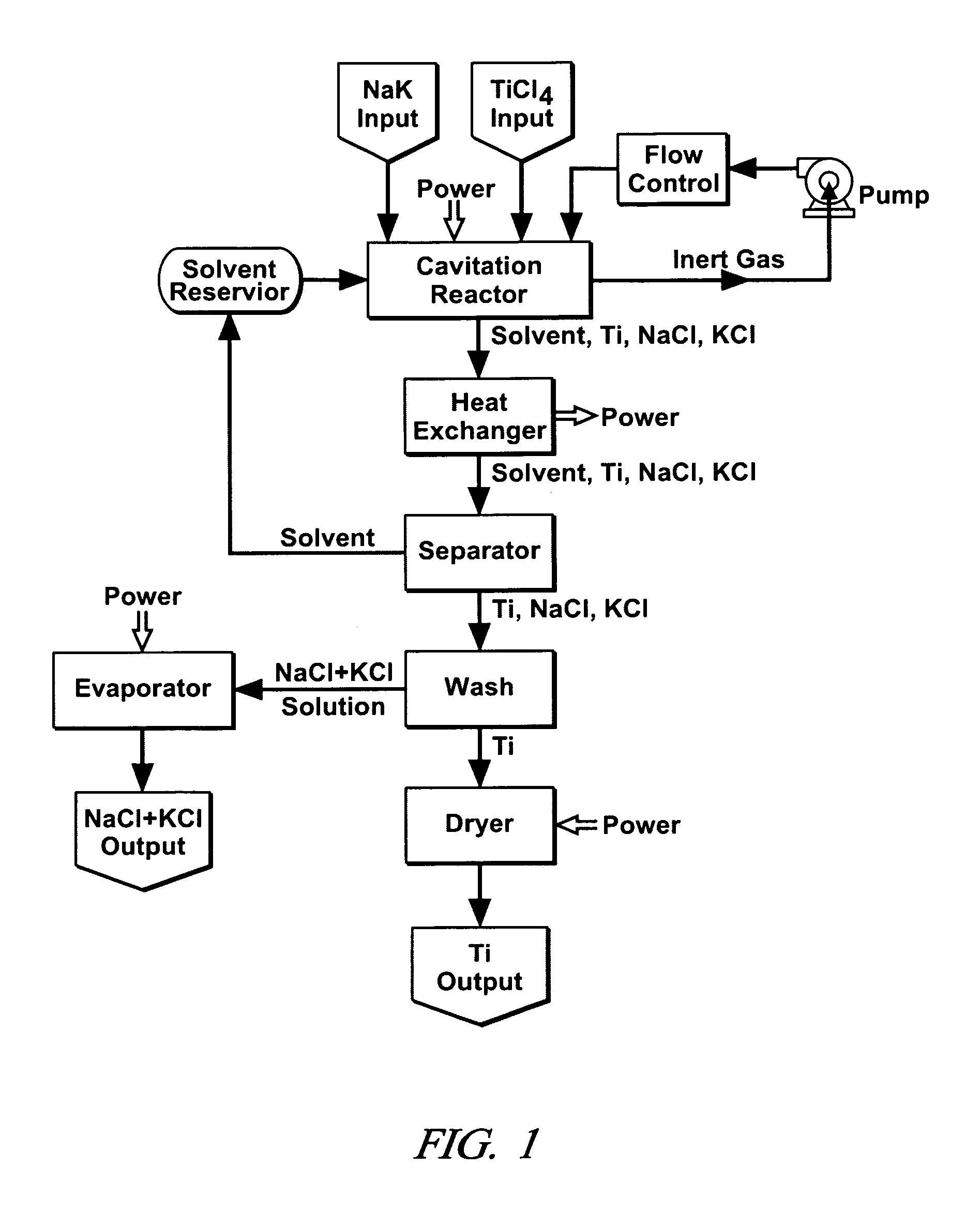
Cavitation process for products from precursor halides
ActiveUS7465333B1Promote lowerLow vapor pressureMagnesium halidesUsing liquid separation agentLiquid mediumCavitation
A precursor halide compound is reduced to a predetermined product at substantially ambient conditions. The halide is added to an anhydrous liquid reaction medium containing one or more alkali metals or alkaline earth metals as reductants. The metal reductants are dispersed as very small globules in the liquid by cavitation of the liquid, such as by application of high intensity ultrasonic vibrations or high-shear mixing to the reaction vessel. Continued cavitation of the liquid medium affects low temperature reduction of the precursor halide(s) to produce a metal, metal alloy, metal compound, ceramic material, metal matrix-ceramic composite material, or the like. The practice may be applied, for example, to titanium tetrachloride, alone or with other chlorides, to produce titanium metal, titanium alloys (for example Ti-6Al-4V), and titanium compounds (TiSi2).
Owner:GM GLOBAL TECH OPERATIONS LLC
Methods for solubilizing and recovering fluorinate compounds
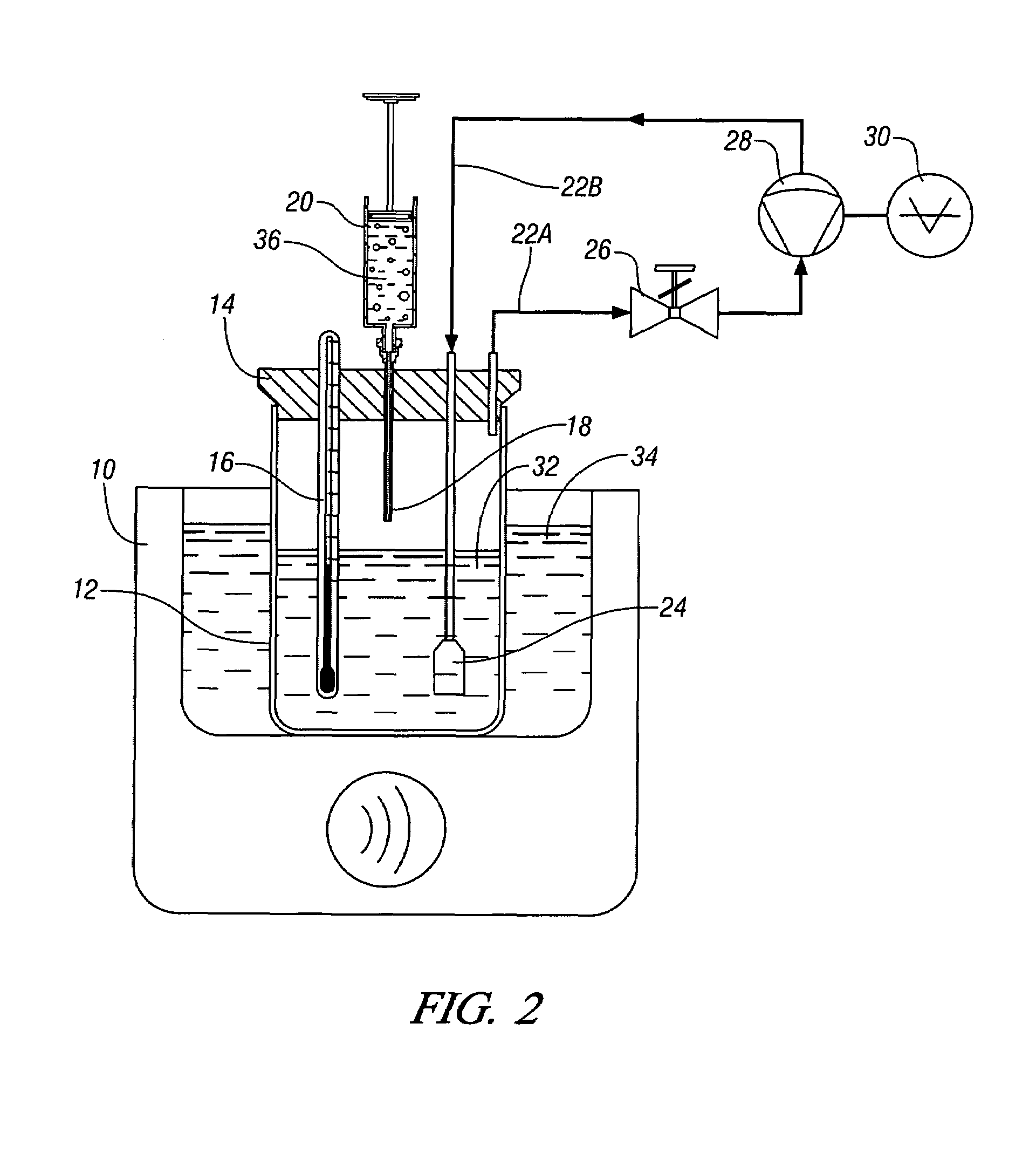
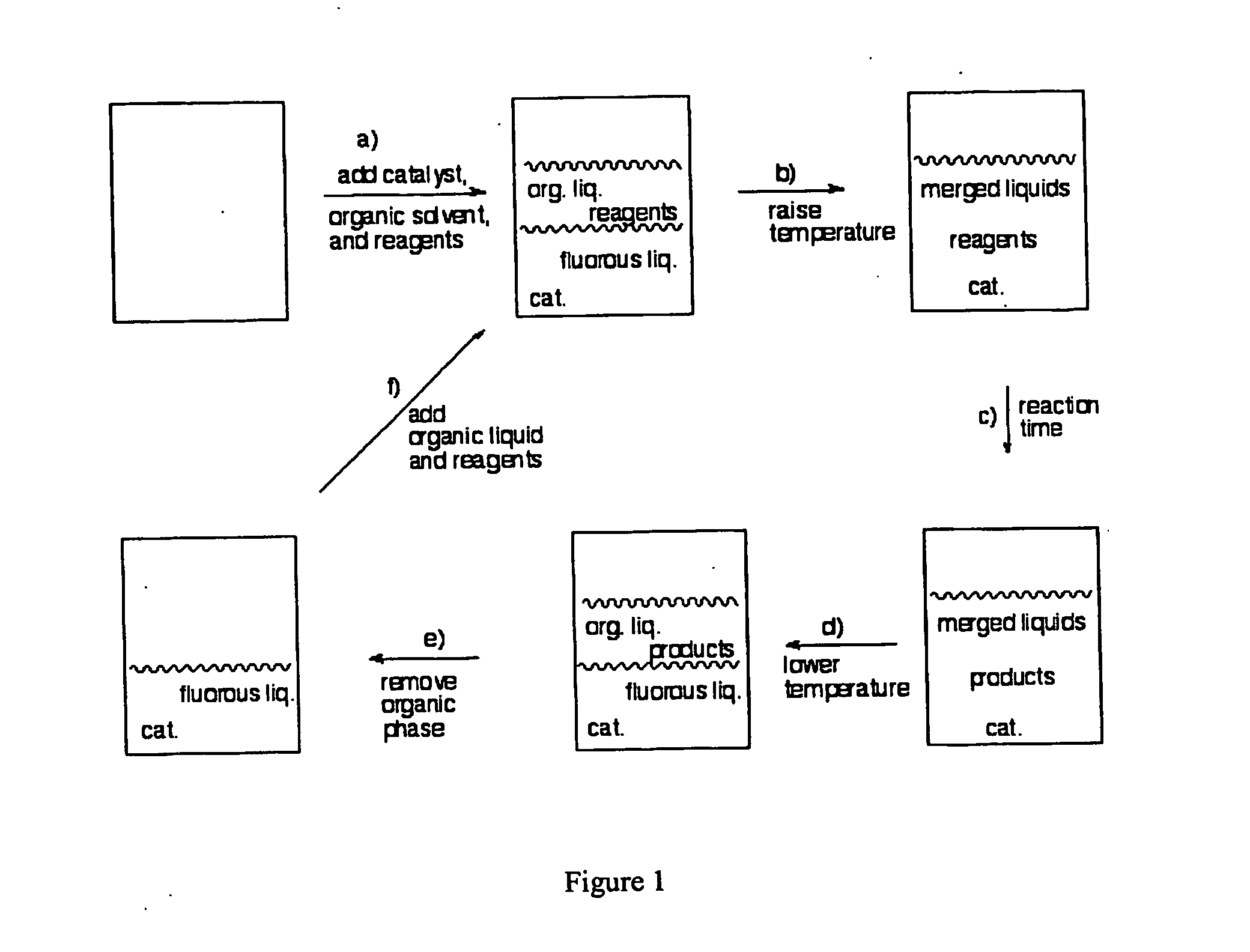
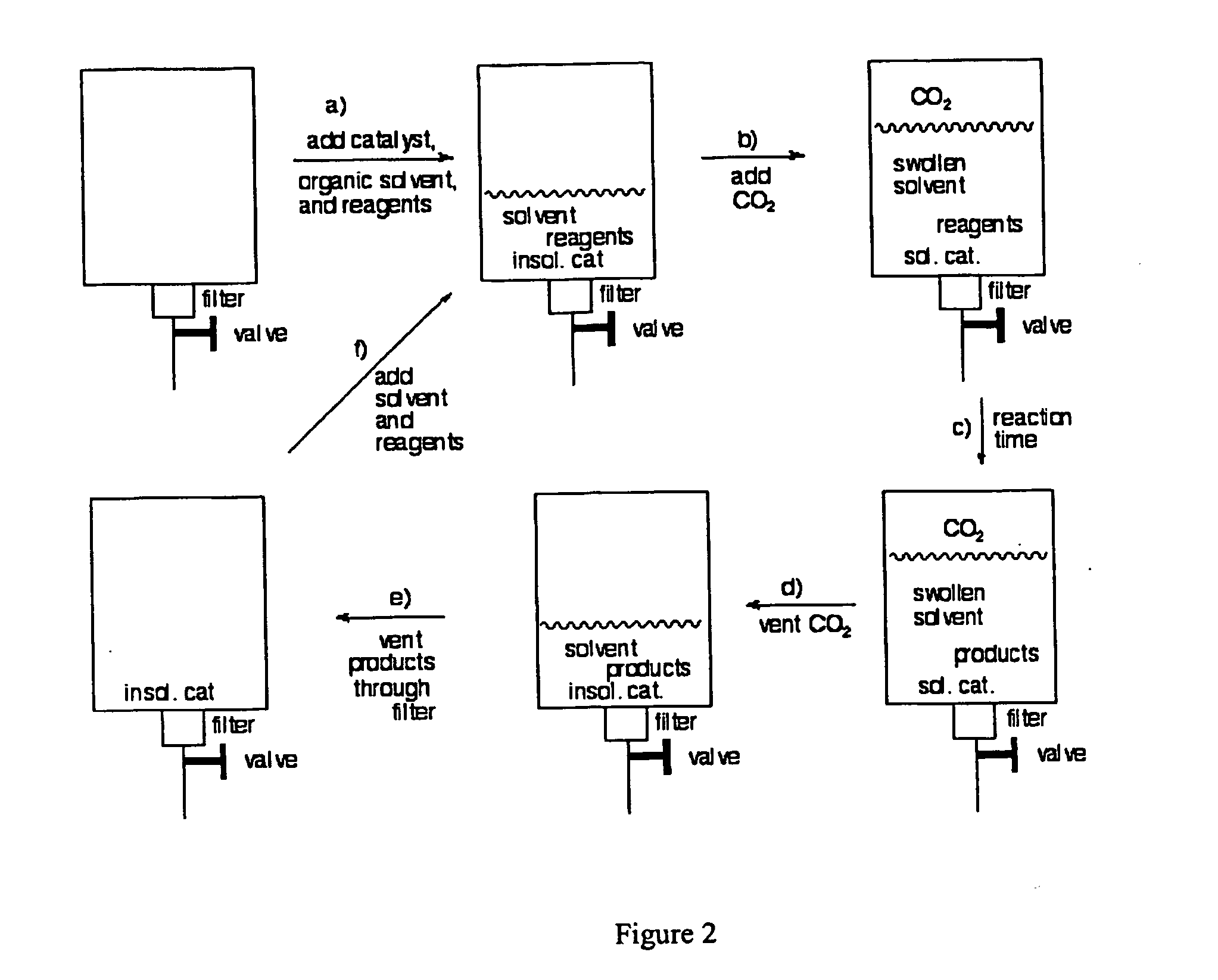
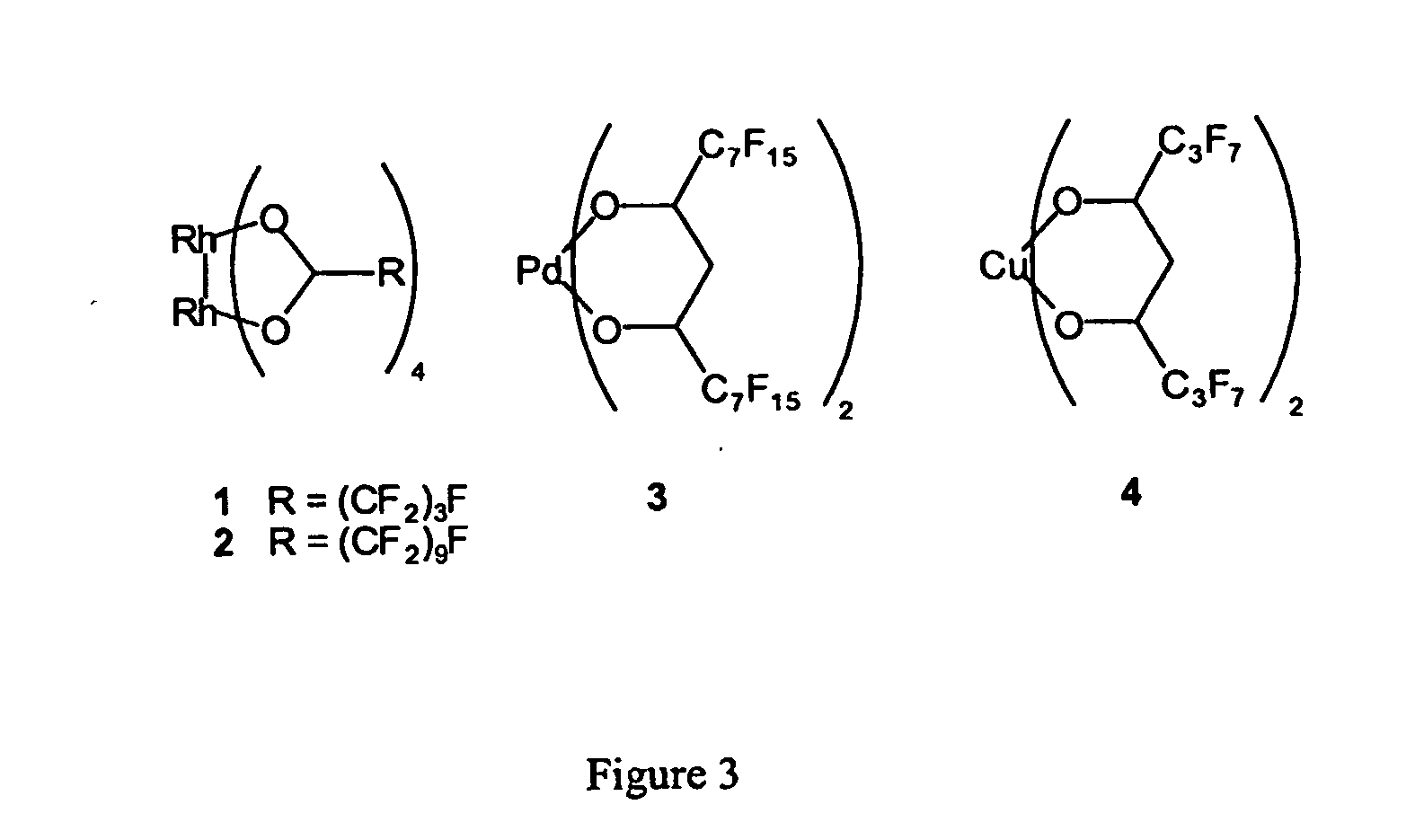
InactiveUS20050015936A1Reducing carbon dioxide gaseous pressureEffective recoveryPlatinum group organic compoundsMagnesium halidesSolubilityOrganic solvent
Methods of enhancing the solubility of a fluorinated compound in an organic solvent are provided. In one embodiment, carbon dioxide gas pressure is applied to the solvent at a pressure effective to enhance the solubility of the fluorinated compound. The method may further include recrystallizing the fluorinated compound by reducing the pressure of the carbon dioxide gas. Also provided are methods of conducting a reaction using a fluorinated compound in an organic solvent In one embodiment, the method comprises applying carbon dioxide pressure to an organic solvent comprising at least one substrate and a fluorinated catalyst, in an effective amount to solubilize the catalyst; and permitting the fluorinated catalyst to catalyze the reaction of the substrate to form a product. The catalyst is optionally separated from the reaction product and solvent after the reaction by the release of the pressure.
Owner:GEORGIA TECH RES CORP +1
Halide-containing stimulable phosphor precursor, halide-containing stimulable phosphor, radiation image conversion panel and production method thereof
InactiveUS20090121140A1Improve luminanceQuality improvementX-ray/infra-red processesLayered productsHalogenImaging quality
This invention relates to a halide-containing stimulable phosphor having improved moisture resistance and luminance, and a process for producing the same. The halide-containing stimulable phosphor is characterized in that, among elements constituting the outermost surface and inside of the phosphor, there is a difference in composition ratio of a halogen element between the outermost surface and the inside of the phosphor. A radiation image conversion panel, which has been improved, for example, in moisture resistance, luminance and image quality by using the phosphor, and a process for producing the same are also provided.
Owner:KONICA MINOLTA MEDICAL & GRAPHICS INC
Process for removal of pollutants
A process for the removal of pollutants from a combustion process and, more particularly, a process for removing pollutants such as carbon dioxide, mercury, sulphur dioxide, nitrogen compounds and oxygen compounds from a combustion process. The process includes the removal of pollutants from a combustion process that produces an emission comprising: cooling the emission to a temperature of about 200° C.; removing nitrogen, water and oxygen from the emission to produce a gas containing a concentration of pollutants; contacting the gas with an aqueous magnesium chloride solution, wherein a slurry mixture is formed; and cooling the gas and the slurry mixture, wherein hydrochloric acid vapour and a sludge are formed.
Owner:CLEAN WORLD STRATEGIES CORP
Snow and ice-melting granules and method for preparing same
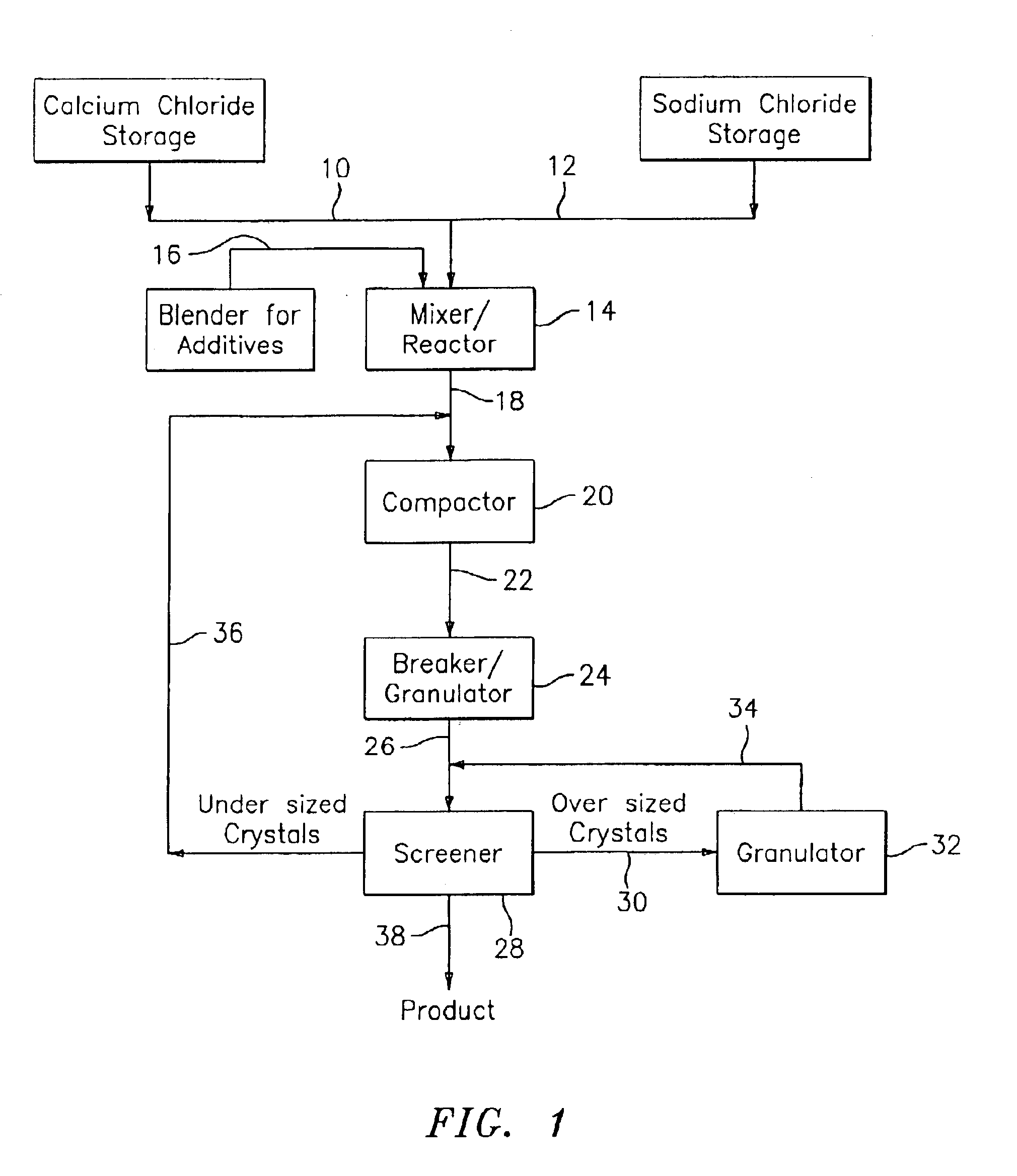
Snow and ice-melting granules prepared from compacted blends of salts of alkali and / or alkaline earth metals and a method for preparing such granules, are provided. The inventive granules have improved mechanical properties and, as such, are not readily reduced to a powder when subjected to mechanical loadings during transit and storage. In a preferred embodiment, the inventive granules employ one or more corrosion inhibitors homogeneously distributed throughout the granules.
Owner:GRO WELL BRANDS INC
Method for water sanitisation
InactiveUS20120267257A1Reduce decreaseGood choiceBiocideMagnesium chloridesAlkaline earth metalPotassium
The present invention provides a method for sanitising water in a swimming pool or the like, which method uses sources of ionic chlorine at significantly lower levels than conventional systems. The method comprises the steps of (i) forming, in the swimming pool water, an electrolyte solution containing from 500 ppm to 9000 ppm of a soluble magnesium halide salt, (ii) treating the electrolyte solution in an electrolytic halogenation cell to form an aqueous solution of hypohalous acid, and (iii) returning the water so treated back to the swimming pool. A mixture of magnesium, potassium and sodium chloride salts with small quantities of a soluble alkaline earth metal bromide, zinc halide, ascorbate, and / or zinc ascorbate may be particularly effective in the sanitisation process.
Owner:ZODIAC GROUP AUSTRALIA
Method and apparatus for treating exhaust gas
ActiveUS8420034B2Reduce the amount requiredCurb energy consumptionGas treatmentMagnesium halidesAmmoniaMetal
Owner:MITSUBISHI HEAVY IND LTD
Processes for preparing highly pure lithium carbonate and other highly pure lithium containing compounds
ActiveUS20120100056A1Electrolysis componentsLithium organic compoundsLithium carbonateLithium hydroxide
Owner:TERRALITHIUM LLC
Method of cutting single crystals
ActiveUS20090283761A1Quality improvementImprove accuracyGrinding machine componentsNitrogen compoundsClassical mechanicsSingle crystal
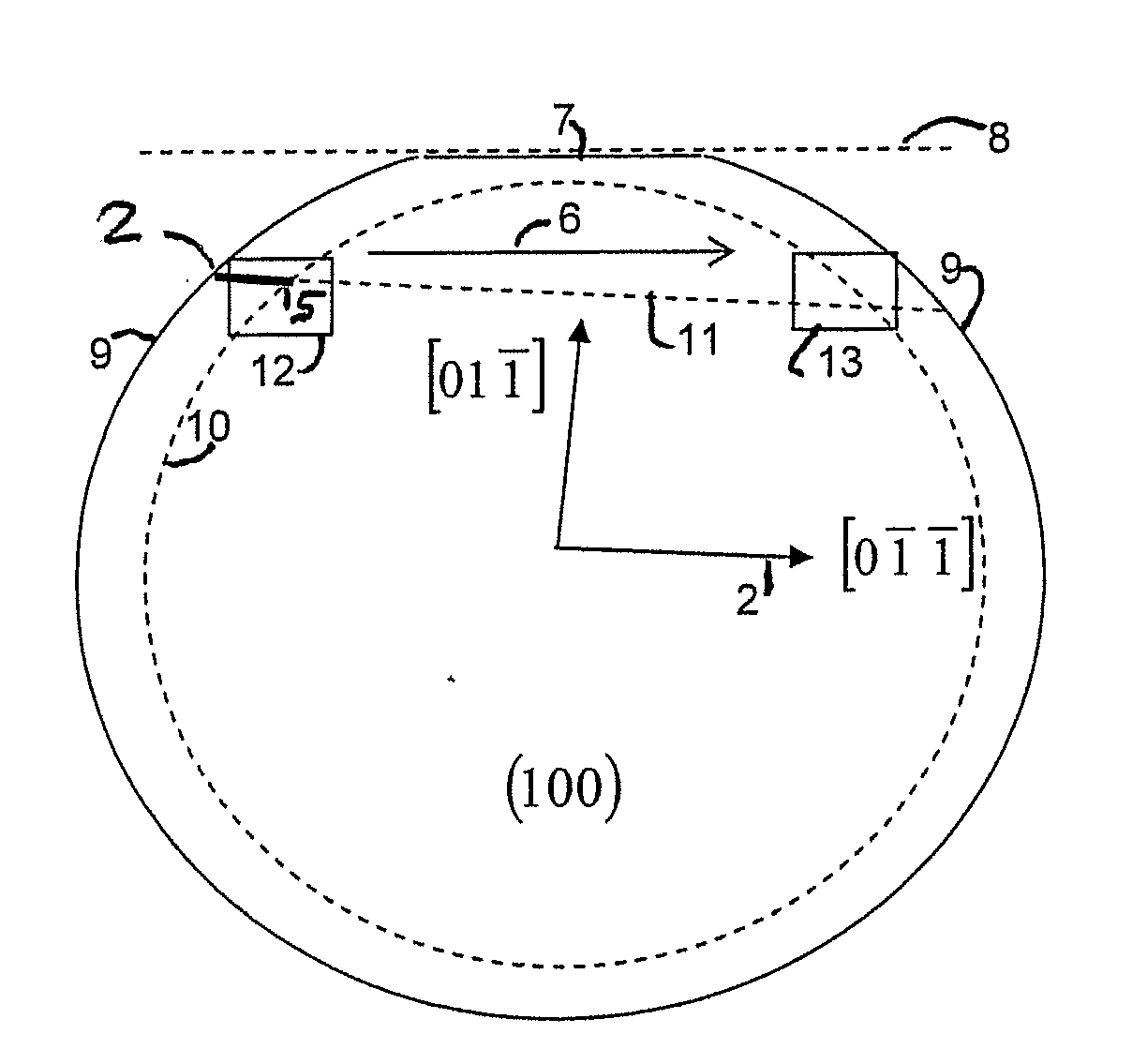
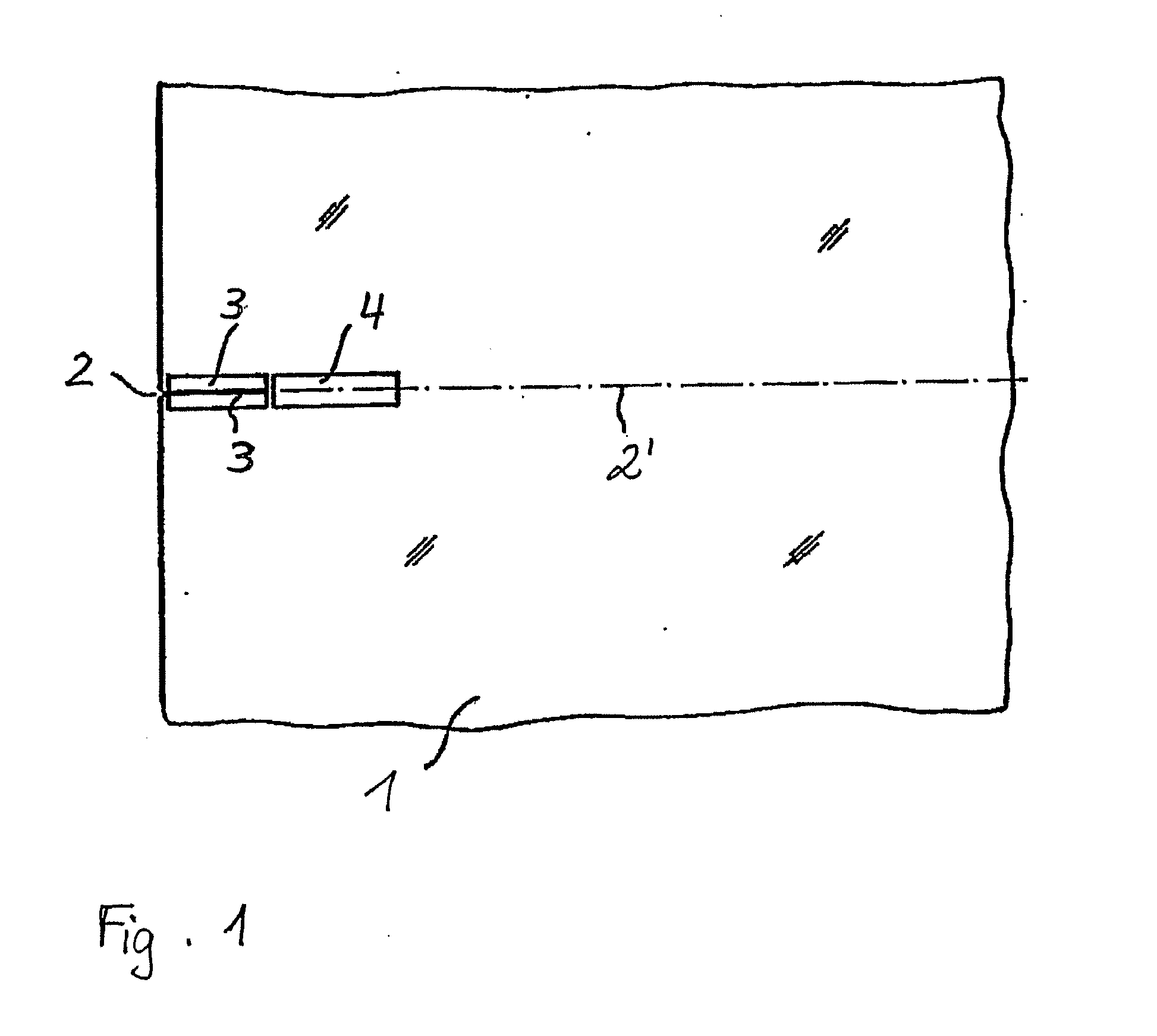
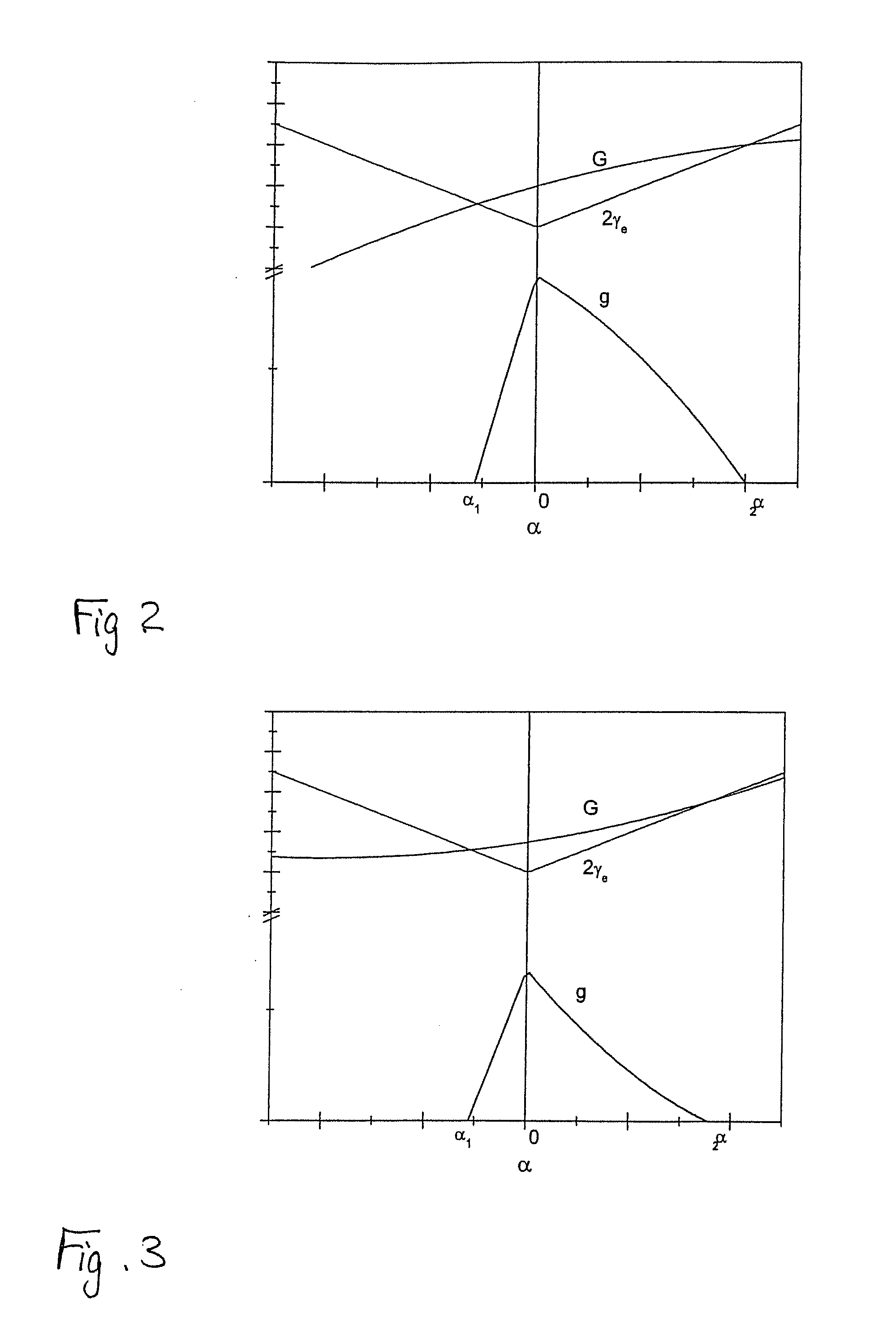
A method of dividing single crystals, particularly of plates of parts thereof, is proposed, which can comprise: pre-adjusting the crystallographic cleavage plane (2′) relative to the cleavage device, setting a tensional intensity (K) by means of tensional fields (3′, 4′), determining an energy release rate G(α) in dependence from a possible deflection angle (α) from the cleavage plane (2′) upon crack propagation, controlling the tensional fields (3′, 4′) such that the crack further propagates in the single crystal, wherein G(0)≧2γe(0) and simultaneously at least one of the following conditions is satisfied:∂G∂αα=0≤2βehif∂2G∂α2≤0or(2.1)∂G∂α≤2βeh∀α:α1<α<α2,(2.2)
Owner:FREIBERGER COMPOUND MATERIALS
Method for preparing high-purity anhydrous strontium iodide
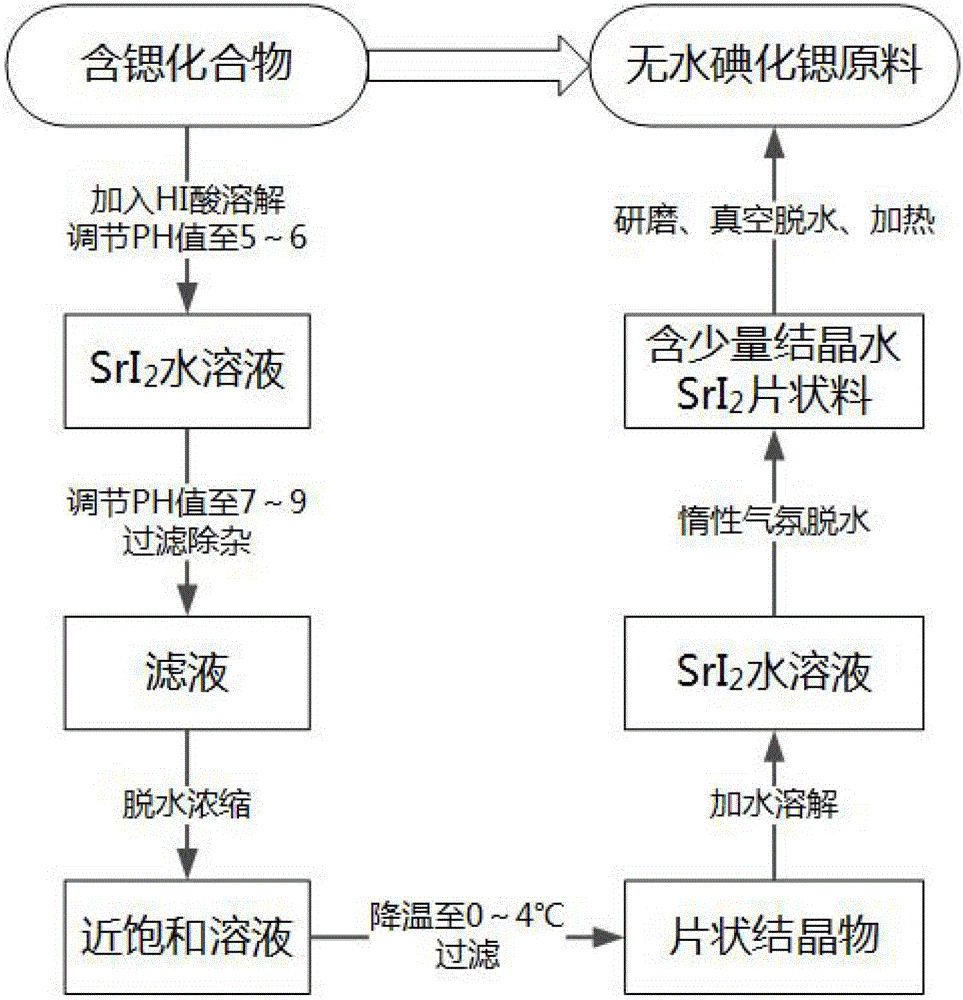
The invention relate to a method for preparing high-purity anhydrous strontium iodide. The method comprises the following steps: dropwise adding hydroiodic acid into a reaction vessel containing an aqueous solution containing a strontium compound under stirring until the pH of the solution is 5-6 to obtain an iodide-strontium aqueous solution; regulating the pH value of the iodide-strontium aqueous solution to 7-9 for filtering; after concentrating filtrate to near saturation, quickly lowering the temperature to 0-4 DEG C and precipitating crystal substances; filtering to obtain the crystal substances dissolving in water, and obtaining a purified iodide-strontium aqueous solution; heating the purified strontium iodide aqueous solution to 150-200 DEG C under the flowing inert shielding gas, and preserving heat for 1-30 hours to dehydrate to obtain a SrI2.xH2O white flurry material, wherein x is less than or equal to 1; grinding the white flurry material into powder in an anhydrous and oxygen-free inert atmosphere, and putting in a glass vessel; under vacuum, heating the glass vessel to 350-450 DEG C and preserving heat for 1-20 hours to remove crystal water in the powder, and naturally lowering the temperature to room temperature in the condition of keeping vacuum; heat-sealing the glass vessel in the condition of keeping vacuum.
Owner:SHANGHAI INST OF CERAMIC CHEM & TECH CHINESE ACAD OF SCI
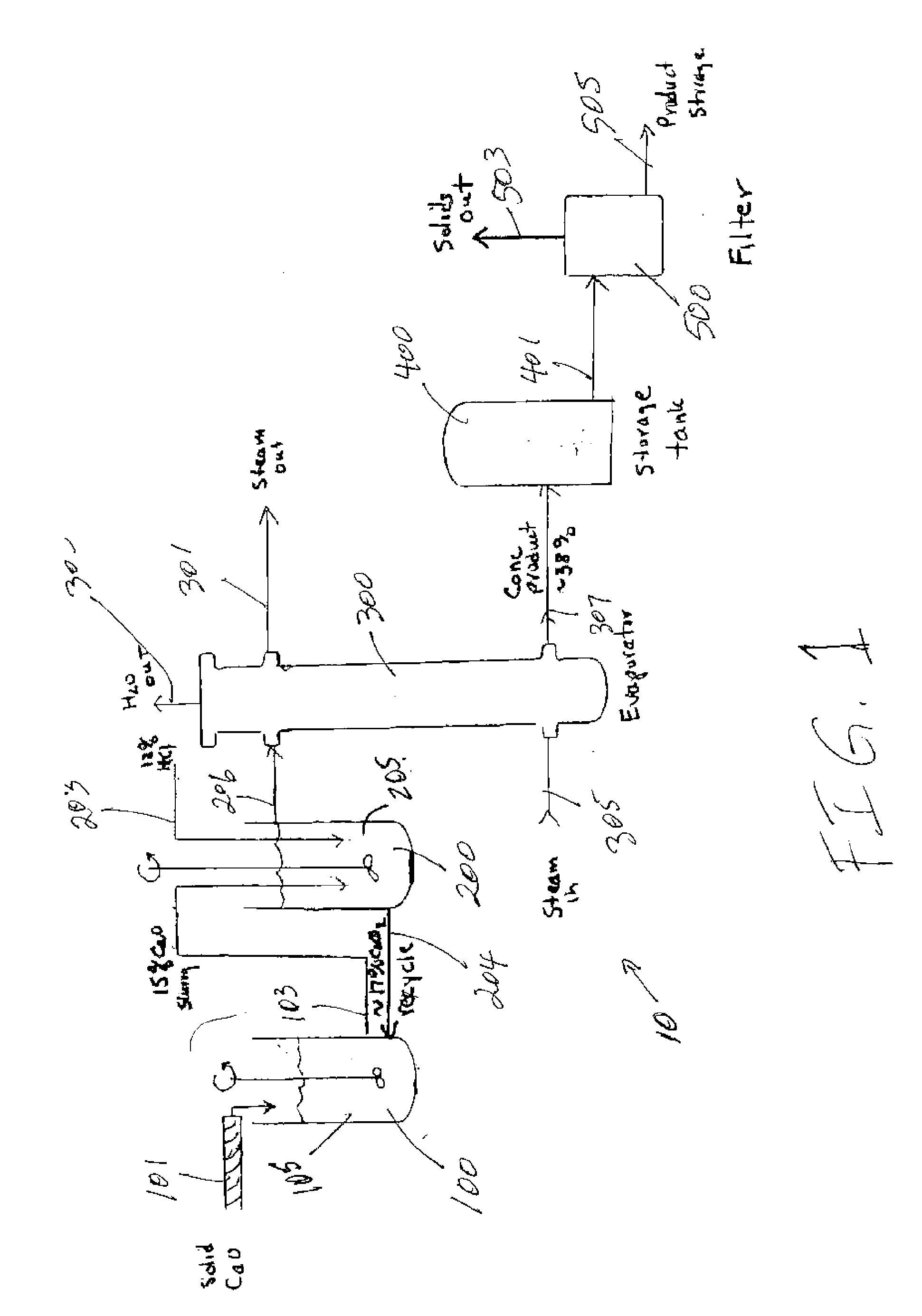
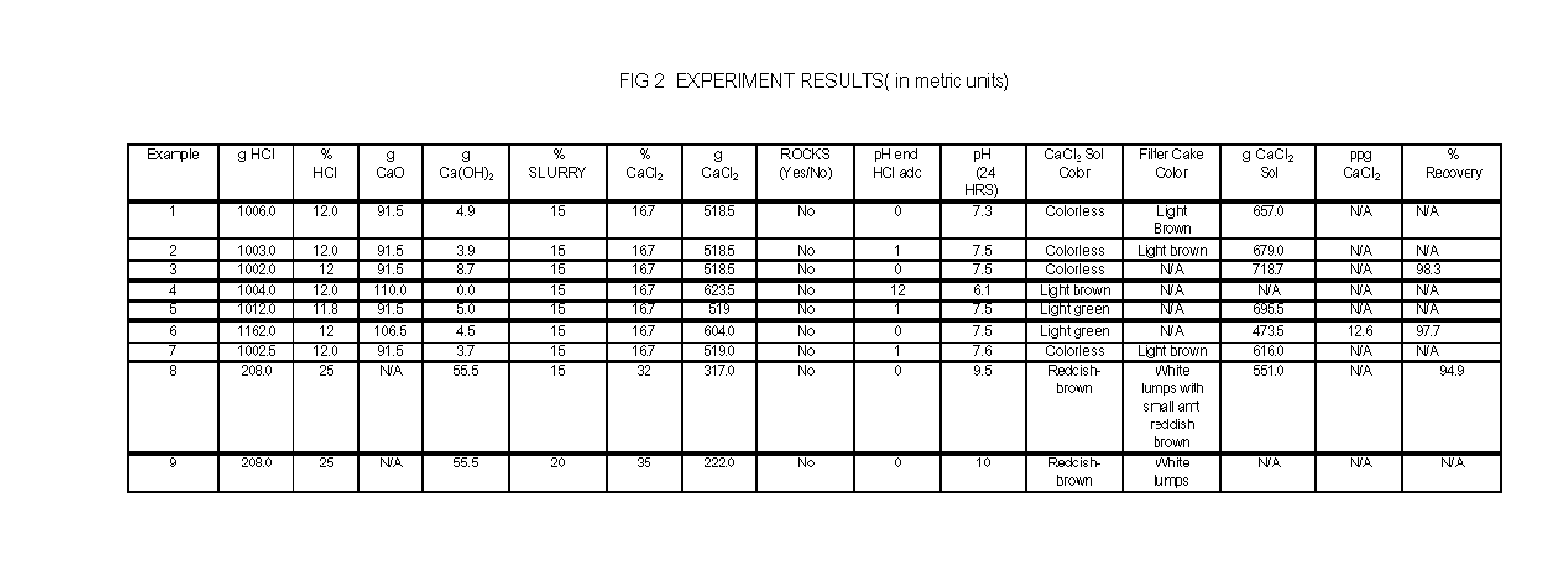
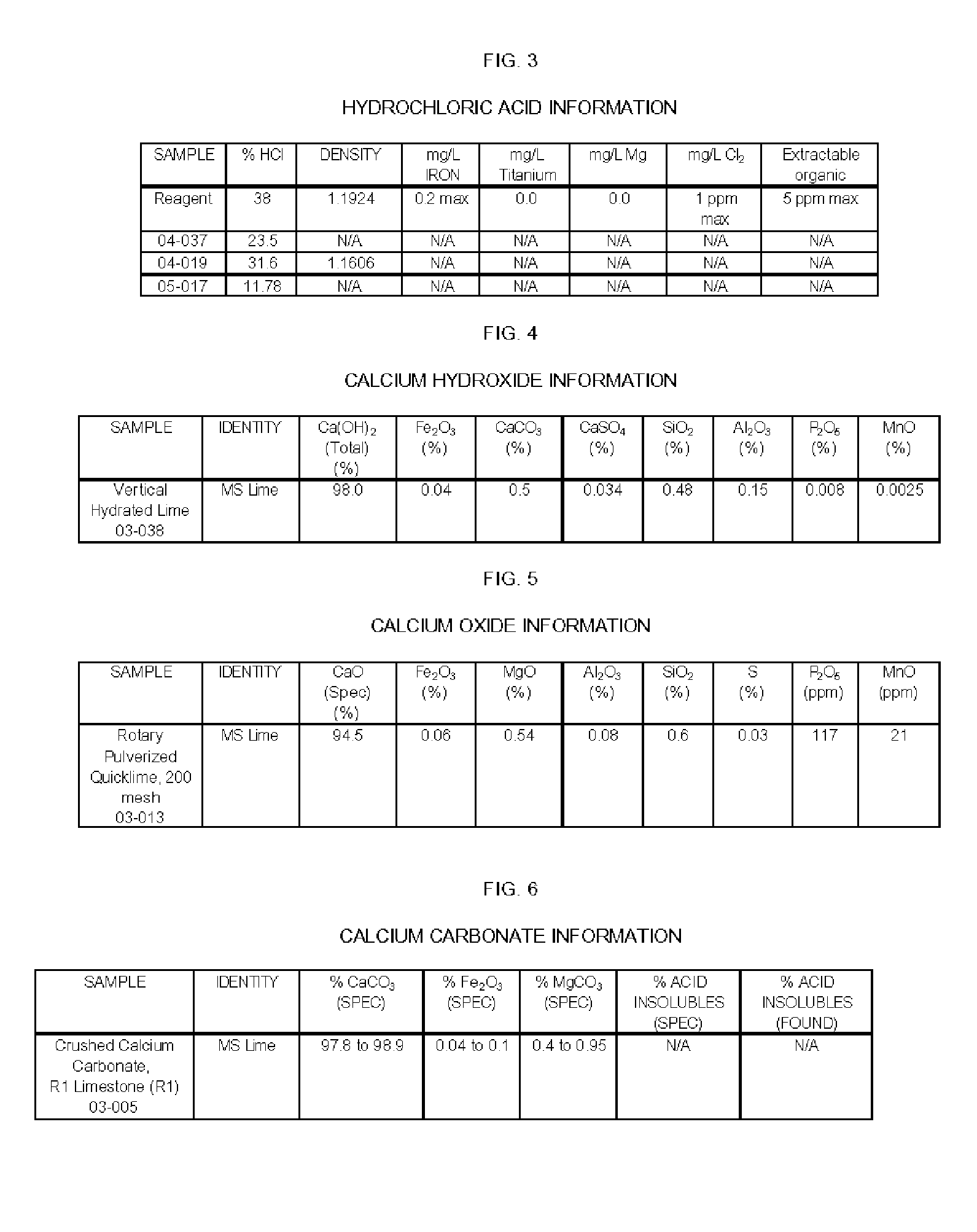
Apparatus and Methods For Producing Calcium Chloride, and Compositions and Products Made Therefrom
InactiveUS20070009423A1Reduce evaporative loadIncrease flow rateMagnesium halidesFerrous oxidesSlurryChloride
A method of producing calcium chloride including first forming a slurry of solid calcium oxide in an aqueous solution of calcium chloride, and then contacting the slurry with hydrochloric acid to convert at least a portion of the calcium oxide into calcium chloride.
Owner:ECO PROD SOLUTIONS LP
Ammonium chloride circulation method for producing magnesium hydroxide and calcium chloride from carbide slag and salt lake magnesium chloride
InactiveCN103011209AIncrease cycle rateSolve pollutionMagnesium hydroxideCalcium/strontium/barium halidesMagnesium saltSlag
The invention belongs to the field of waste reuse. Carbide slag is a waste generated in an acetylene production process using a calcium carbide method; and the main component is Ca(OH)2 the content of which exceeds 85%. The byproduct magnesium salt of the salt lake sylvite industry is mainly used for producing cheap magnesium chloride hexahydrate and magnesium sulfate heptahydrate, or is exported directly in a mineral form, causing waste of resources. According to the invention, high-purity magnesium hydroxide as well as anhydrous calcium chloride is produced by use of carbide slag and salt lake magnesium chloride by an ammonium chloride circulation method; and the method not only can obtain high-purity magnesium hydroxide and industrial-grade calcium chloride, but also can effectively solve the problem of environmental pollution caused by ammonia gas in magnesium hydroxide produced by an ammonia method. The study promotes energy conservation, emission reduction and low-carbon economy, and realizes great environmental benefits, social benefits and economic benefits.
Owner:但建明
Popular searches
Nitrogen-metal/silicon/boron binary compounds Semiconductor/solid-state device manufacturing From melt solutions From frozen solutions Using seed in melt Eutectic material solidification Single crystal growth details Lithium compounds Electrolytic organic production Group 3/13 element organic compounds
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com