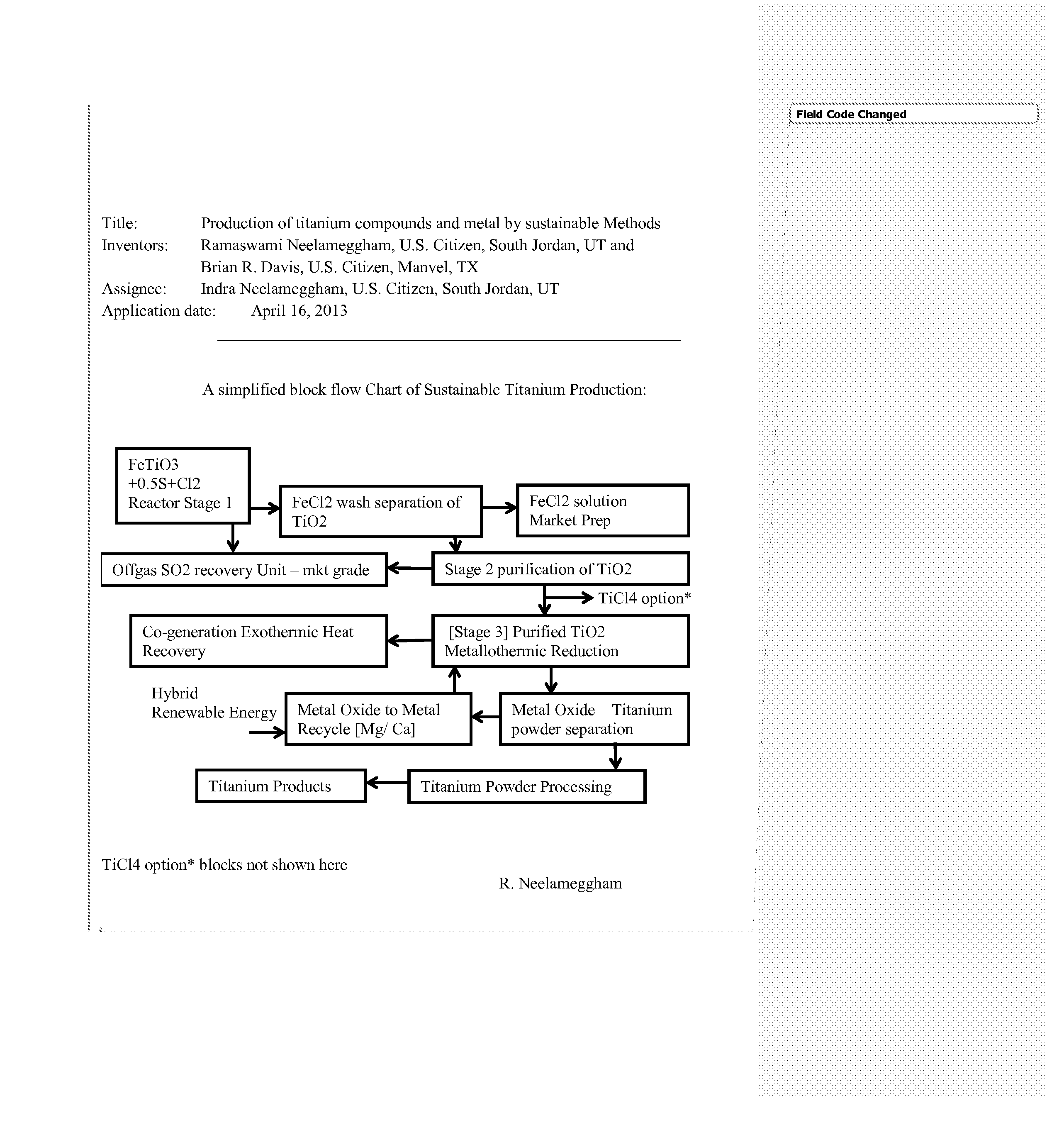
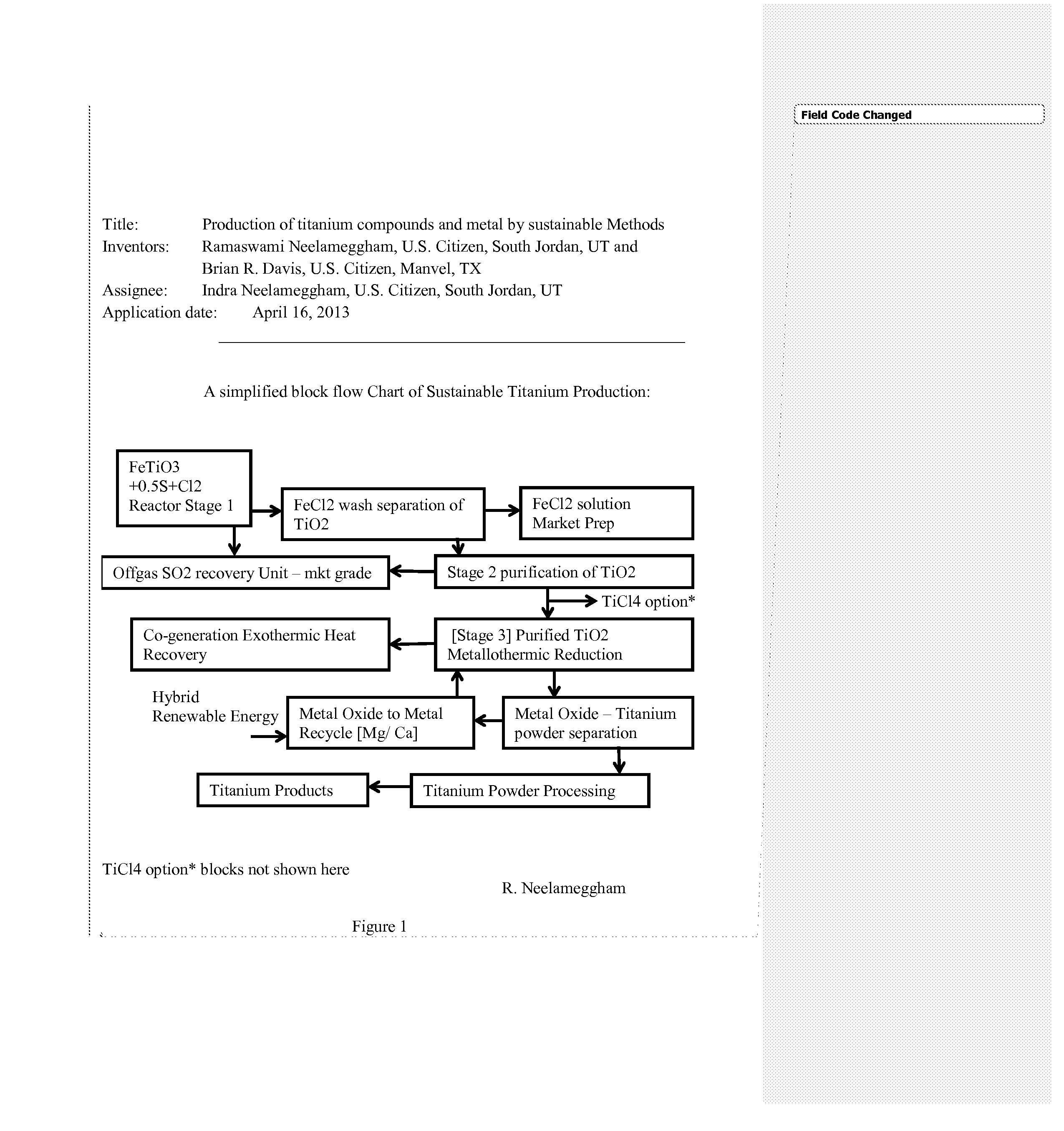
Production of titanium compounds and metal by sustainable Methods
a technology of titanium compounds and metals, applied in the direction of magnesium halide, furnace details, furnaces, etc., can solve the problem of not being considered as a sustainable process, and achieve the effect of low carbon dioxide footprint, low cost and low cos
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 2
[0024]In one embodiment of the present invention, it is converted to pure titanium tetrachloride in a third stage sulfo-chlorination reactor using one mole of sulfur per mole of titanium dioxide, and two moles of chlorine per mole of upgraded titanium dioxide. The titanium tetrachloride from the third reactor would have its own off-gas system to recover condensed TiCl4 and send the sulfur dioxide to sulfuric acid recovery plant. This makes a low carbon dioxide foot print and ‘a sustainable’ TiCl4
TiO2+S+2Cl2==TiCl4[v]+SO2 [g] [C]
TiCl4[v][condensing scrubber→]TiCl4[1] [D]
SO2[g]→Sulfuric acid [E]
Reaction of chlorination TiO2 is endothermic, added heat if necessary is supplied by co-burning sulfur with oxygen which produces sulfur dioxide which is another product handled by the process.
example 3
[0025]In another embodiment of the present invention, the second stage pure titanium dioxide is converted to titanium metal by reaction with magnesium metal in a specially designed reactor where the temperature is controlled—in producing initially a mixture of titanium powder and magnesium oxide. The temperature is controlled in the 400 to 900° C. range and preferably around 500 to 600° C.
[0026]The mixture of titanium powder and magnesium oxide is first processed by slurrying in water, followed by flotation of magnesium hydroxide to a froth stream in flotation equipment such as a flotation column, while leaving the titanium powder to the heavier tail stream. The heavier tail stream titanium powder is analyzed for its purity from inclusions, and unremoved magnesium and titanium oxides. Following such analysis sufficient amount of a suitable acid is added avoiding dissolution of titanium powder. Then the powder is filtered, filter cake dried with heated gas inert to the powder—thus ma...
example 4
[0027]The process shown in example 3 is carried out using calcium instead of magnesium providing similar products.
PUM
| Property | Measurement | Unit |
|---|---|---|
| Temperature | aaaaa | aaaaa |
| Temperature | aaaaa | aaaaa |
| Temperature | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com