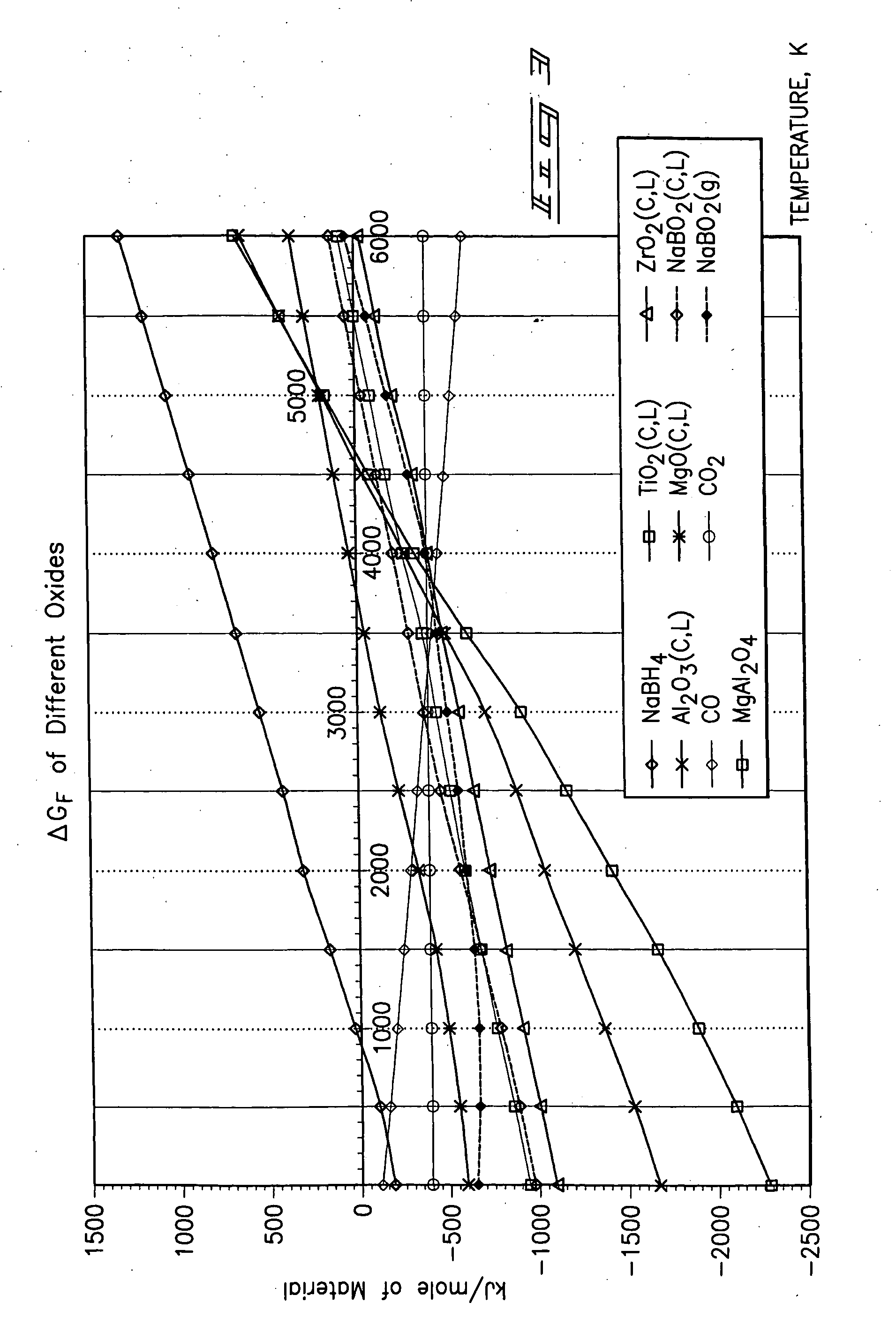
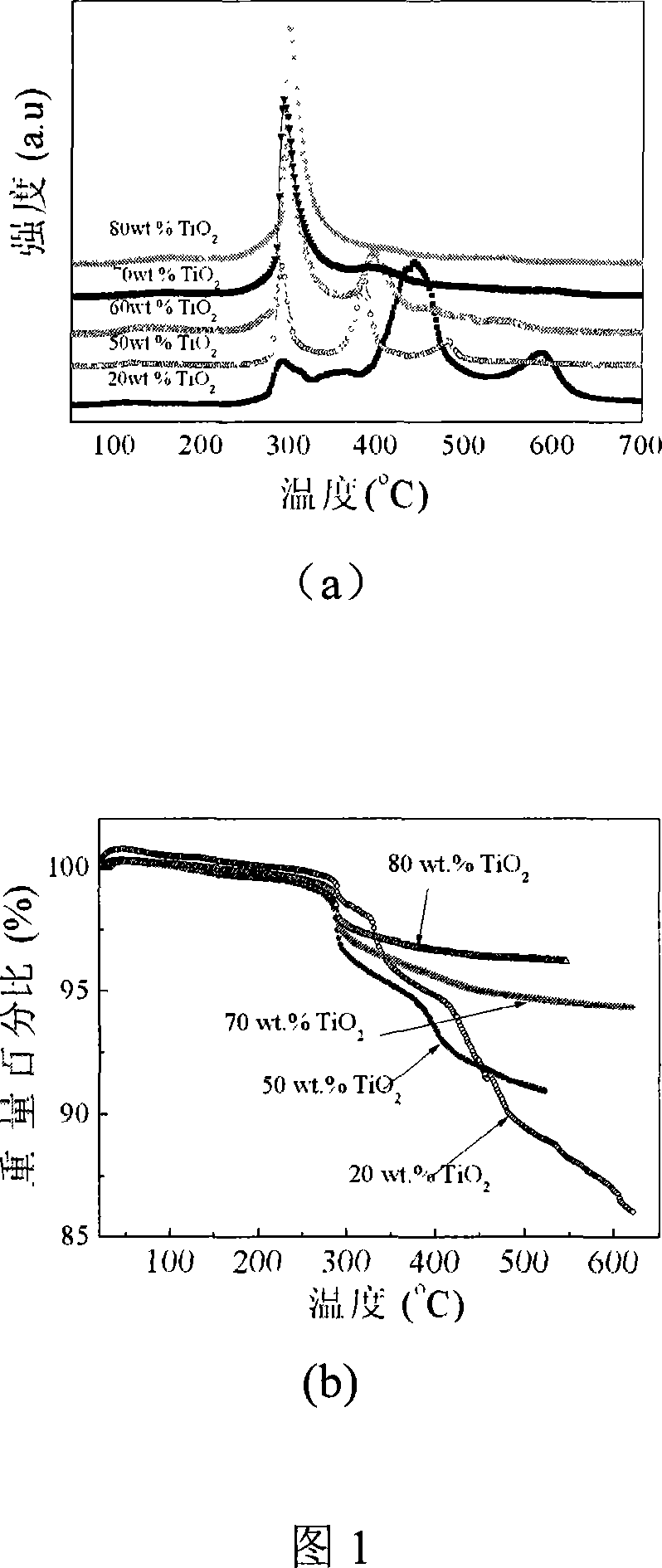
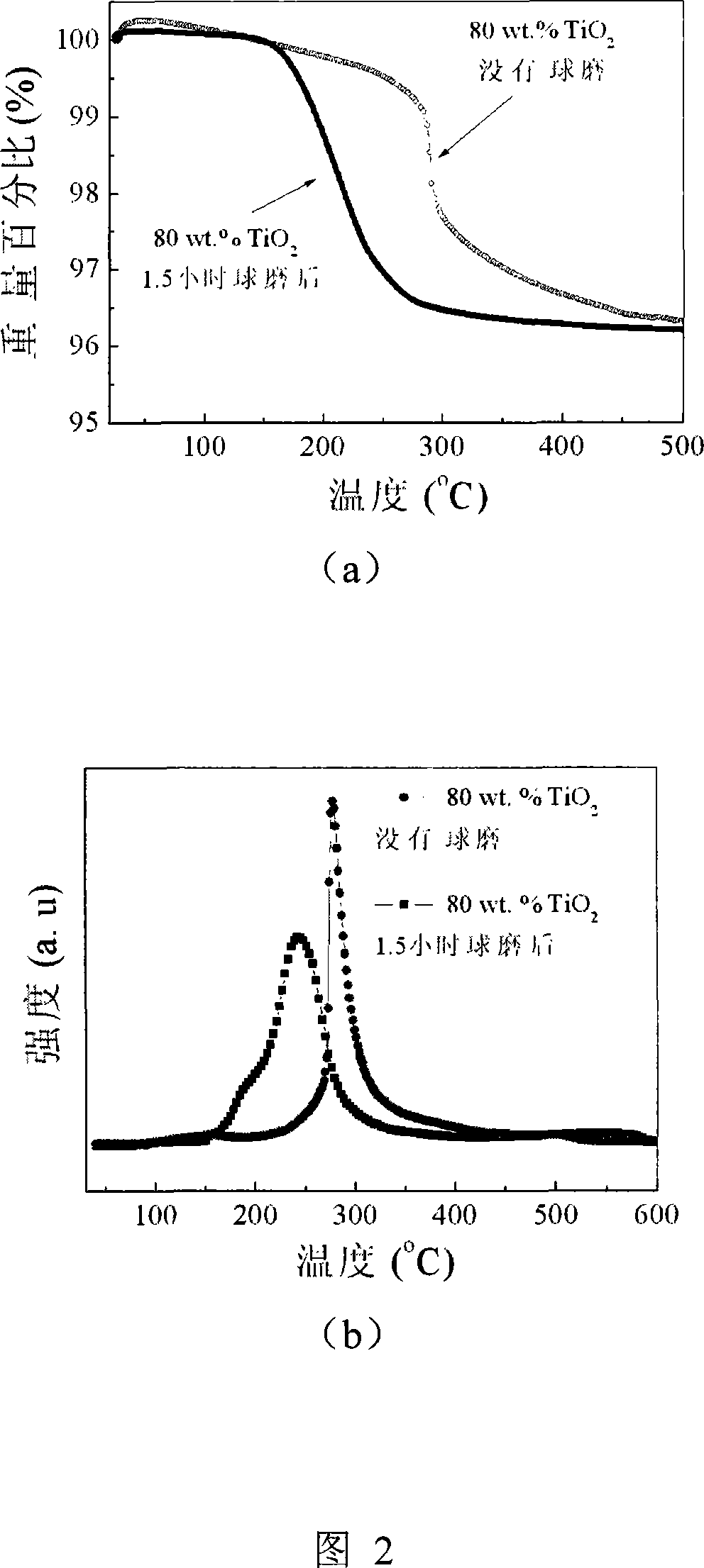
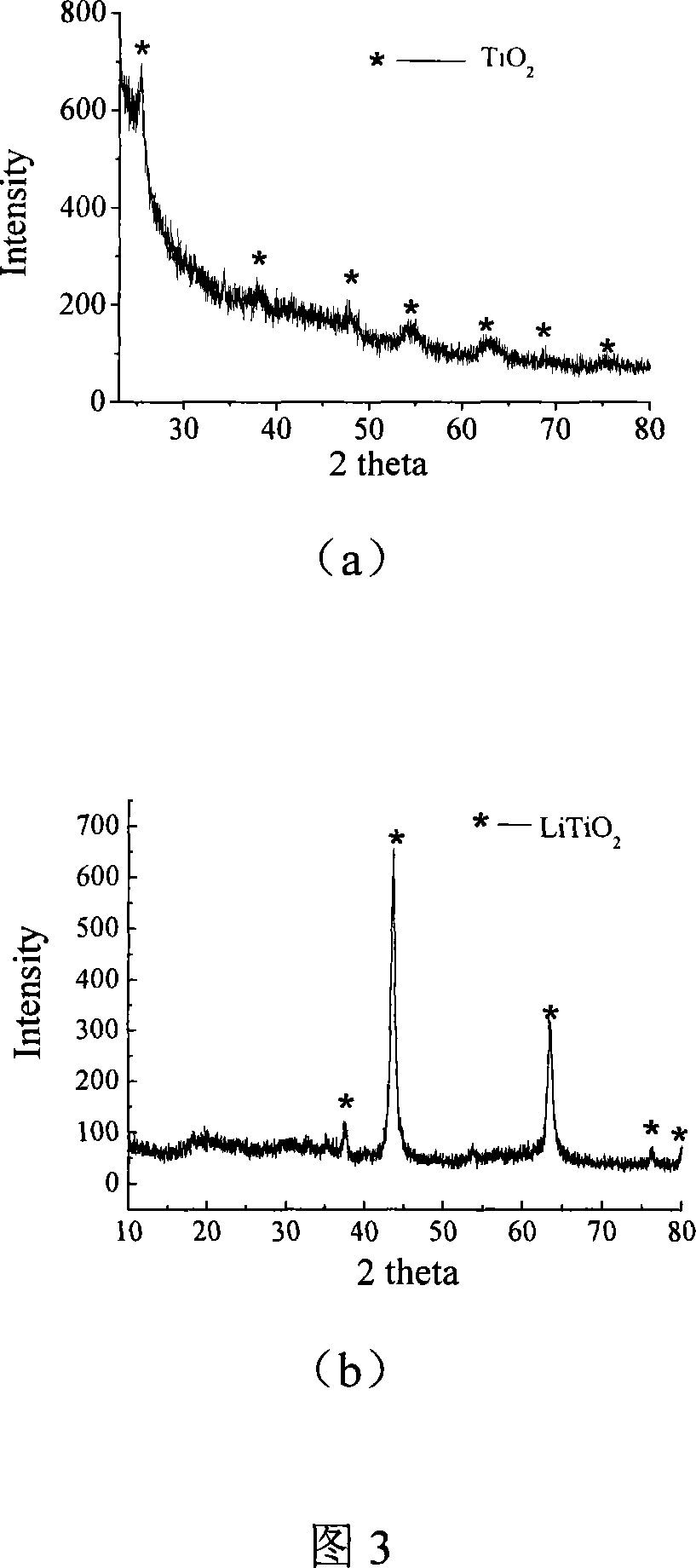
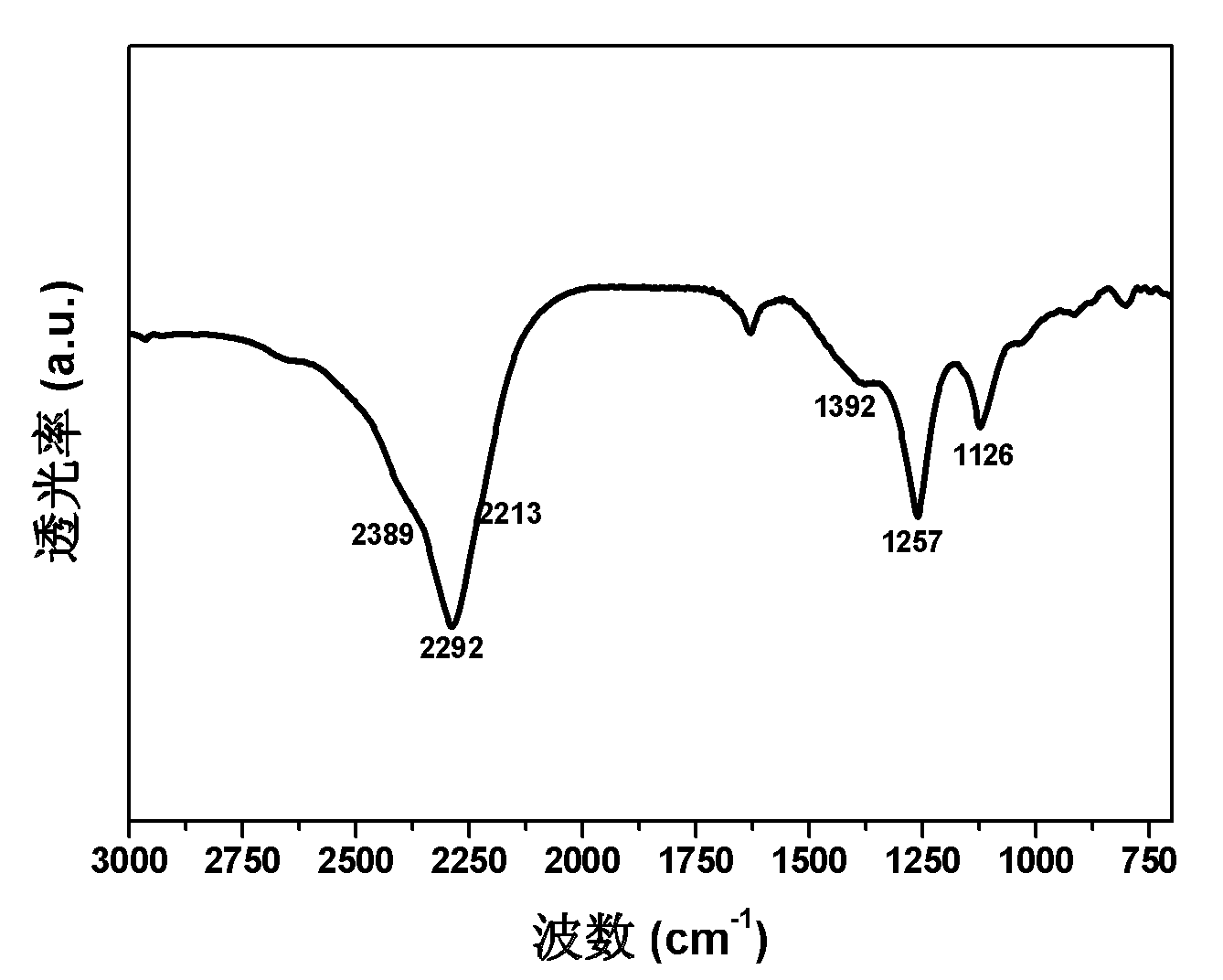
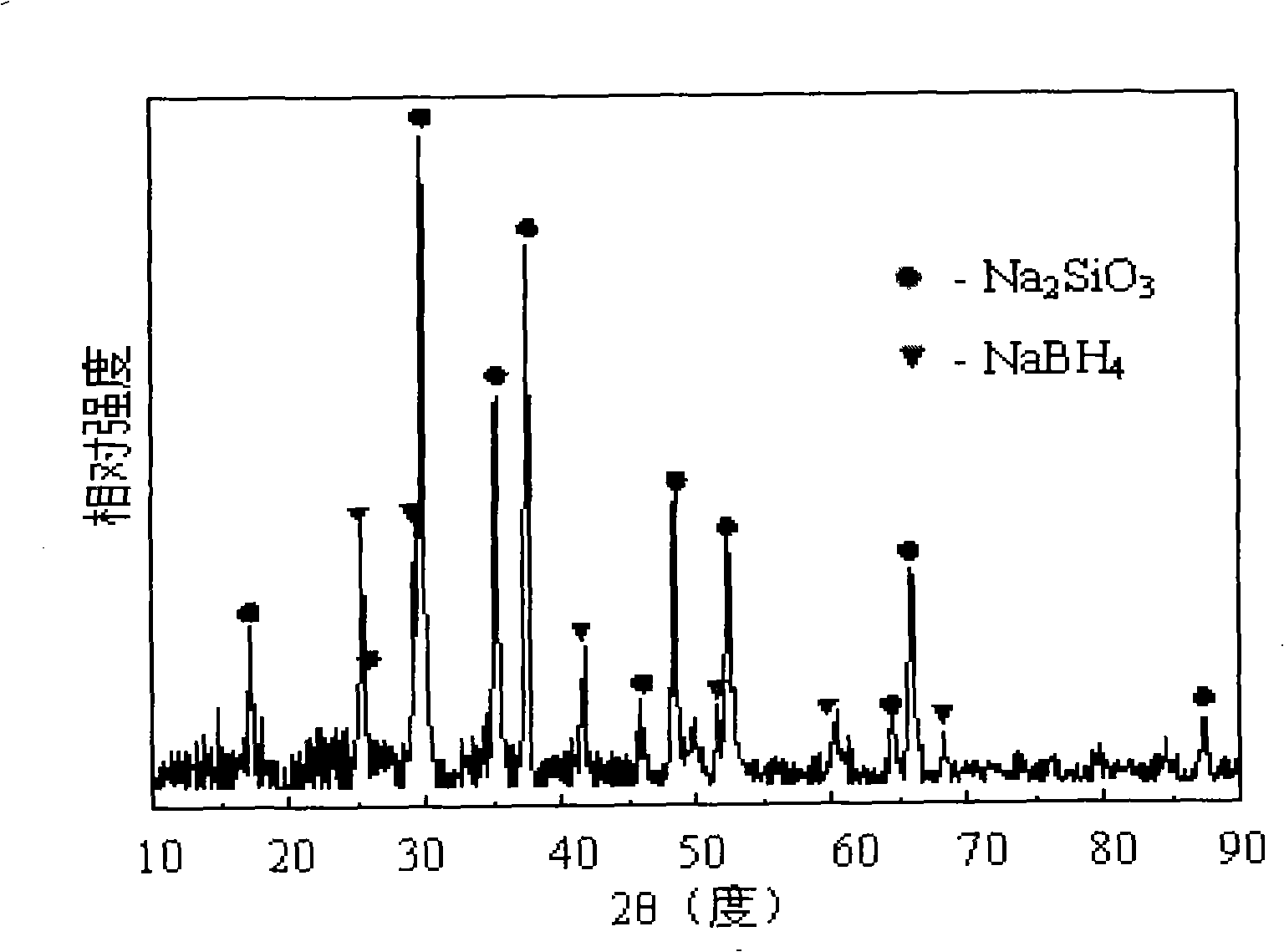
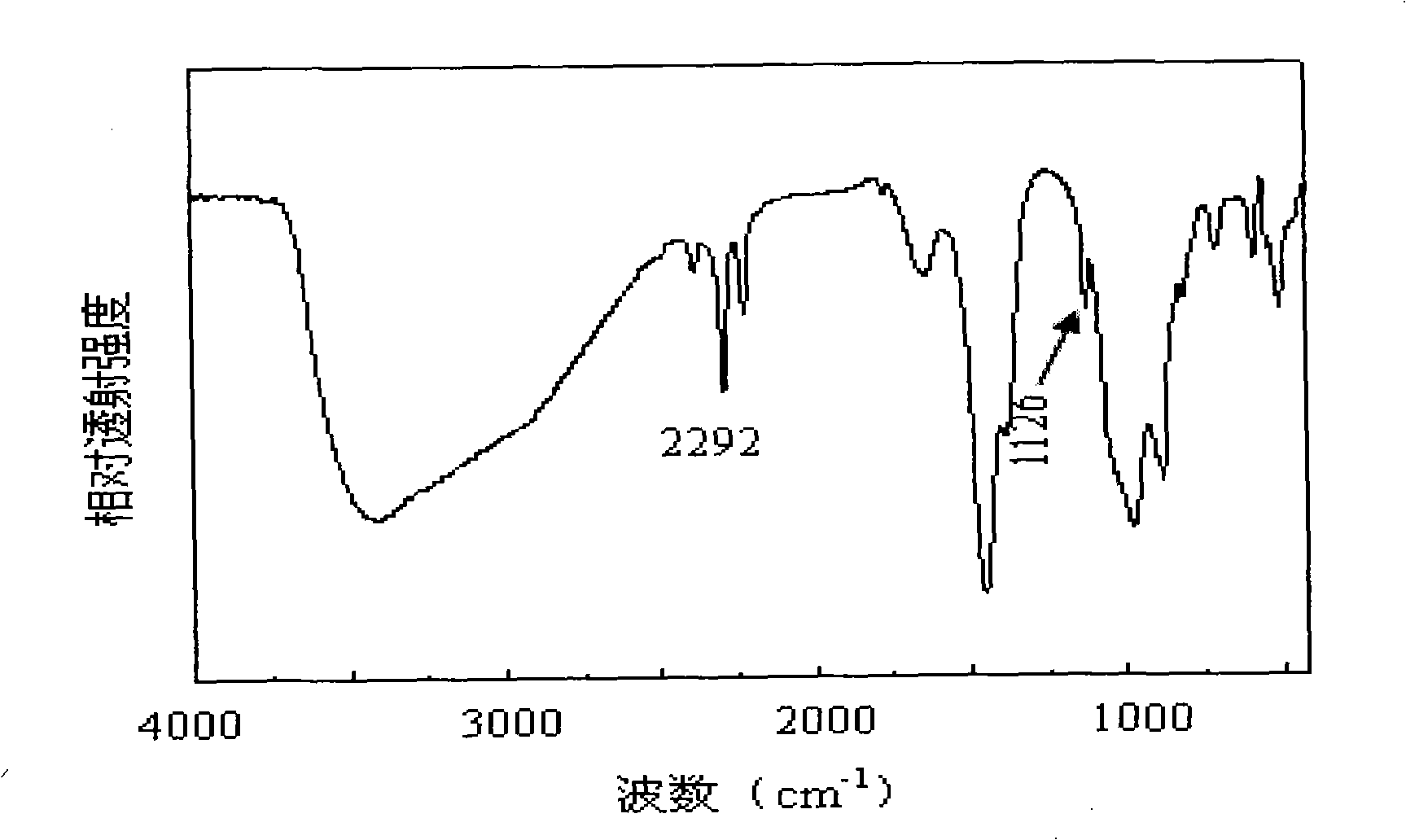
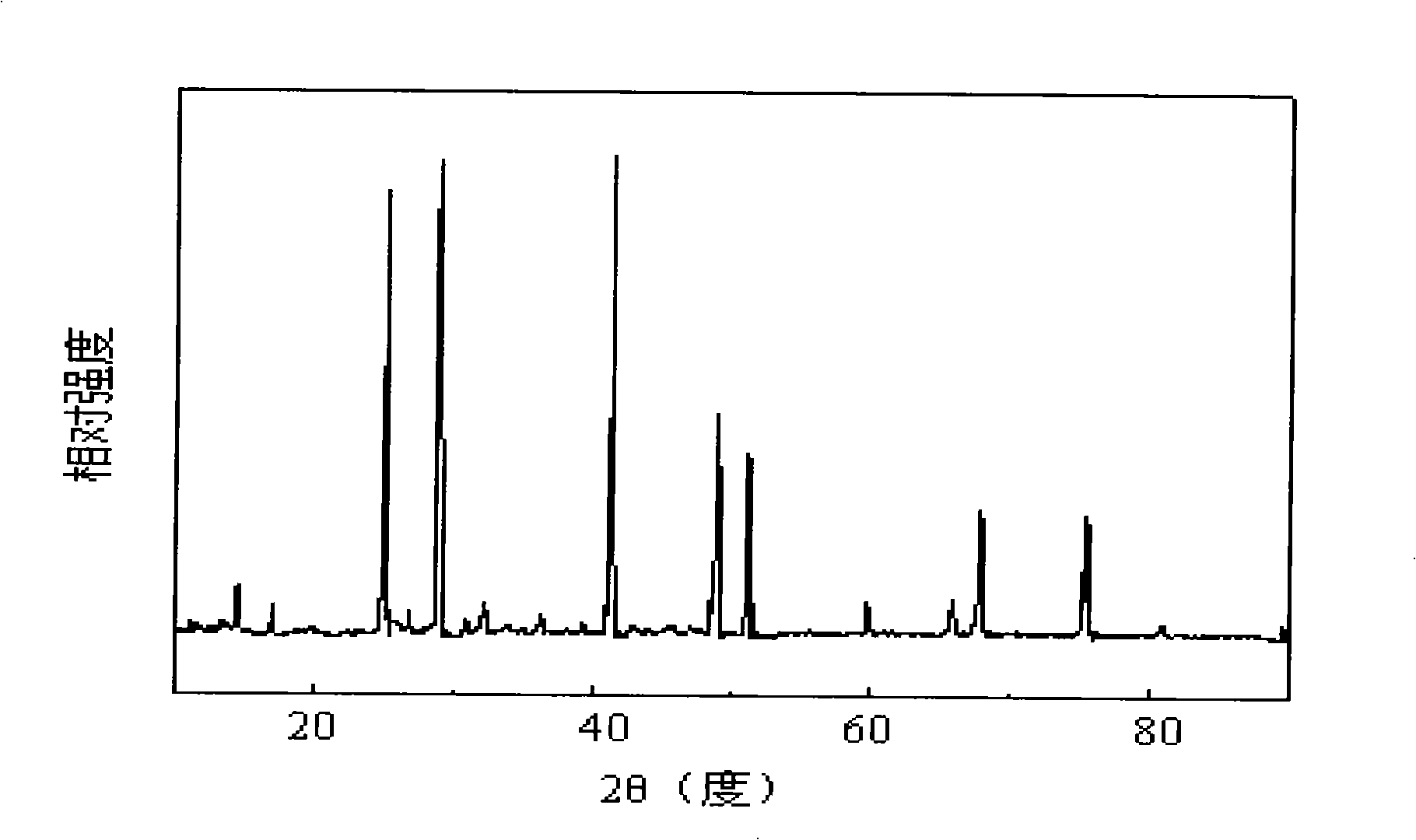
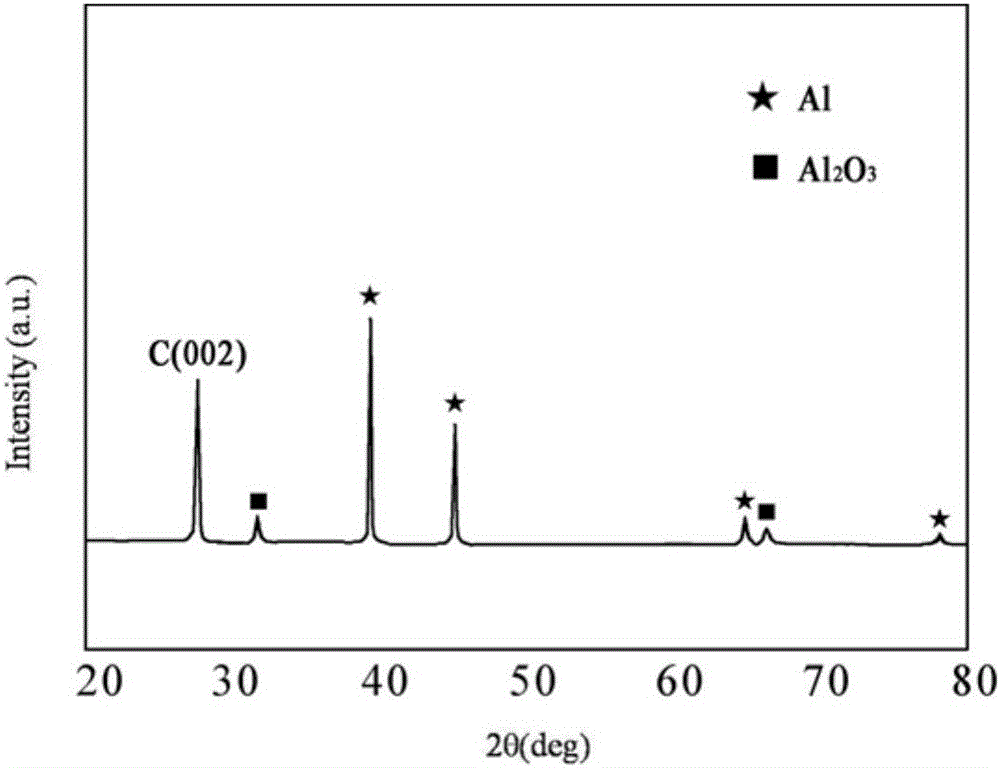
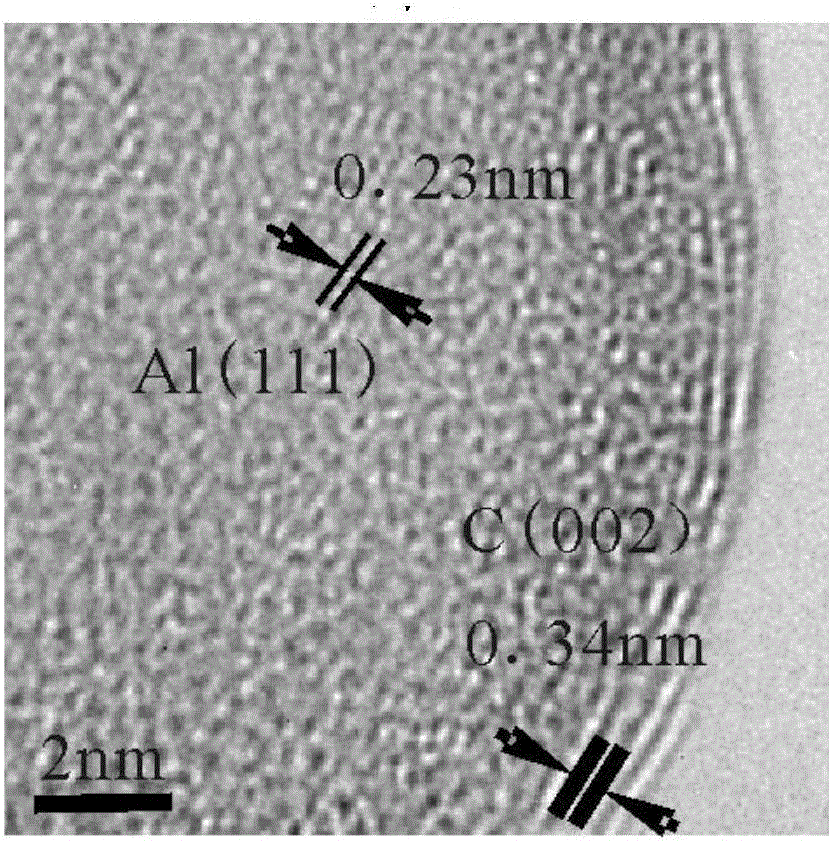
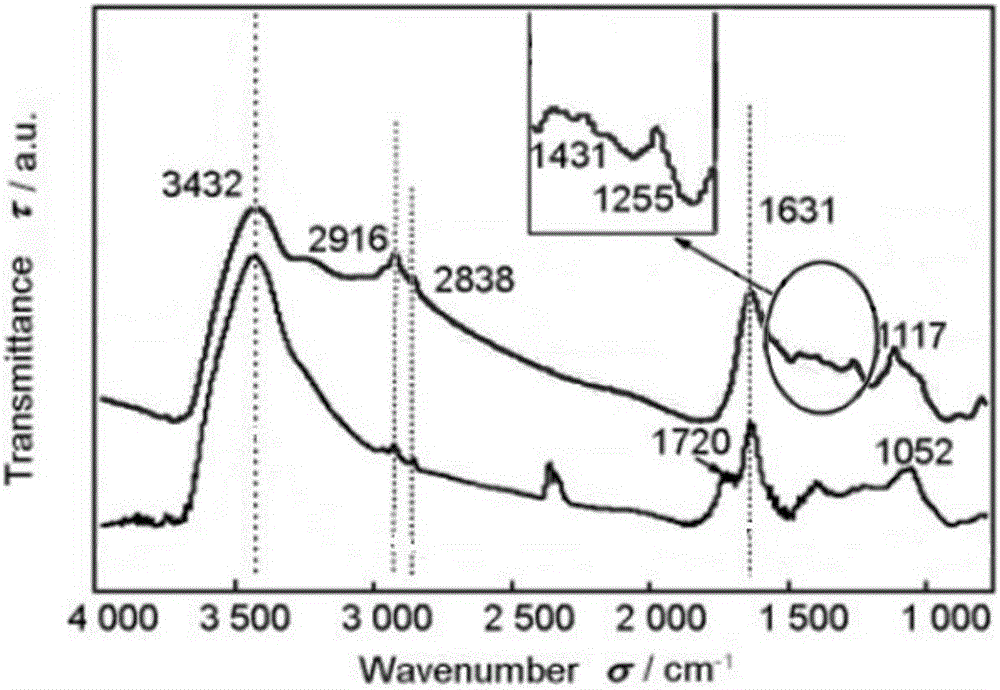
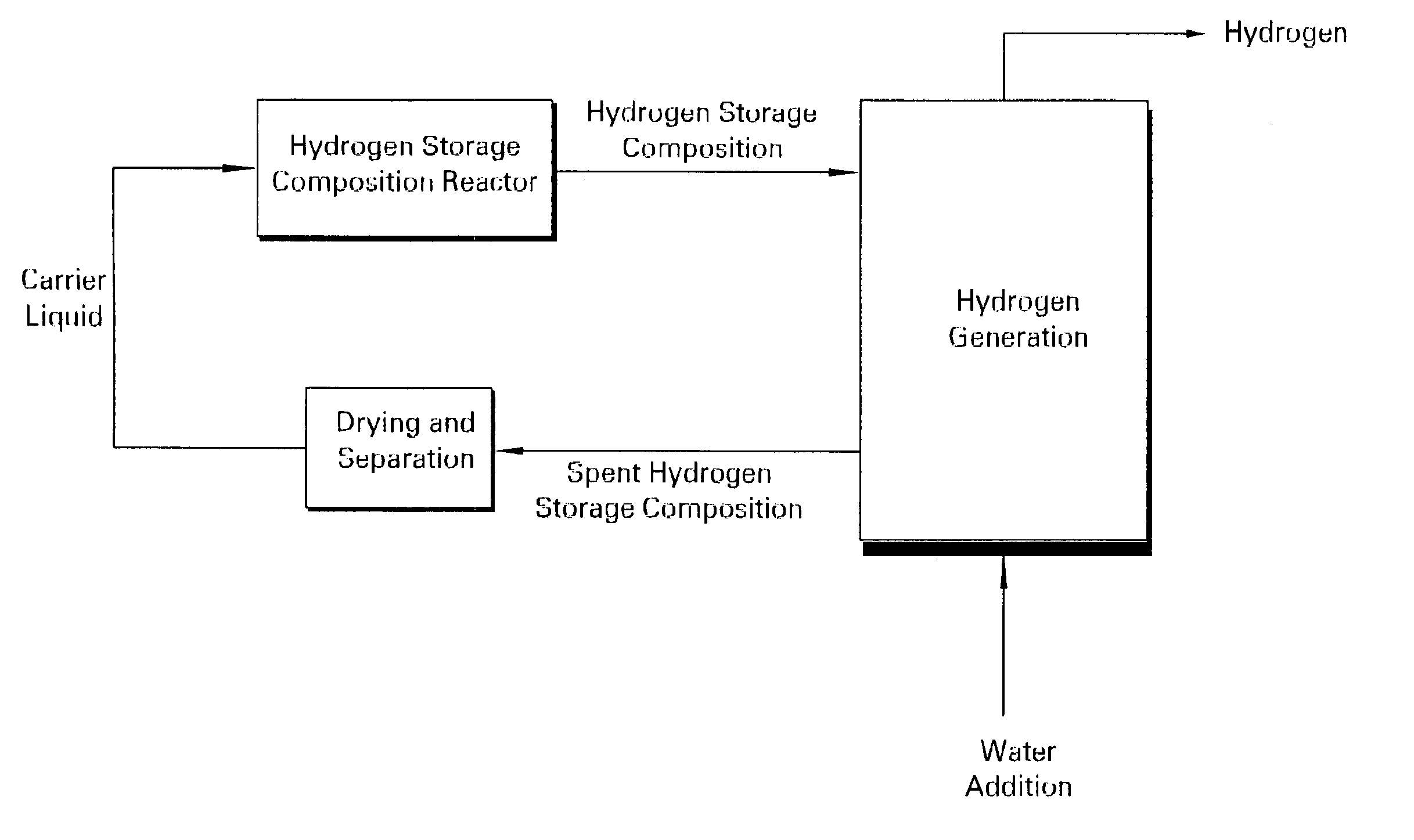
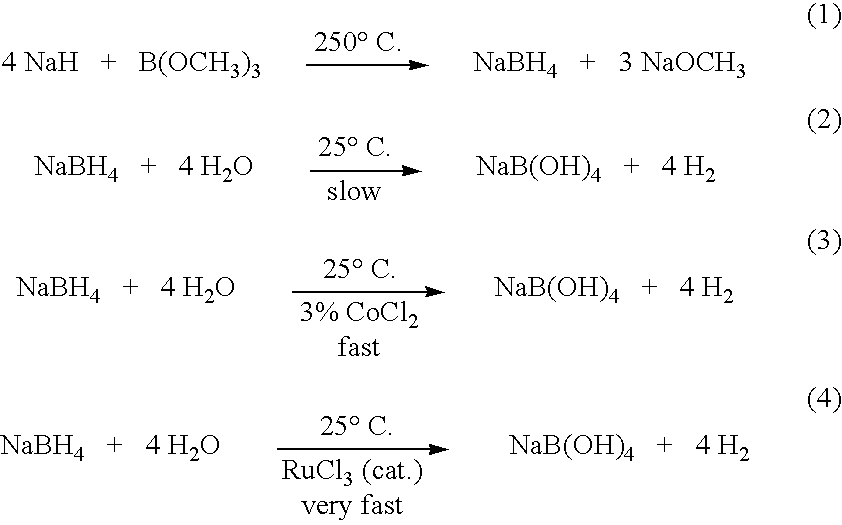Patents
Literature
259results about "Monoborane/diborane hydrides" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
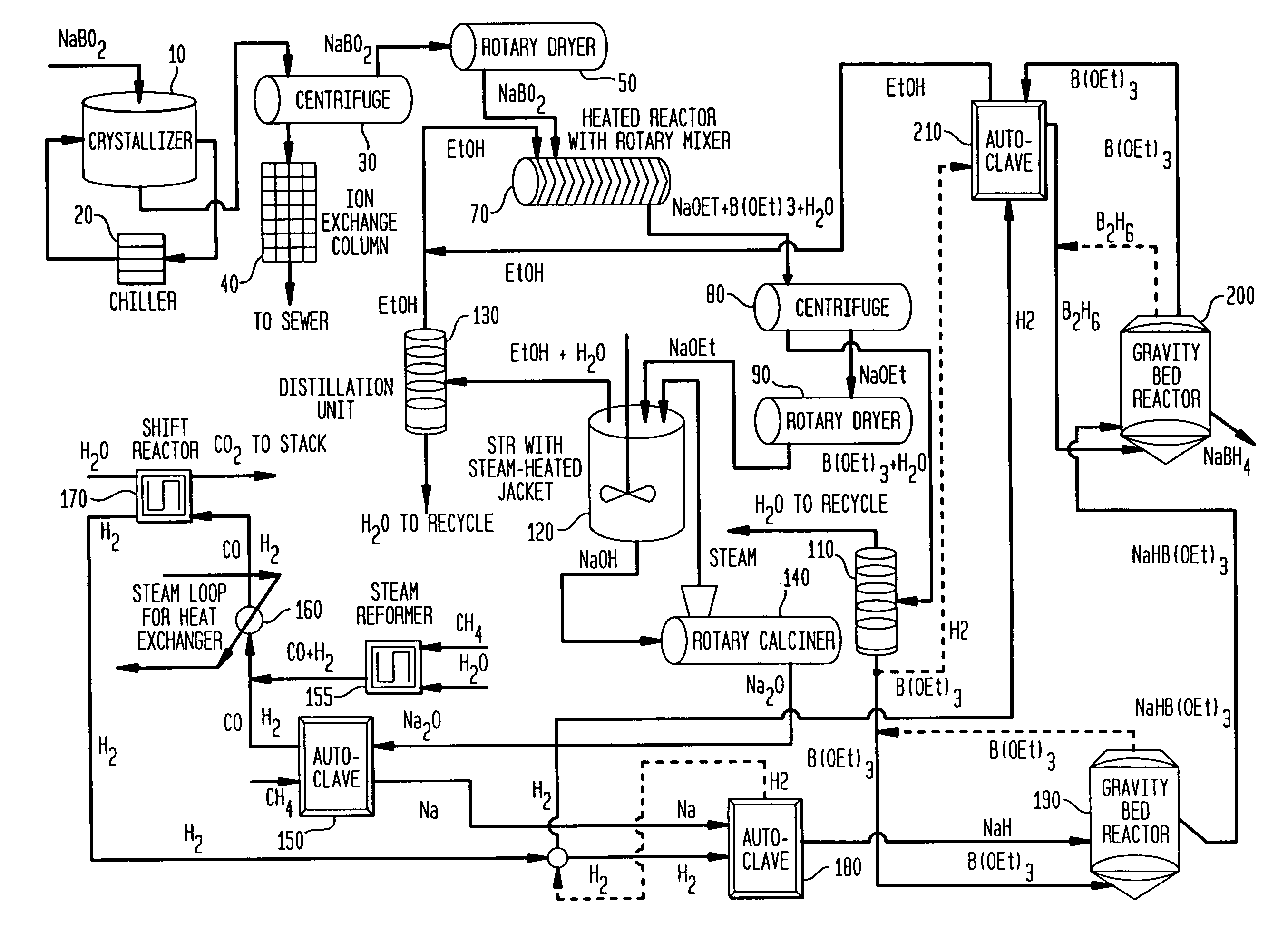
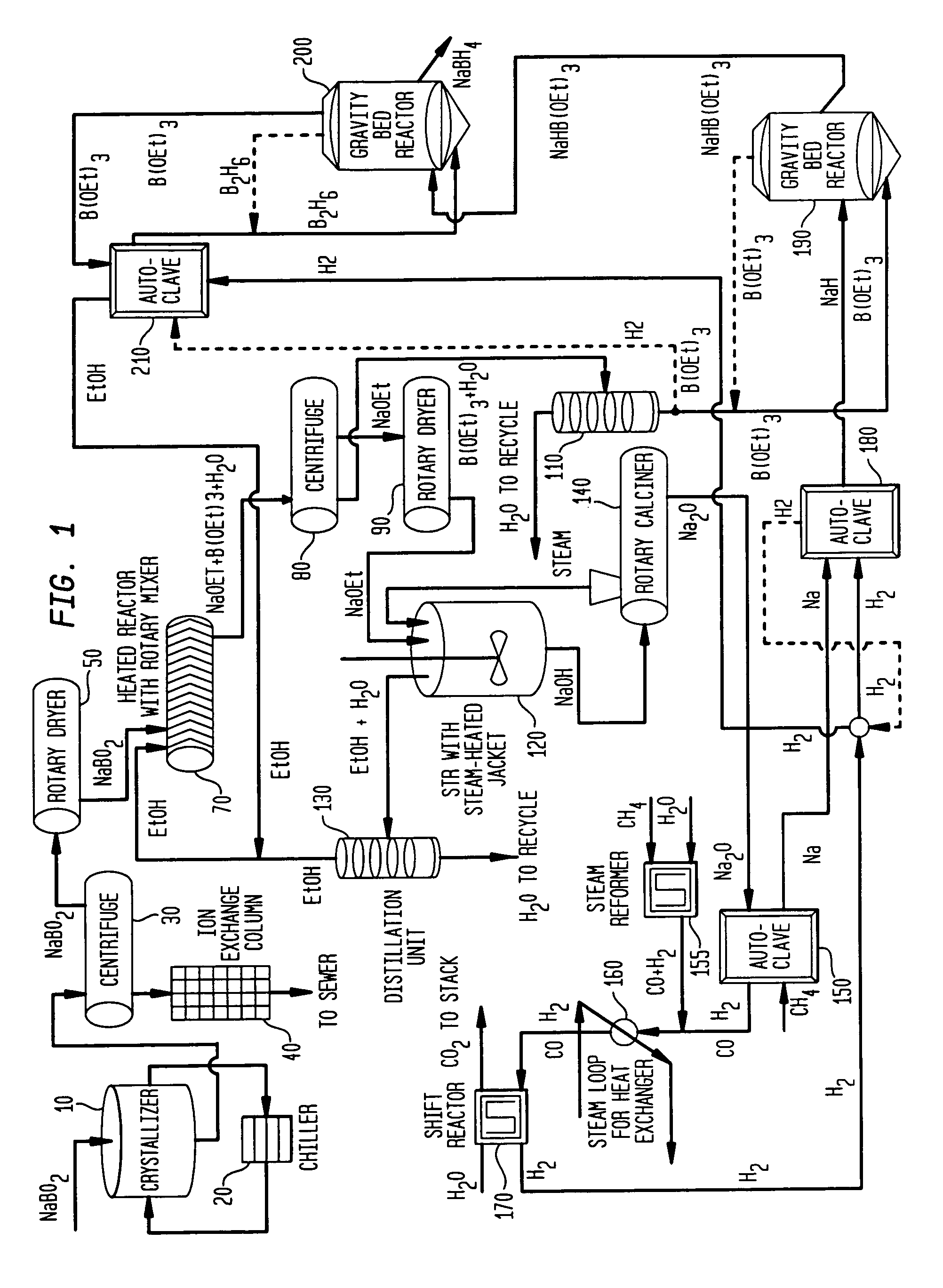
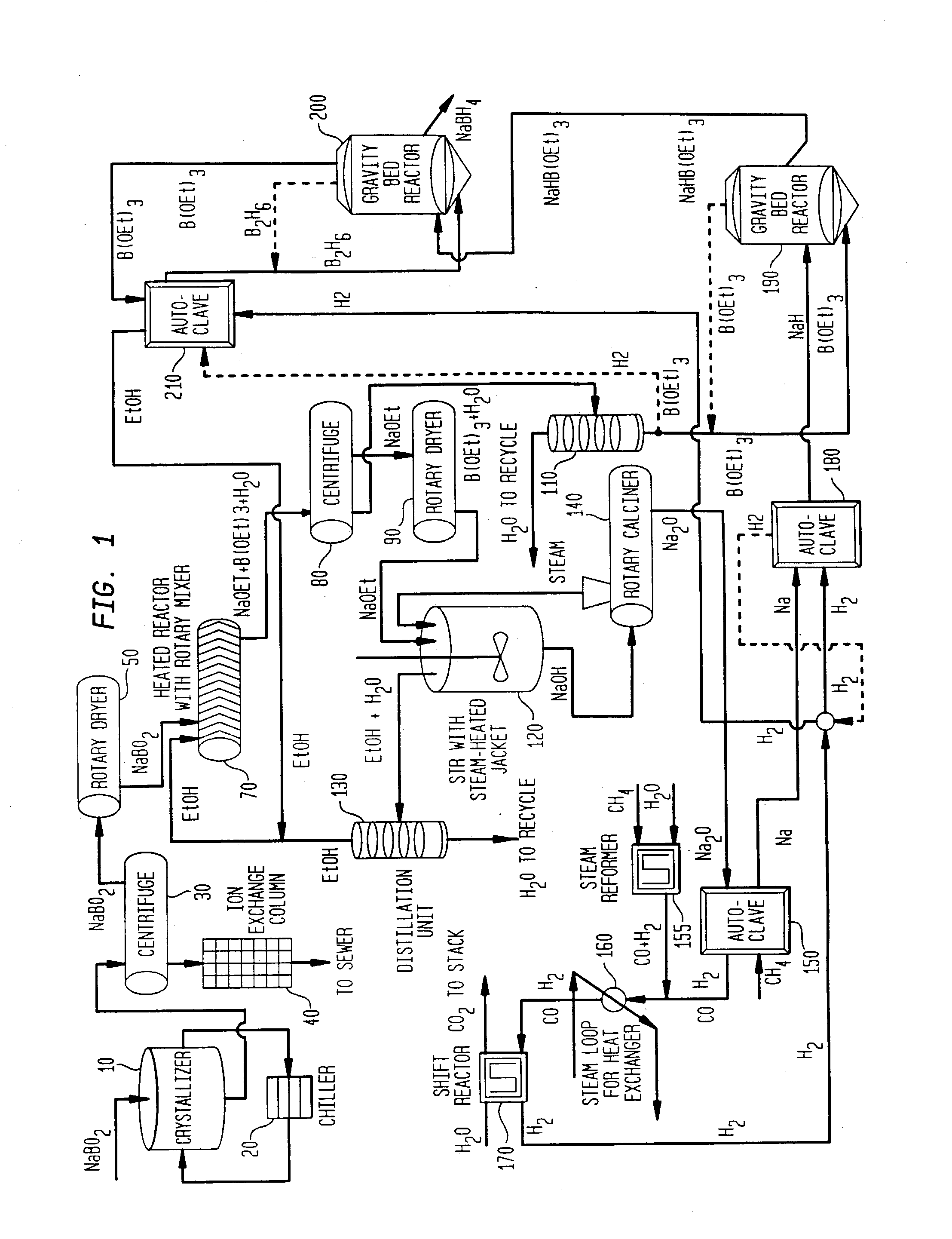
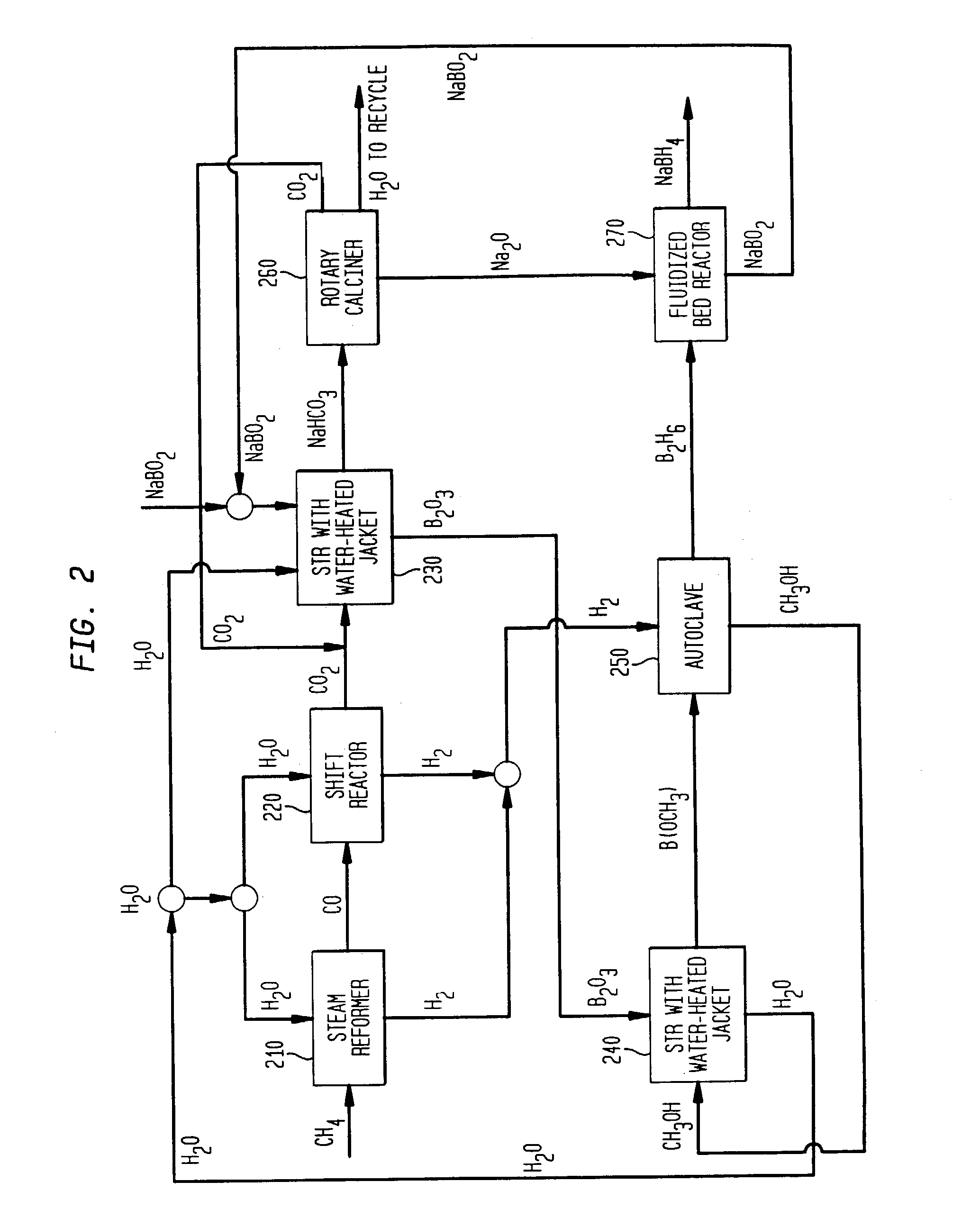
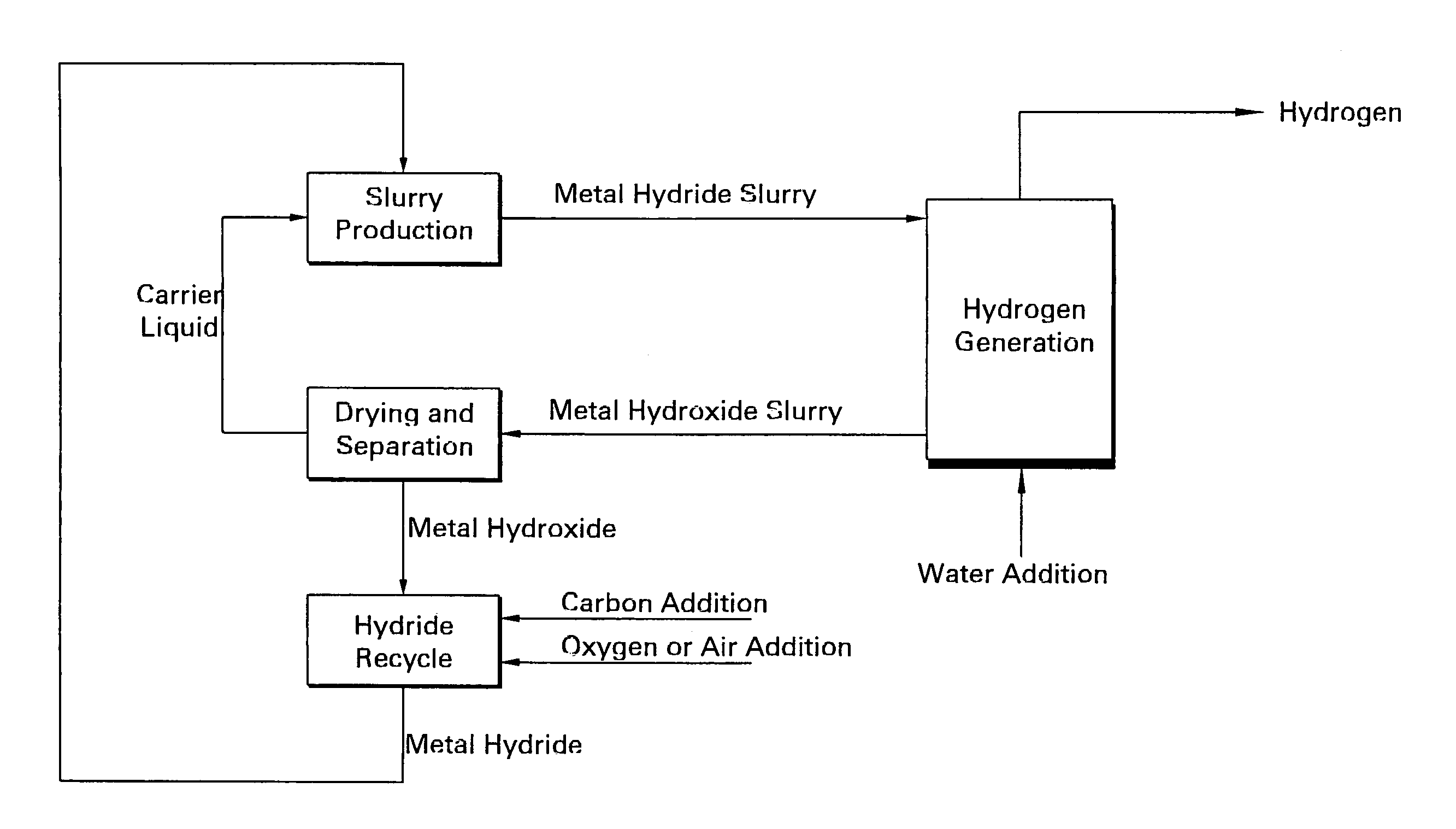
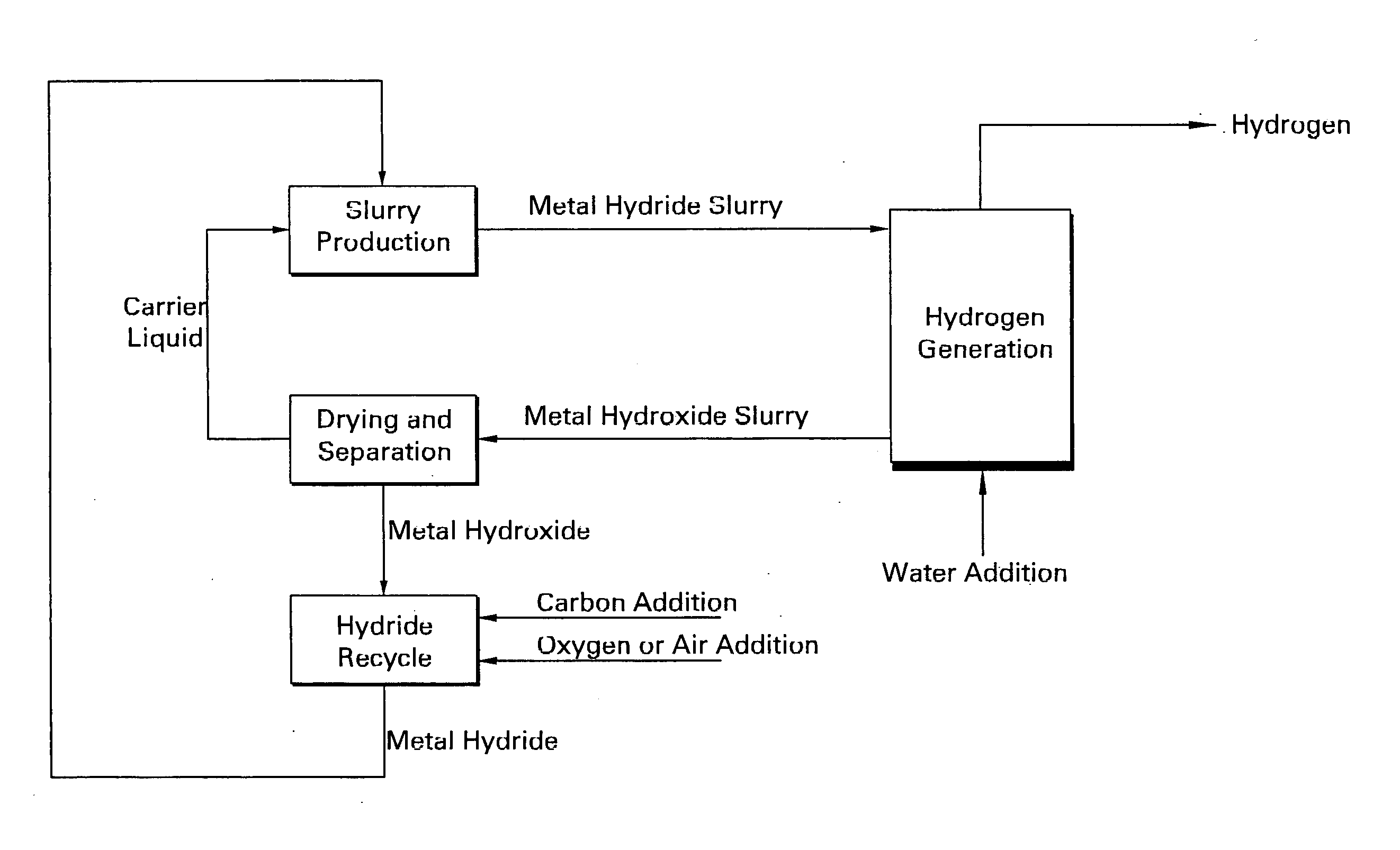
Processes for synthesizing alkali metal borohydride compounds
Processes for synthesizing borohydride compounds with reduced energy requirements and high efficiency are disclosed. The processes include the reaction of a base with a borane complex or diborane to produce a borohydride compound of formula YBH4, where Y is a monovalent cationic moiety.
Owner:MILLENNIUM CELL
Compositions and processes for synthesizing borohydride compounds
InactiveUS7019105B2Improve processing efficiencyOvercome deficienciesHydrogenMonoborane/diborane hydridesOrganic chemistryBorohydride
The present invention relates to compositions and processes for producing borohydride compounds. In particular, the present invention provides efficient processes and compositions for the large-scale production of borohydride compounds.
Owner:MILLENNIUM CELL
Destabilized and catalyzed borohydrided for reversible hydrogen storage
InactiveUS20060194695A1Reduce the temperatureImproved hydrogen binding/release kineticsHydrogenPhysical/chemical process catalystsIndiumCobalt
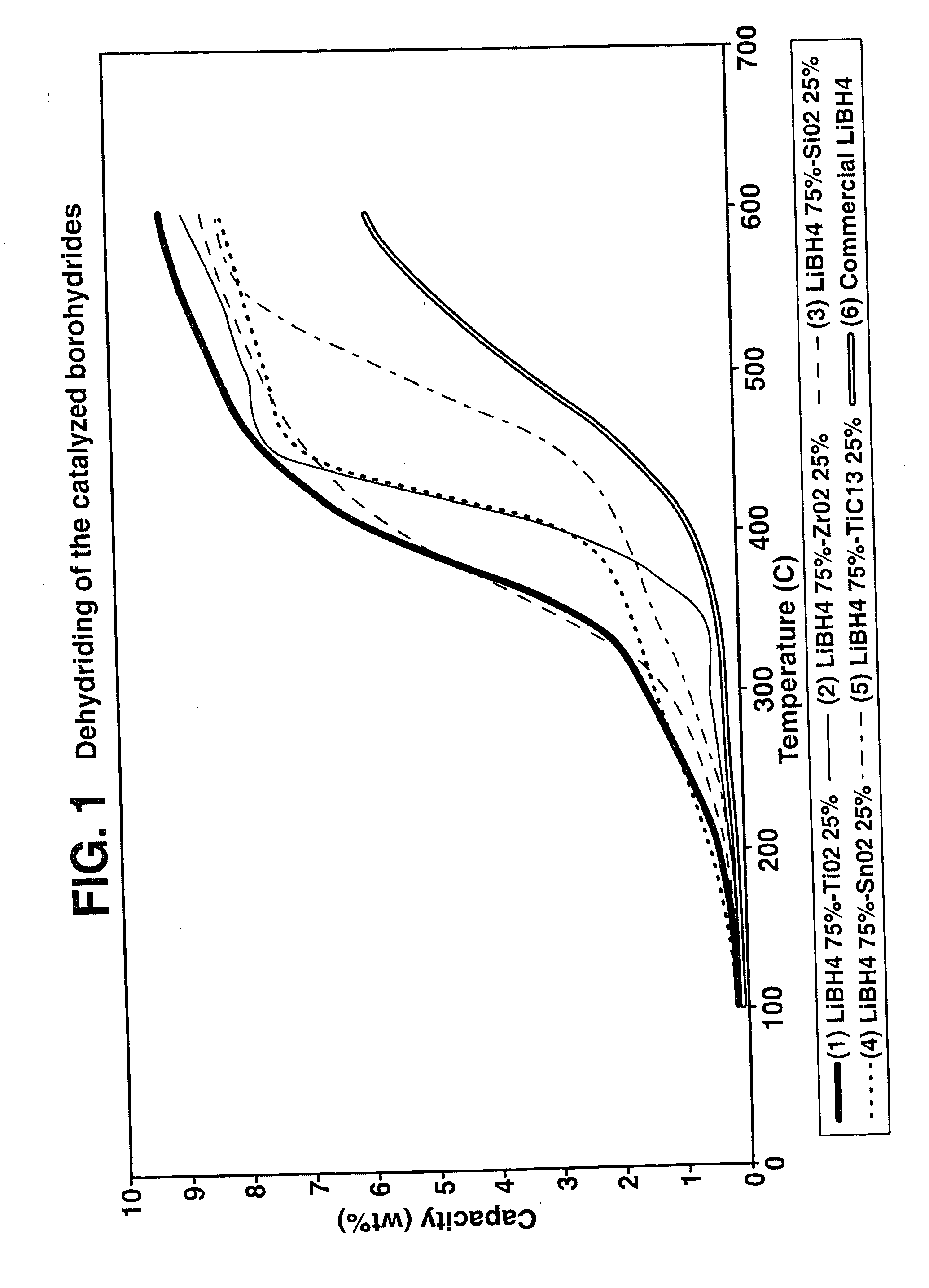
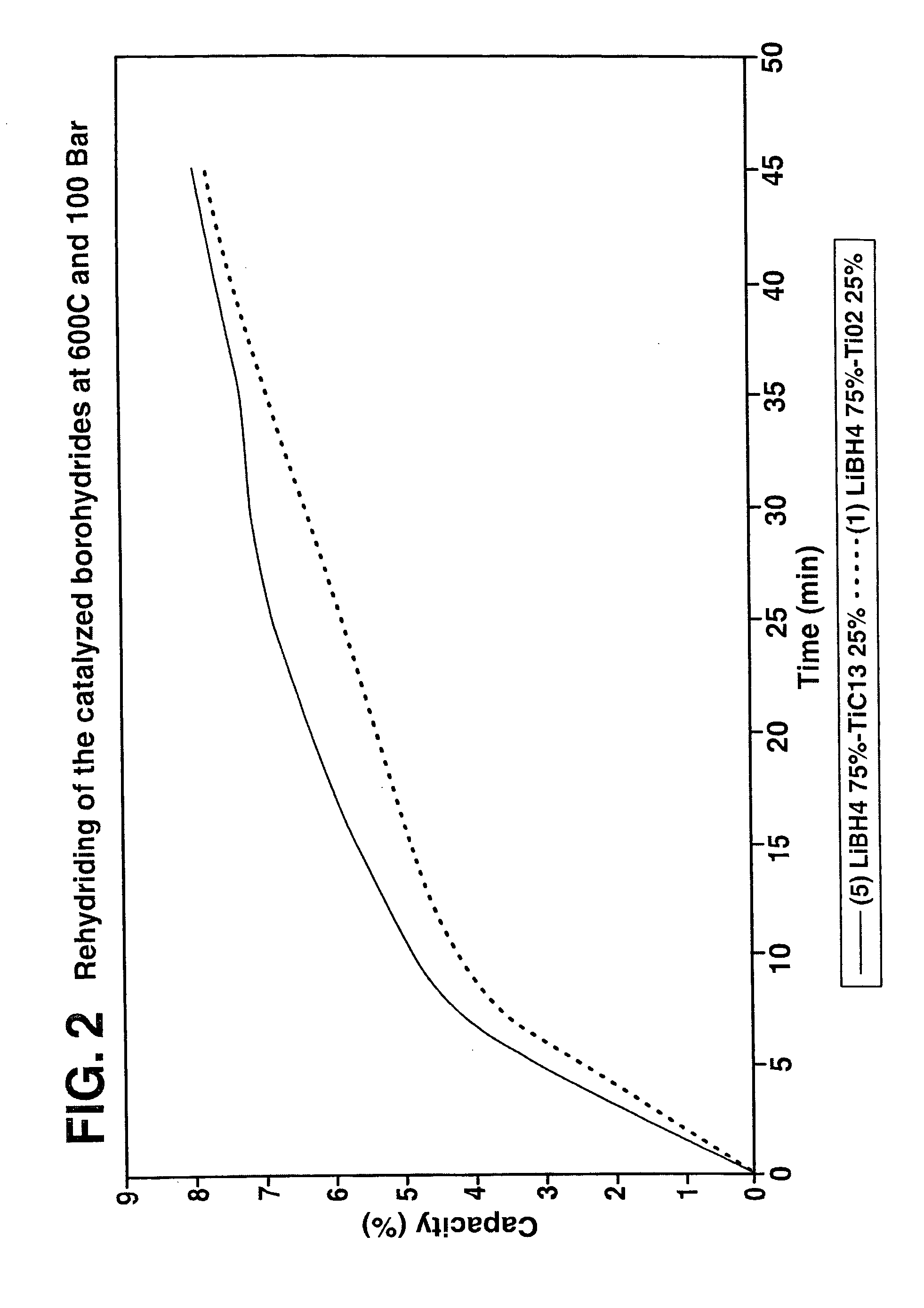
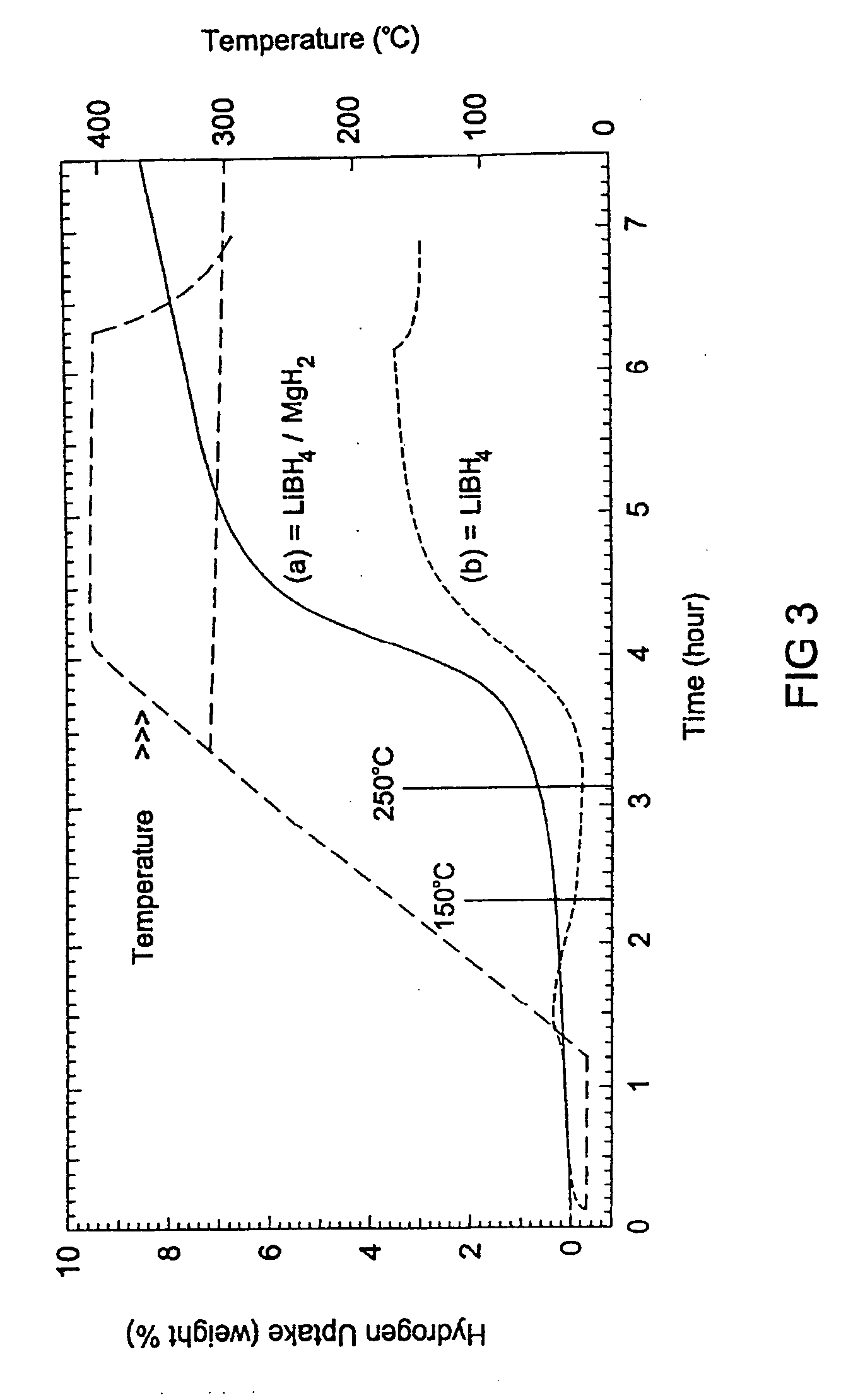
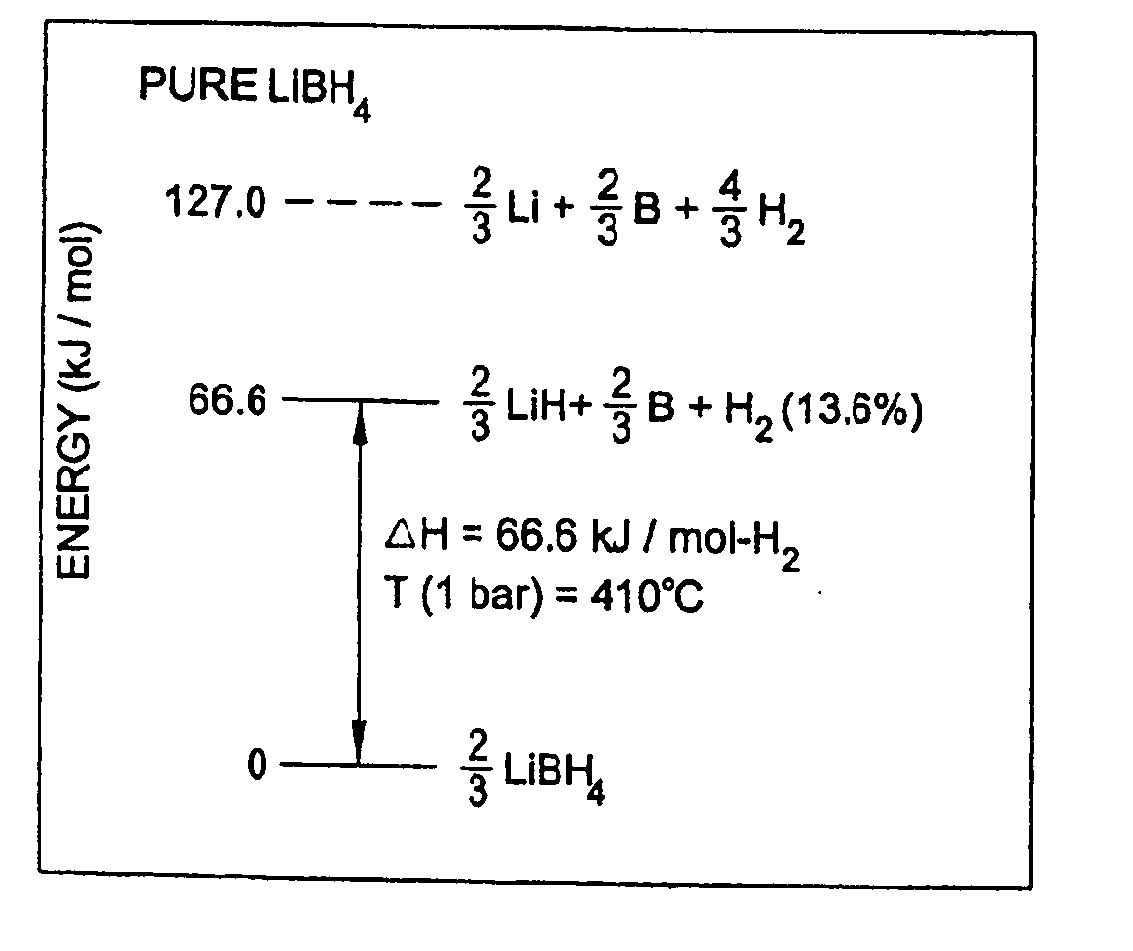
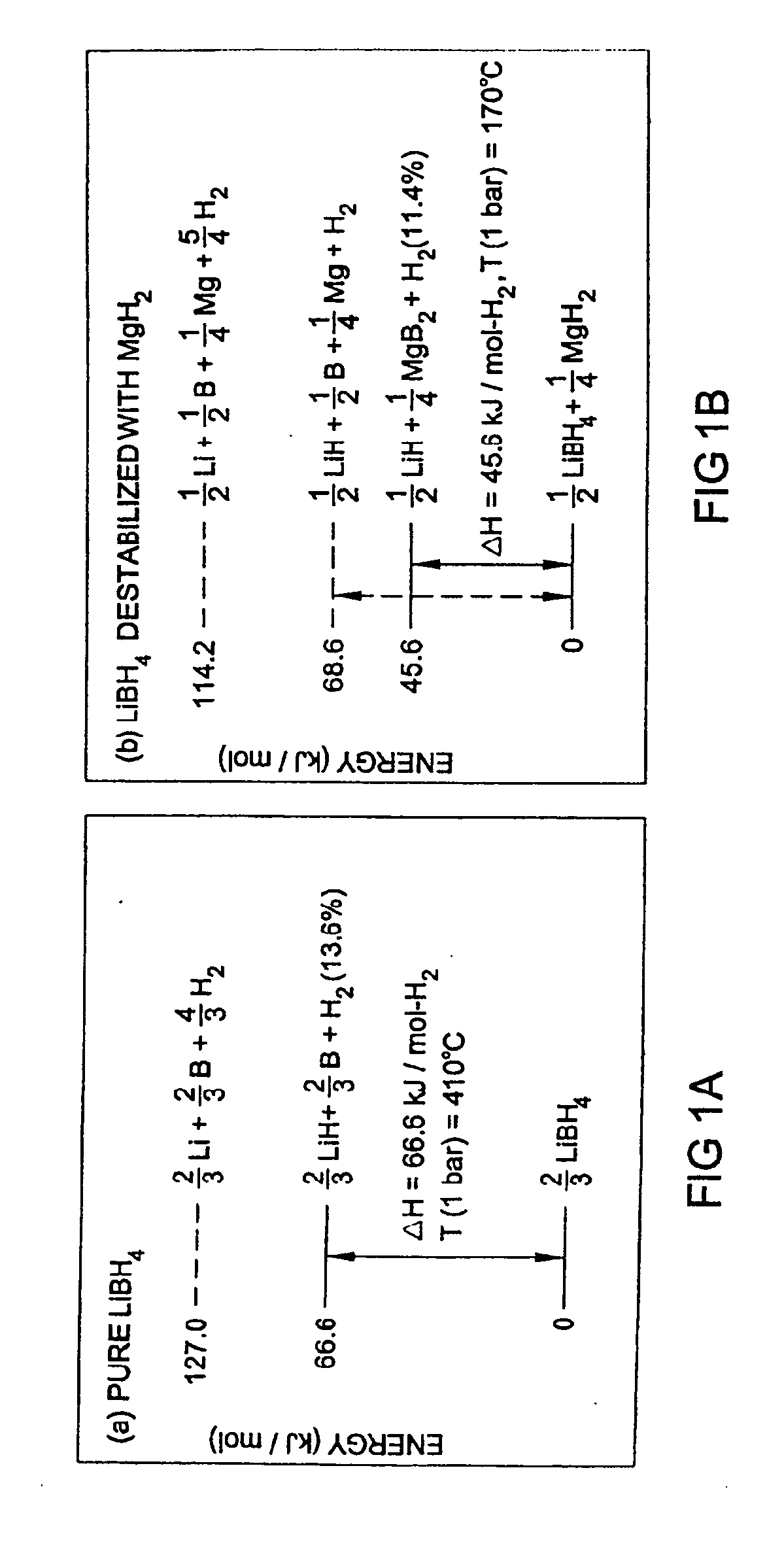
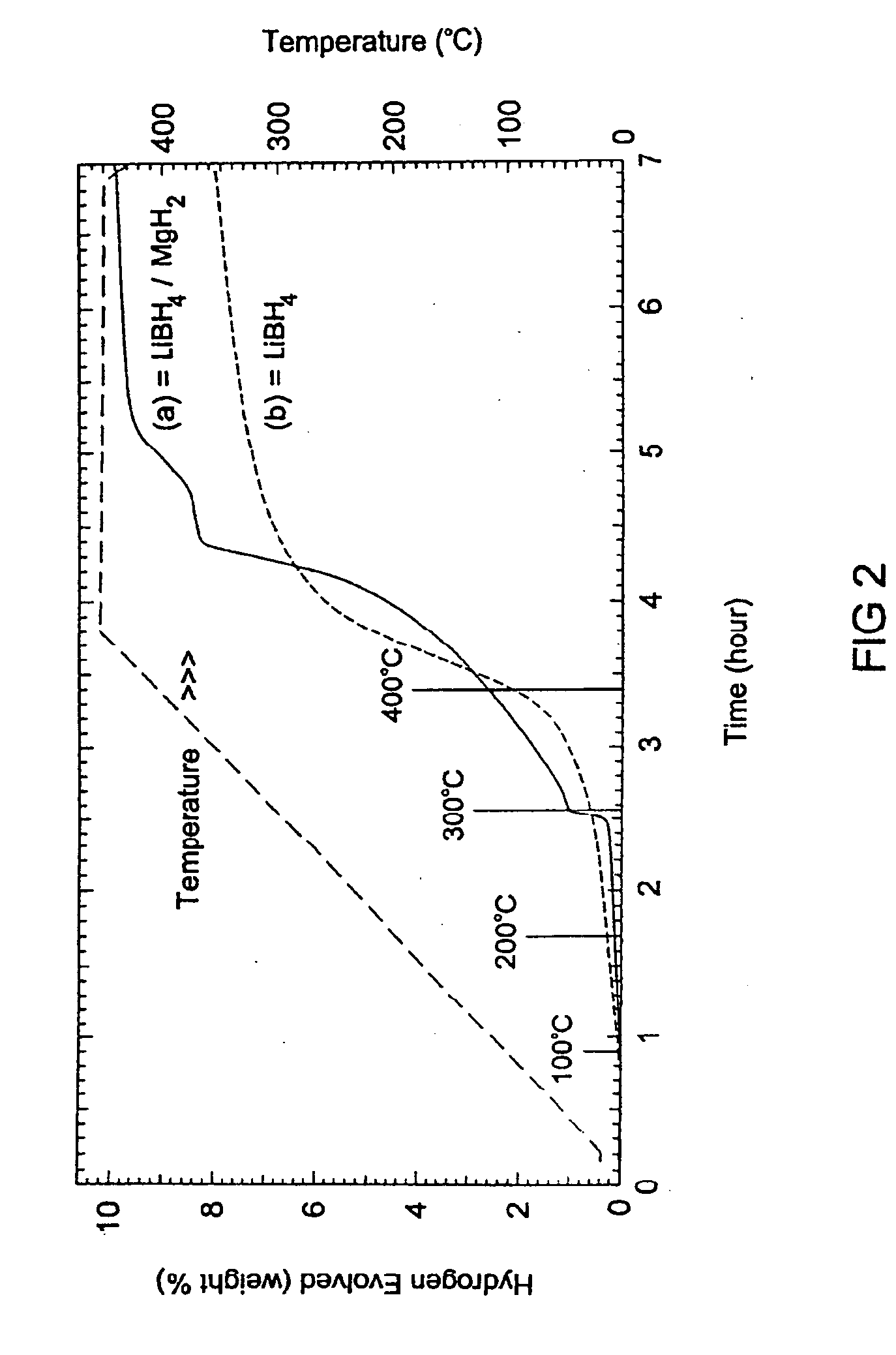
A hydrogen storage material and process is provided in which catalyzed alkali borohydride materials and partially substituted borohydride materials are created and which may contain effective amounts of catalyst(s) which include transition metal oxides, halides, and chlorides of titanium, zirconium, tin, vanadium, iron, cobalt and combinations of the various catalysts and the destabilization agents which include metals, metal hydrides, metal chlorides and complex hydrides of magnesium, calcium, strontium, barium, aluminum, gallium, indium, thallium and combinations of the various destabilization agents. When the catalysts and destabilization agents are added to an alkali borodydride such as a lithium borohydride, the initial hydrogen release point of the resulting mixture is substantially lowered. Additionally, the hydrogen storage material may be rehydrided with weight percent values of hydrogen of at least about nine percent.
Owner:SAVANNAH RIVER NUCLEAR SOLUTIONS
Triborohydride salts as hydrogen storage materials and preparation thereof
InactiveUS20050135996A1Physical/chemical process catalystsAlkali/alkaline-earth/beryllium/magnesium hydridesFuel cellsSlurry
The present invention relates to the use of triborohydride salts as hydrogen storage materials. The present invention also relates to a system of using triborohydride salts to generate hydrogen gas for use in a fuel cell or other hydrogen-consuming device. A novel method of preparing triborohydride salts is also disclosed, wherein gaseous diborane is reacted with a carbonate suspended in a non-aqueous solvent in a suitable vessel with agitation. The process is typically carried out utilizing sodium carbonate to form sodium triborohydride. Other triborohydride salts can then be formed by cationic exchange. Hydrogen generating fuels according to the present invention include aqueous or hydroalcoholic solutions or slurries of a triborohydride salt, which may additionally contain a borohydride salt to provide operation over a broader temperature range.
Owner:MILLENNIUM CELL
Reversible hydrogen storage systems
InactiveUS20060013753A1Maintain electroneutralityRelease of hydrogenAlkali/alkaline-earth/beryllium/magnesium hydridesHydrogen separationHydrogen storage systemMaterial system
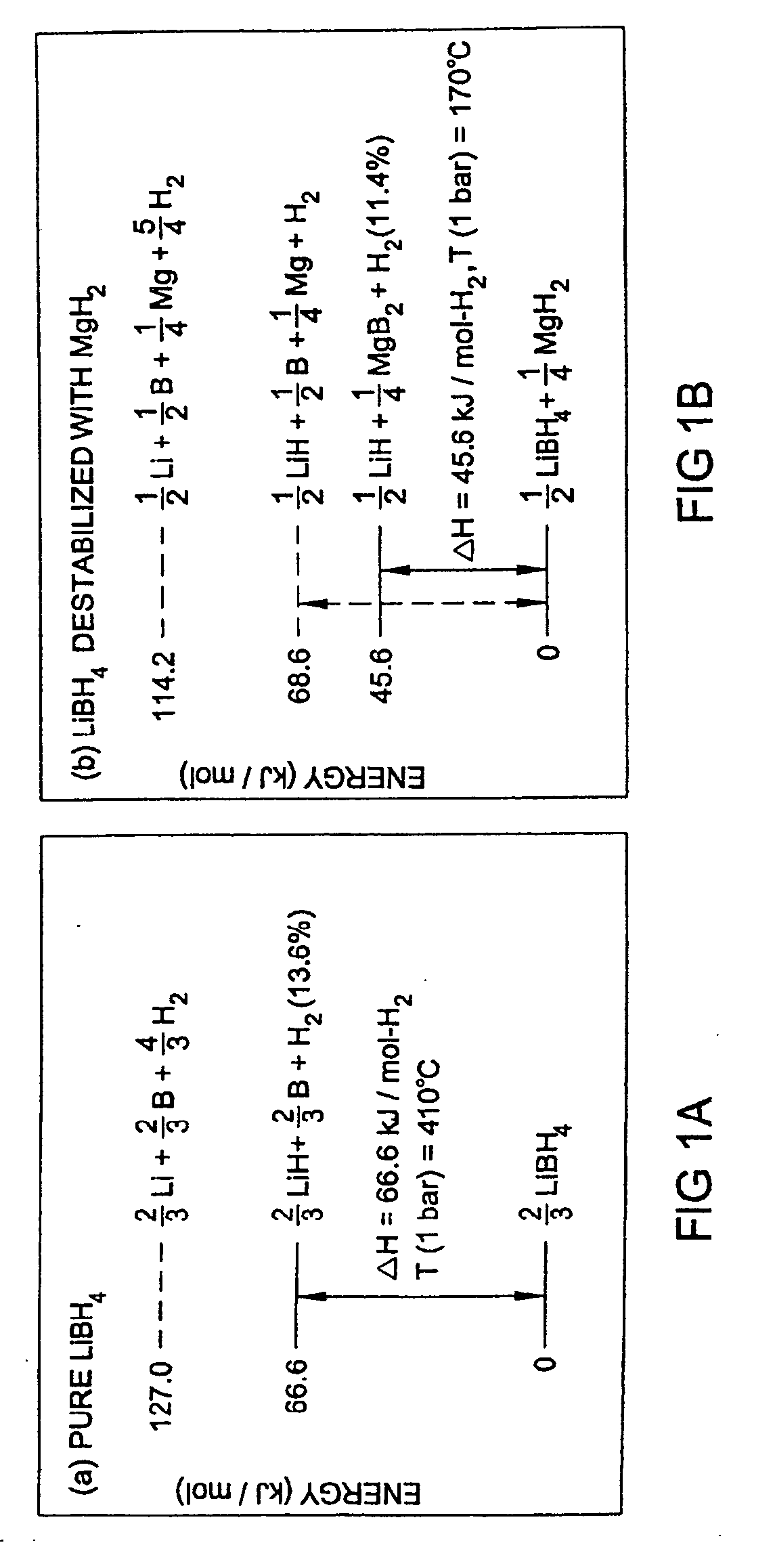
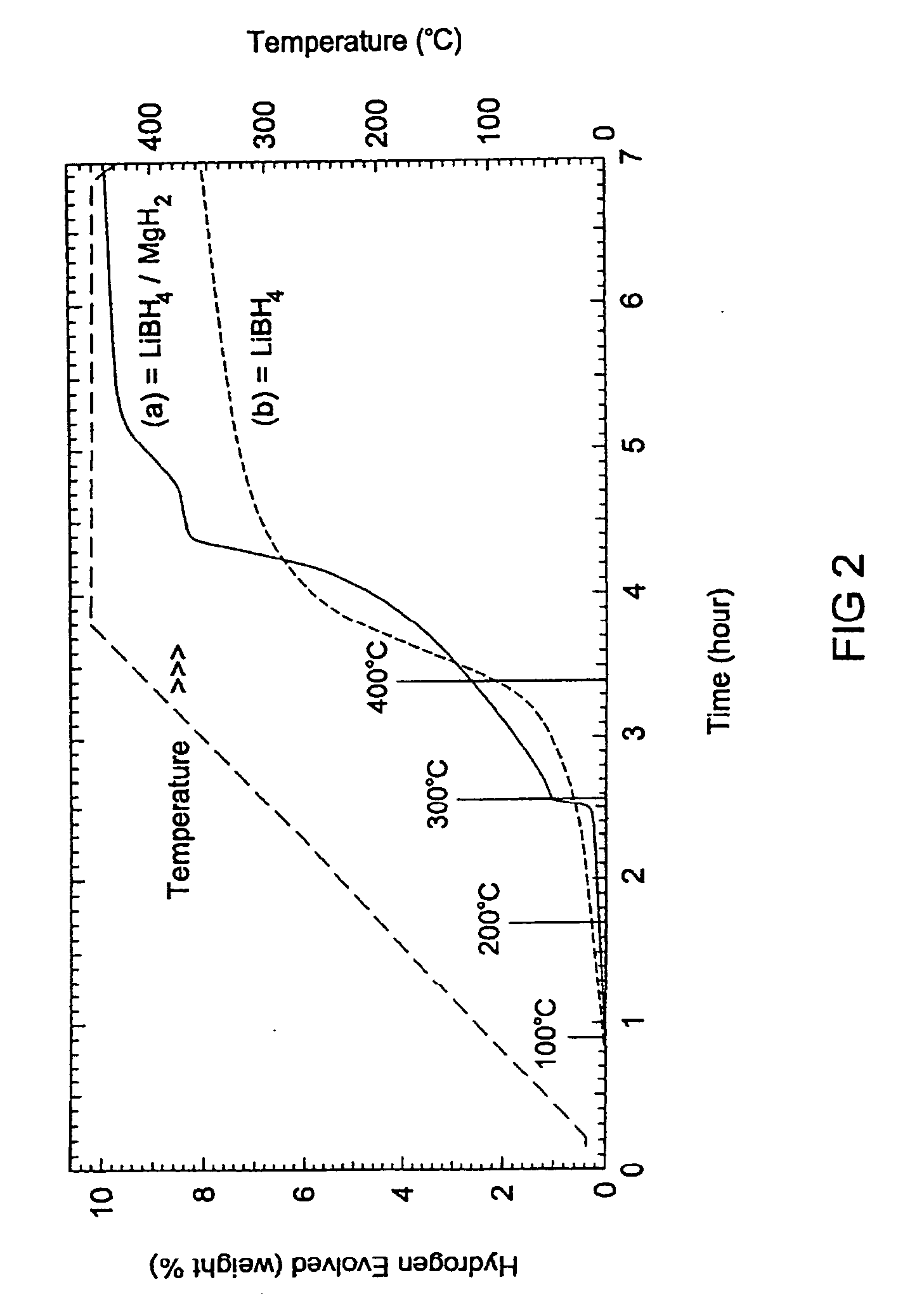
The invention provides compositions for reversible storage of hydrogen at industrially practicable temperature and pressure conditions. Hydrogen storage material systems comprising stable hydrides and destabilizing hydrides. When the stable hydride is in the presence of the destabilizing hydride, the stable hydride releases hydrogen at a lower energy level than it would in the absence of the destabilizing hydride.
Owner:GM GLOBAL TECH OPERATIONS LLC
Processes for synthesizing borohydride compounds
InactiveUS20030092877A1HydrogenMonoborane/diborane hydridesAlkaline earth metalCombinatorial chemistry
The present invention relates to processes for producing borohydride compounds. In particular, the present invention provides efficient processes and compositions for the large-scale production of borohydride compounds of the formula YBH.sub.4 by the reaction of a boron-containing compound represented by the formula BX.sub.3 with hydrogen or an aldehyde to obtain diborane and HX, and reacting the diborane with a Y-containing base selected from those represented by the formula Y.sub.2O, YOH and Y.sub.2CO.sub.3 to obtain YBH.sub.4 and YBO.sub.2. Y is selected from the group consisting of the alkali metals, pseudo-alkali metals, alkaline earth metals, an ammonium ion, and quaternary amines of the formula NR.sub.4.sup.+, wherein each R is independently selected from hydrogen and a straight- or branched-chain C.sub.1-4 alkyl group, and X is selected from the group consisting of halide ions, --OH, --R' and --OR' groups, chalcogens, and chalcogenides, wherein R' is a straight- or branched-chain C.sub.1-4 alkyl group.
Owner:MILLENNIUM CELL
Methods of synthesis of isotopically enriched borohydride and methods of synthesis of isotopically enriched boranes
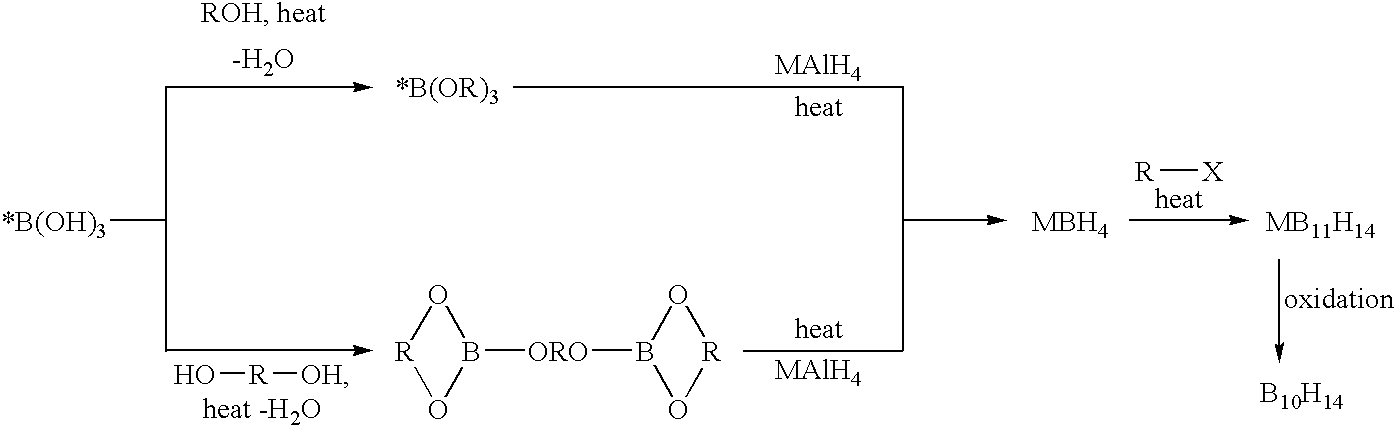
InactiveUS7641879B2High yieldPeroxides/peroxyhydrates/peroxyacids/superoxides/ozonidesMonoborane/diborane hydridesIsotopeBoric acid
The invention provides new methods for the synthesis of isotopically enriched metal borohydrides, metal tetrahydroundecaborate salts, and decaborane from isotopically enriched 10B-boric acid or 11B-boric acid. The invention is particularly useful for synthesis of isotopically enriched sodium or lithium borohydride, MB11H14 (where M is Li, Na, K, or alkylammonium), and decaborane.
Owner:SEMEQUIP
Aqueous borohydride compositions
InactiveUS6866689B2Use minimizedHigh catalytic efficiencyOther chemical processesMonoborane/diborane hydridesHydrogenSodium borohydride
An aqueous fuel for generating hydrogen includes alkaline aqueous composition of about 17 to 37 mole percent of a sodium borohydride, and from about 0.001 to 1 mole percent of sodium hydroxide.
Owner:MONTGOMERY CHEM
Triborohydride salts as hydrogen storage materials and preparation thereof
The present invention relates to the use of triborohydride salts as hydrogen storage materials. The present invention also relates to a system of using triborohydride salts to generate hydrogen gas for use in a fuel cell or other hydrogen-consuming device. A novel method of preparing triborohydride salts is also disclosed, wherein gaseous diborane is reacted with a carbonate suspended in a non-aqueous solvent in a suitable vessel with agitation. The process is typically carried out utilizing sodium carbonate to form sodium triborohydride. Other triborohydride salts can then be formed by cationic exchange. Hydrogen generating fuels according to the present invention include aqueous or hydroalcoholic solutions or slurries of a triborohydride salt, which may additionally contain a borohydride salt to provide operation over a broader temperature range.
Owner:MILLENNIUM CELL
Organic matter and ammonia borane compounded hydrogen storage material and preparation method thereof
ActiveCN102030313ALowering the temperature of thermally liberated hydrogenInhibitionMonoborane/diborane hydridesPolyethylene oxideSolvent
The invention relates to an organic matter and ammonia borane compounded hydrogen storage material. The hydrogen storage material is prepared by compounding the organic matter and the ammonia borane, wherein the organic matter is phthalic anhydride, polyethylene oxide, dextrose, mannitol or mannitol hexaacetic ester. The preparation method comprises the following steps: 1) adding the organic matter to the purified acetonitrile solvent, and stirring for dissolving; 2) dissolving the ammonia borane into the mixing solvent comprising acetonitrile and methanol, and stirring at the temperature of 20 to 70 DEG C to obtain a uniform solution; and 3) carrying out vacuum drying, and removing the solvent, thus obtaining the hydrogen storage material. The invention has the advantages that the ammonia borane and the organic matter are taken as raw materials to prepare the hydrogen storage material at the lower hydrogen discharge temperature; the thermal decomposition and hydrogen discharge temperature of the ammonia borane can be effectively reduced; the generation of harmful gas impurities of borazole, diborane, ammonia and the like is effectively inhibited; the hydrogen storage material has quicker hydrogen discharge kinetics; in addition, the heat discharge amount is less in the hydrogen discharge course; and the enthalpy change of a decomposition reaction approaches to thermal neutrality; and the hydrogen storage material is beneficial to realizing the regeneration of reaction products through a solid-gas reaction or a chemical process under the relatively mild condition.
Owner:NANKAI UNIV
Method for synthesizing metal coordinate hydride hydrogen-storing material directly by reaction ball milling
InactiveCN101264863AIncrease productivityEasy to operateMultiple metal hydridesMonoborane/diborane hydridesRare earthRoom temperature
The invention discloses a method for directly synthesizing a hydrogen storage material of metal coordination hydride by a reaction ball milling, which is characterized in that metal coordination hydride which is expressed in a chemical general formula as MNH4 is directly synthesized in a step by the reaction ball milling method and through controlling the hydrogenation pressure and the time of the ball milling, wherein the chemical general formula, M is one or two of Li and Na, and N is one or two of B and Al, the transition metal such as Ti, Zr and Ni, transition metal halide such as TiF3, HfCl3, TiCl4, and ScCl3, rare-earth chloride such as LaCl3, CeCl3, PrCl3, NdCl3, and SmCl3 are acted as the catalyst during the synthesis process. The method for directly synthesizing the hydrogen storage material has the advantages of only taking one step of the reaction ball milling to directly synthesize the final product of coordinate metal hydride at a room temperature, having simple operation, low energy consumption, safety, and reliability, enjoying high yield rate of the synthesized hydrogen storage material, and possessing high capacity of reversible hydrogen charging and discharging circulation at a low and intermediate temperature.
Owner:ZHEJIANG UNIV
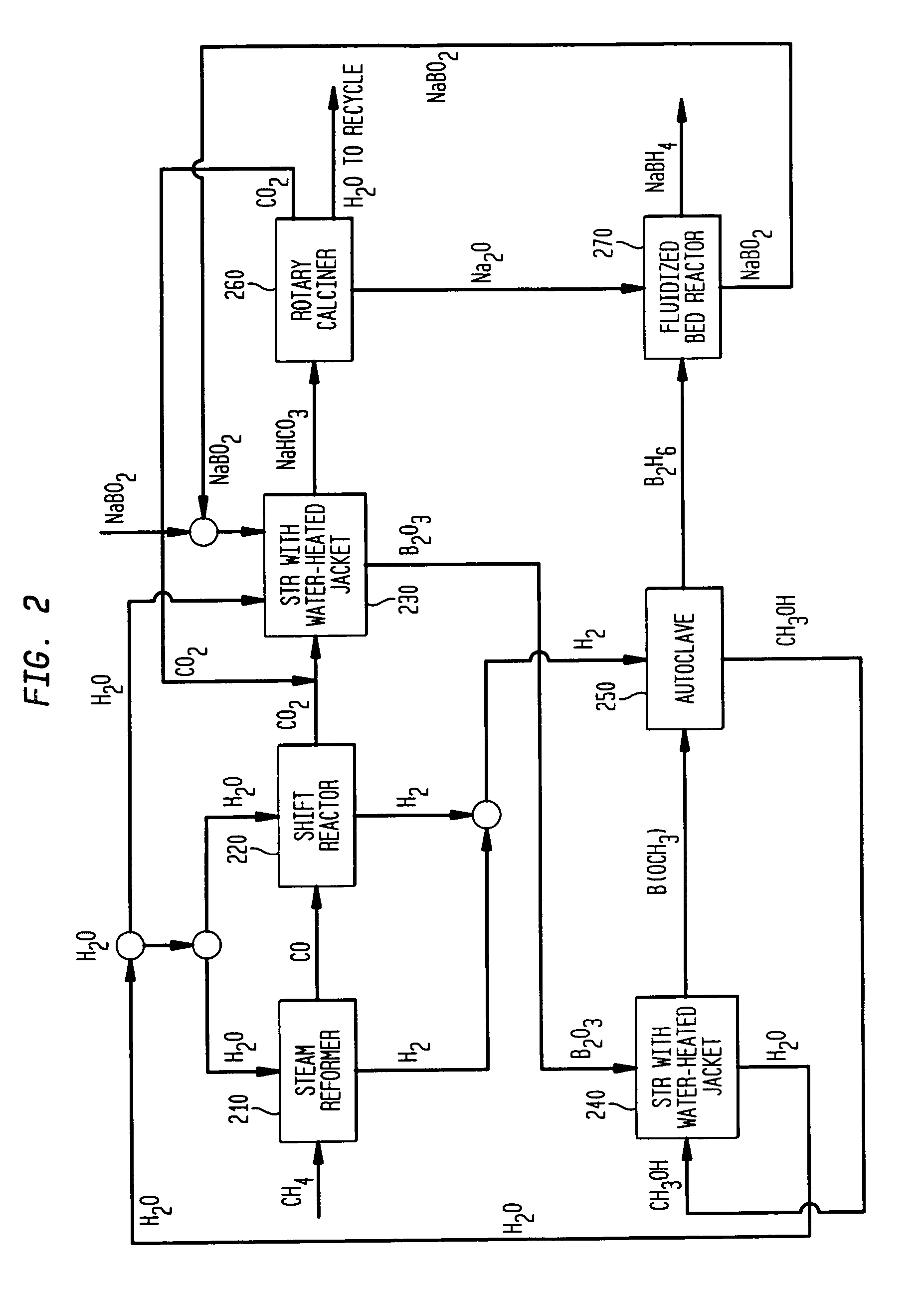
Method for producing a borohydride
InactiveUS20060106195A1Promote conversionProduction of hydrogen gasMonoborane/diborane hydridesFuel cell auxillariesOrganic chemistryBorohydride
A method for producing a borohydride is described and which includes the steps of providing a source of borate; providing a material which chemically reduces the source of the borate to produce a borohydride; and reacting the source of borate and the material by supplying heat at a temperature which substantially effects the production of the borohydride.
Owner:BATTELLE ENERGY ALLIANCE LLC
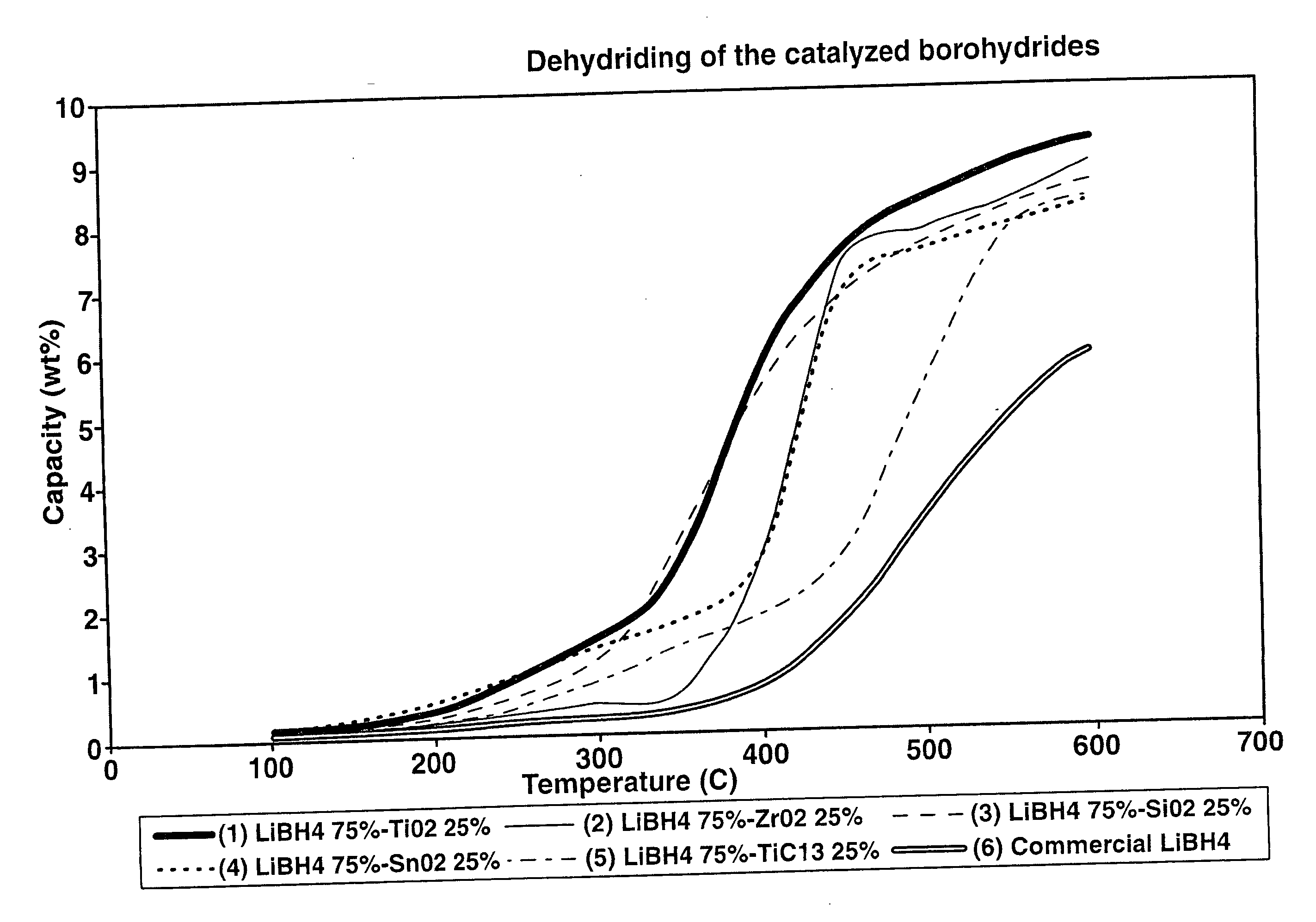
Lithium borohydride hydrogen storage material decorated by oxide and preparation method thereof
InactiveCN101054162ASimple methodLow hydrogen release temperatureOther chemical processesMonoborane/diborane hydridesBall millAtmosphere
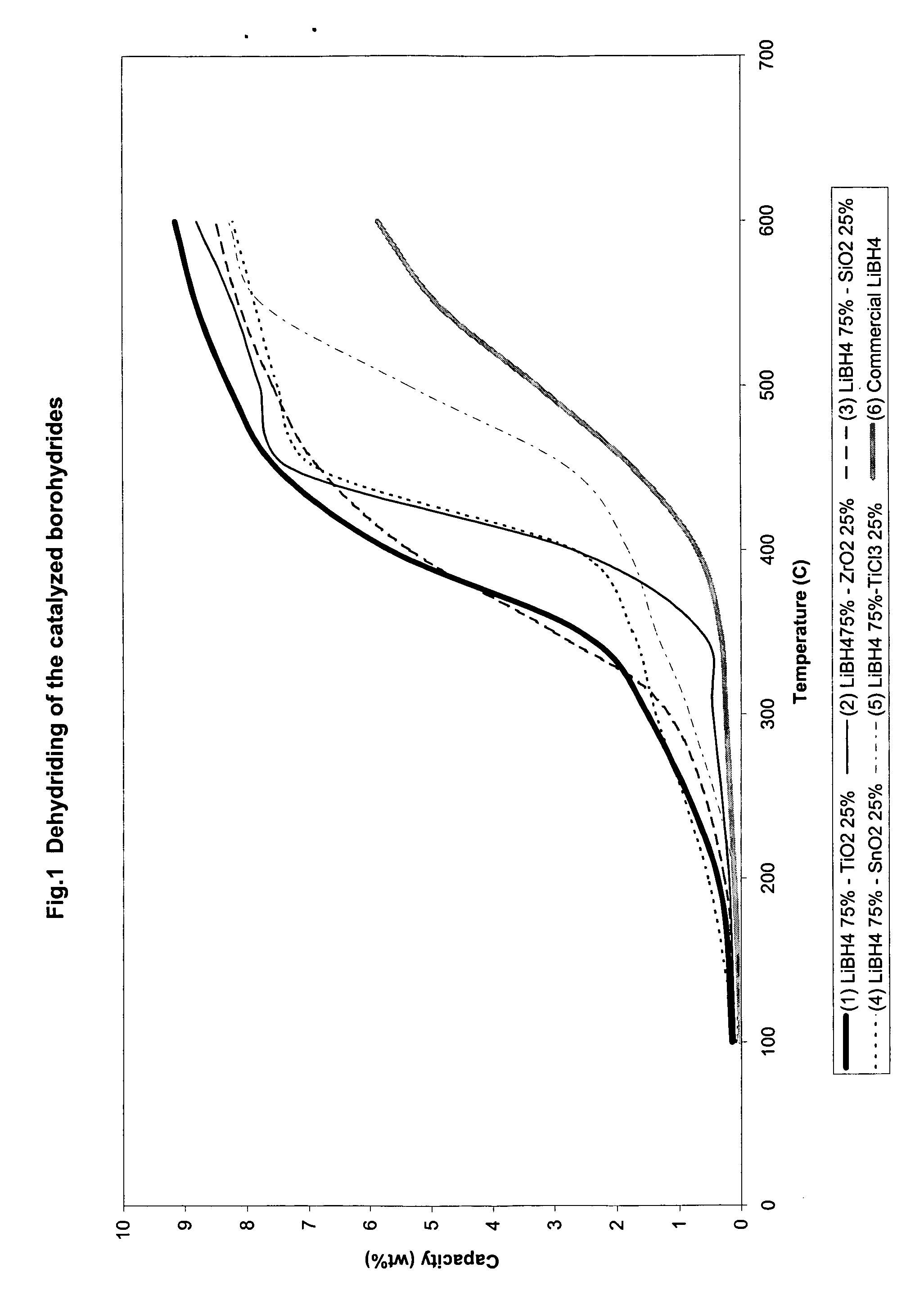
The invention relates to liborohydride hydrogen storage material and a preparation method thereof, which is characterized in that the formula of hydrogen storage material is (100-x)LiBH4+XMeO, wherein MeO is a adorning oxide, weight percent of X is 10-80%. After blending LiBH4 and the oxide according to the formula, the mixture is ball milled in protection of inert atmosphere to process surface treatment. The oxide is optional one of TiO2, Fe2O3, ZrO2, V2O5, SiO2, Al2O3, Al2O3-SiO2 or TiO2-SiO2. The intial hydrogen temperature of hydrogen storage material of the invention is lower than 100 DEG C, hydrogen amount is 3-6.5% at lower than 300 DEG C.
Owner:SHANGHAI INST OF MICROSYSTEM & INFORMATION TECH CHINESE ACAD OF SCI
Methods for reversibly storing hydrogen
InactiveUS20060013766A1Alkali/alkaline-earth/beryllium/magnesium hydridesHydrogen separationTemperature and pressureChemistry
The invention provides a method of reversibly storing hydrogen at industrially practicable temperature and pressure conditions. A stable hydrogen storage hydride is mixed with a destabilizing hydride. The stable hydride is capable of releasing hydrogen at a first energy level. When the stable hydride is in the presence of the destabilizing hydride, the stable hydride releases hydrogen at a second energy level. The second energy level is significantly reduced from the first energy level.
Owner:GM GLOBAL TECH OPERATIONS LLC
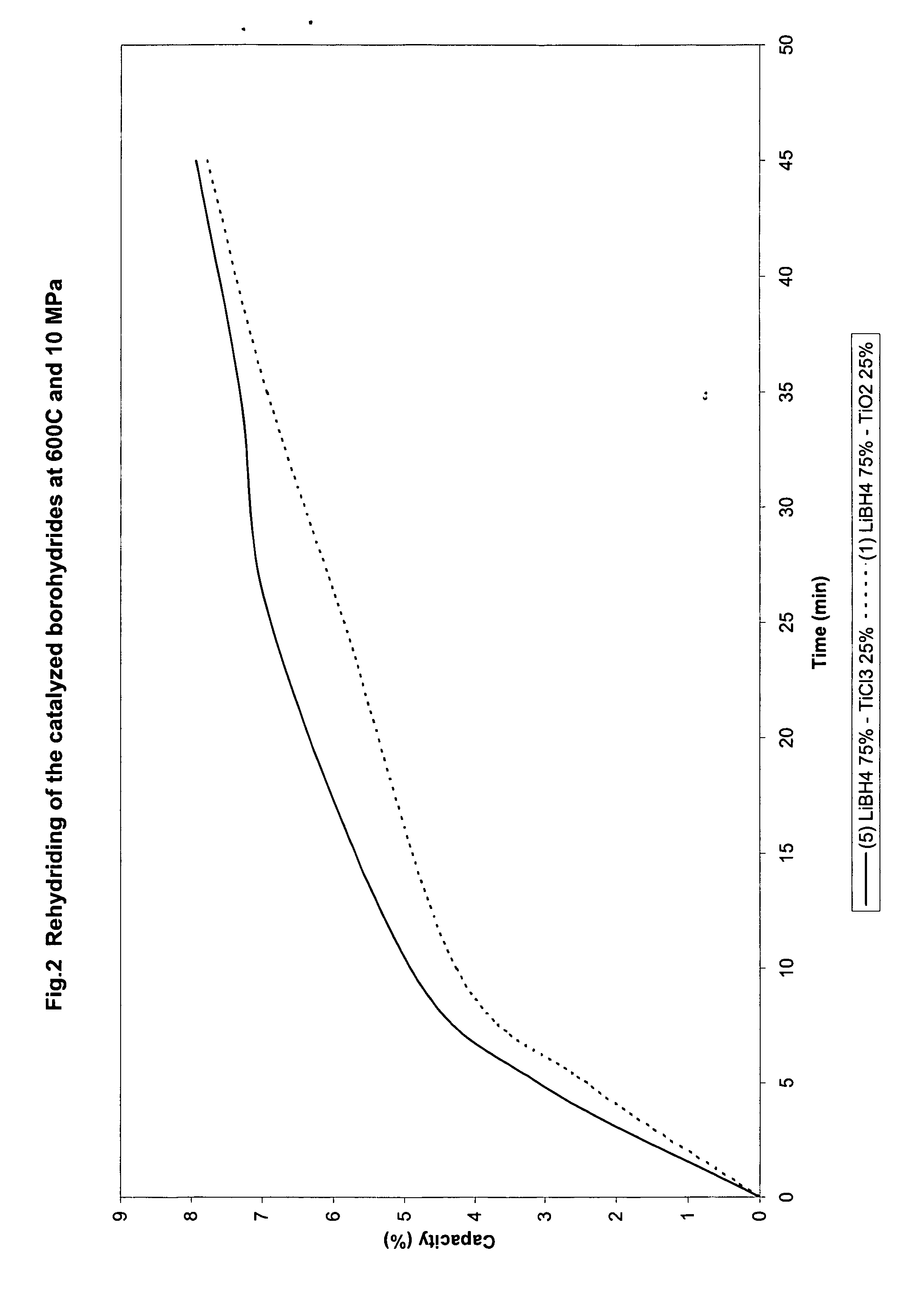
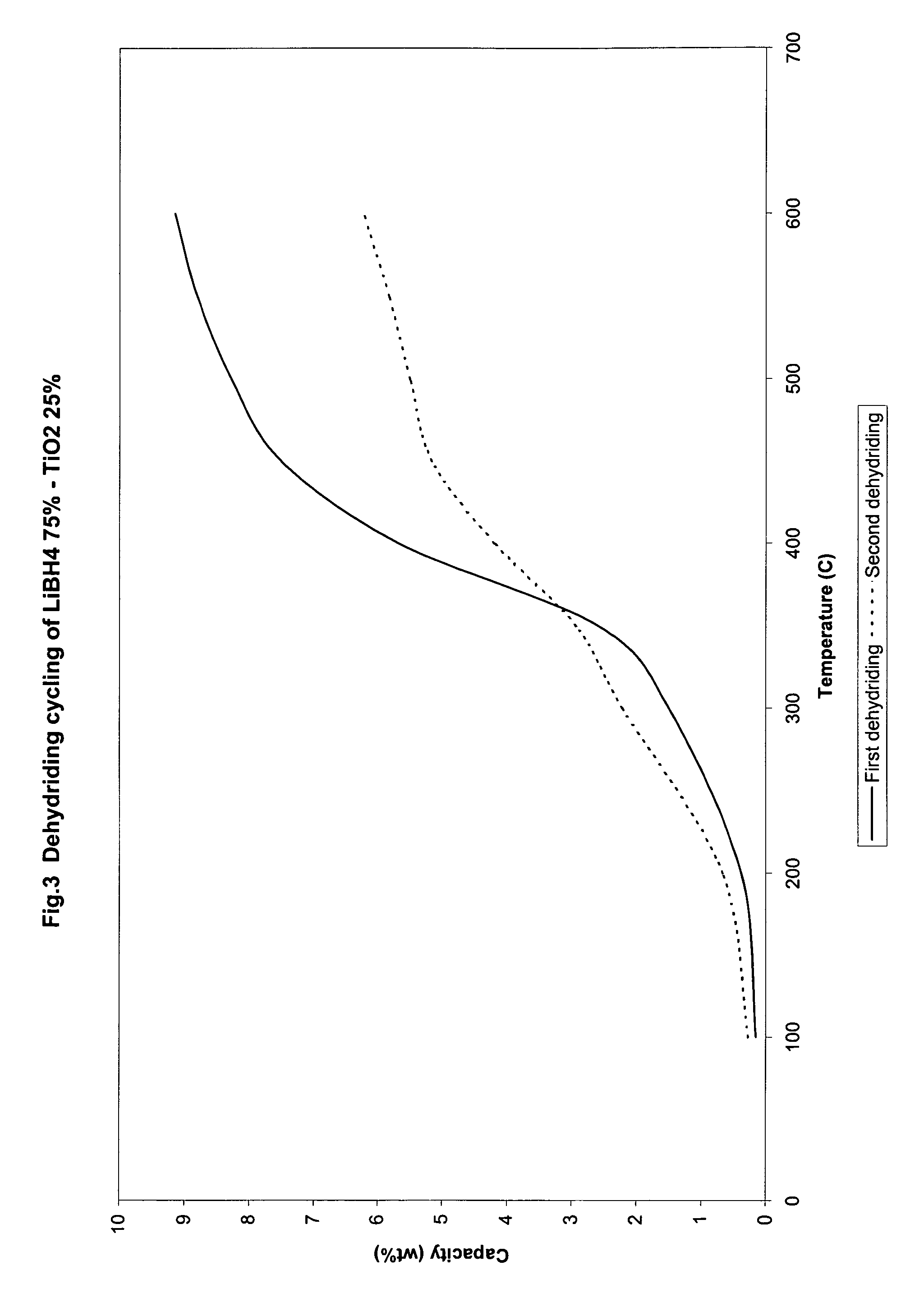
Catalyzed borohydrides for hydrogen storage
InactiveUS20060046930A1Reduce the temperatureOther chemical processesMonoborane/diborane hydridesChlorideTitanium
A hydrogen storage material and process is provided in which alkali borohydride materials are created which contain effective amounts of catalyst(s) which include transition metal oxides, halides, and chlorides of titanium, zirconium, tin, and combinations of the various catalysts. When the catalysts are added to an alkali borodydride such as a lithium borohydride, the initial hydrogen release point of the resulting mixture is substantially lowered. Additionally, the hydrogen storage material may be rehydrided with weight percent values of hydrogen at least about 9 percent.
Owner:SAVANNAH RIVER NUCLEAR SOLUTIONS
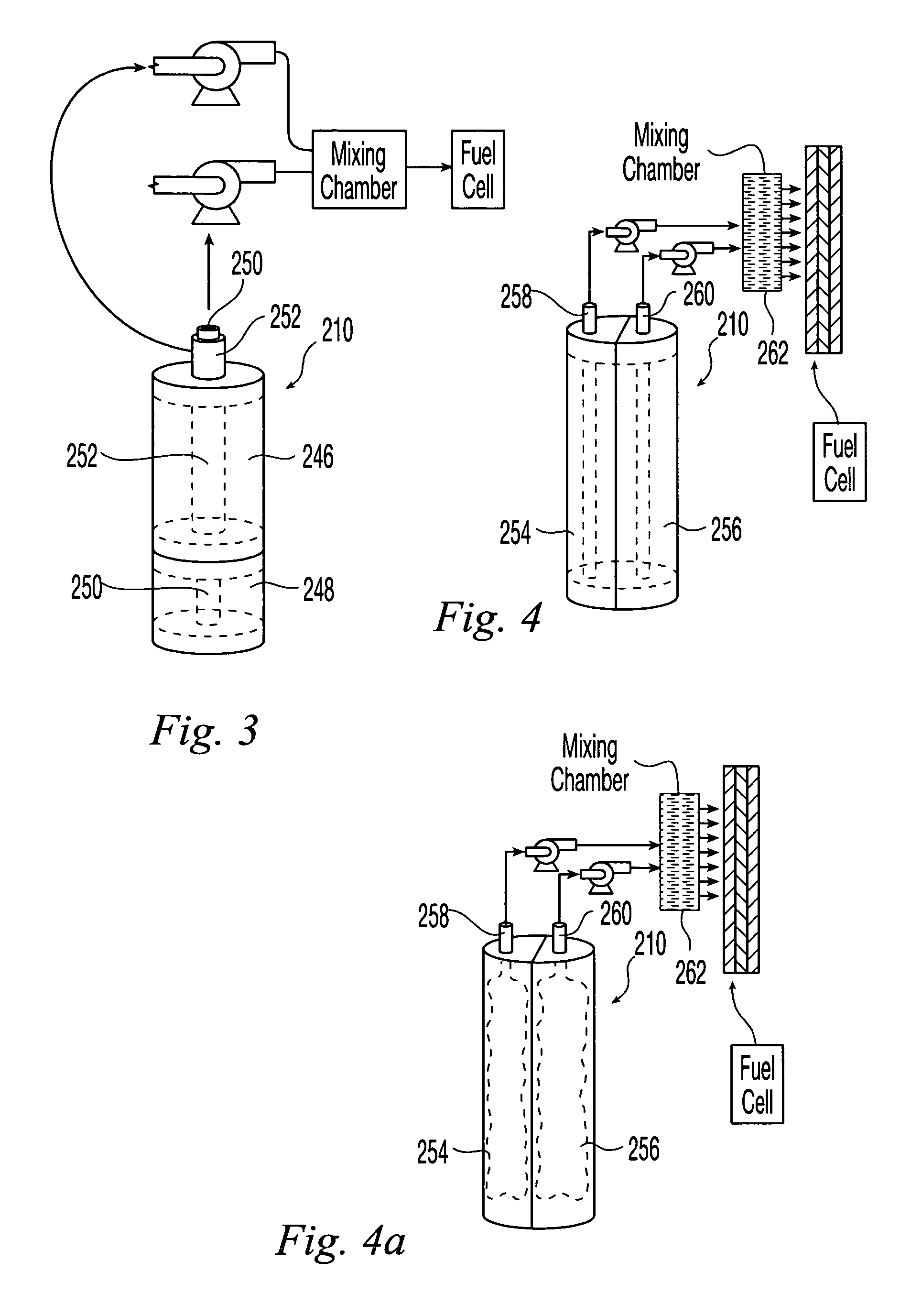
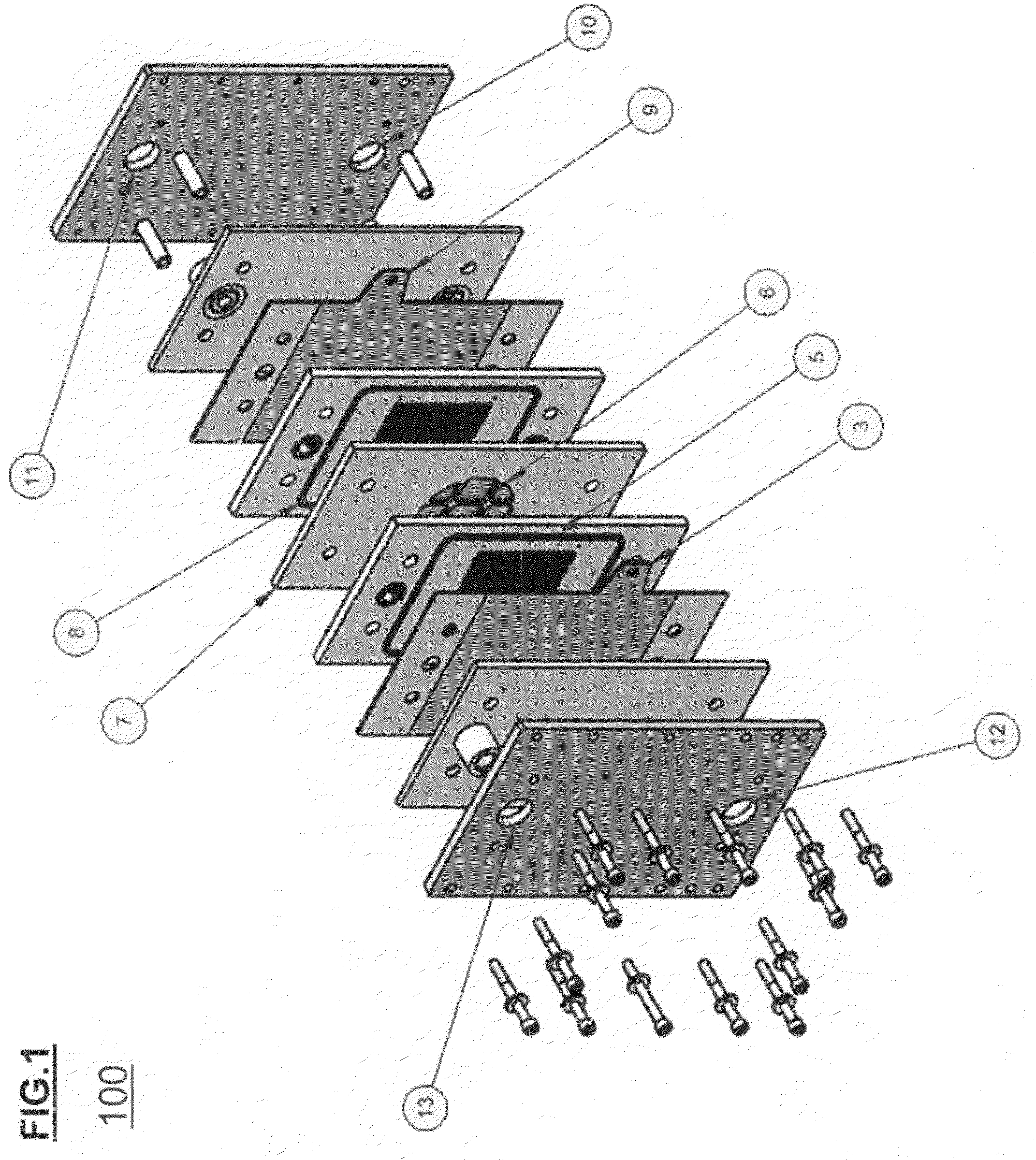
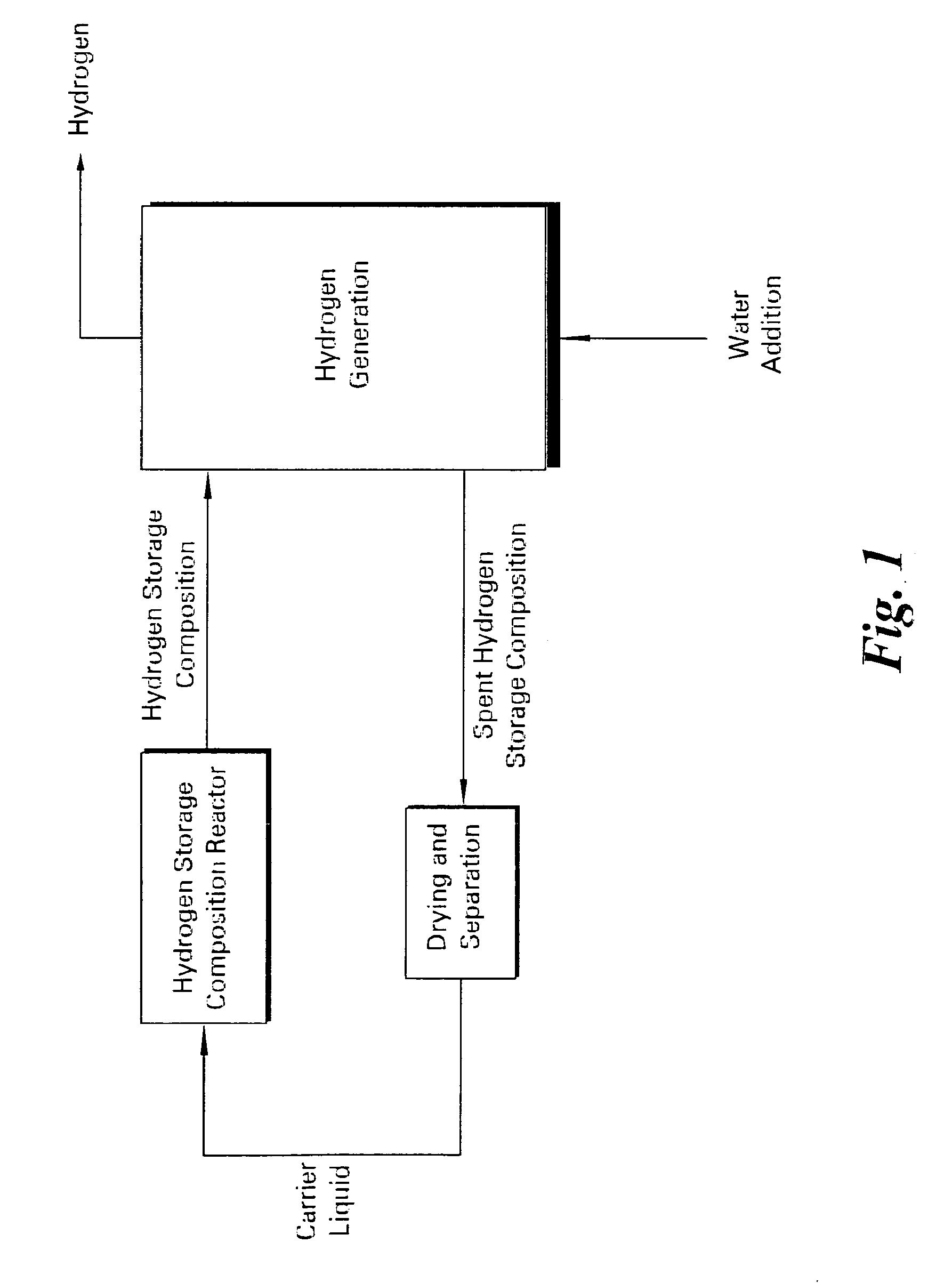
Apparatus and method for in situ production of fuel for a fuel cell
Fuel cell fuel supplies having single and multiple compartments for storing and containing fuel cell fuel precursor reagents. These fuel supplies allow storage and packaging of precursors for in situ production and use of fuel cell fuel. A method for making fuel cell fuel and a fuel cell system is also disclosed.
Owner:INTELLIGENT ENERGY LTD
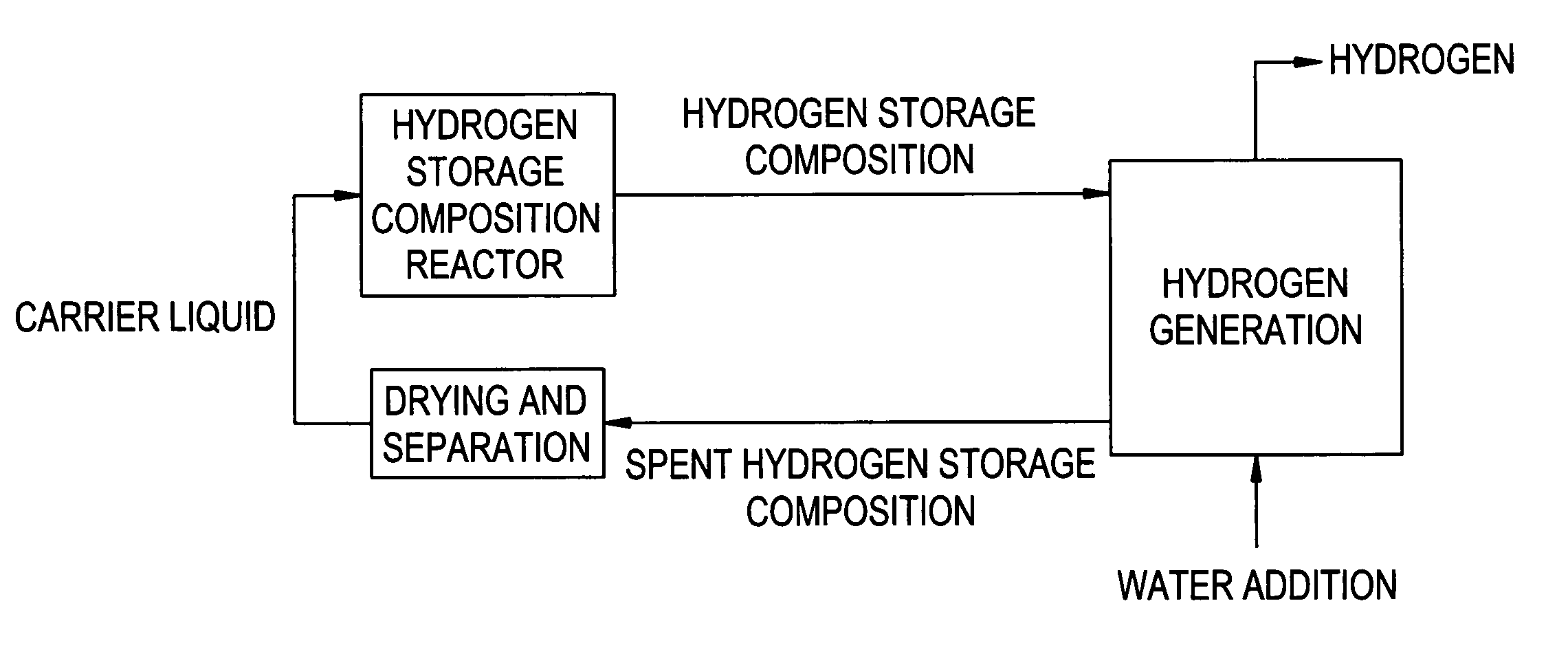
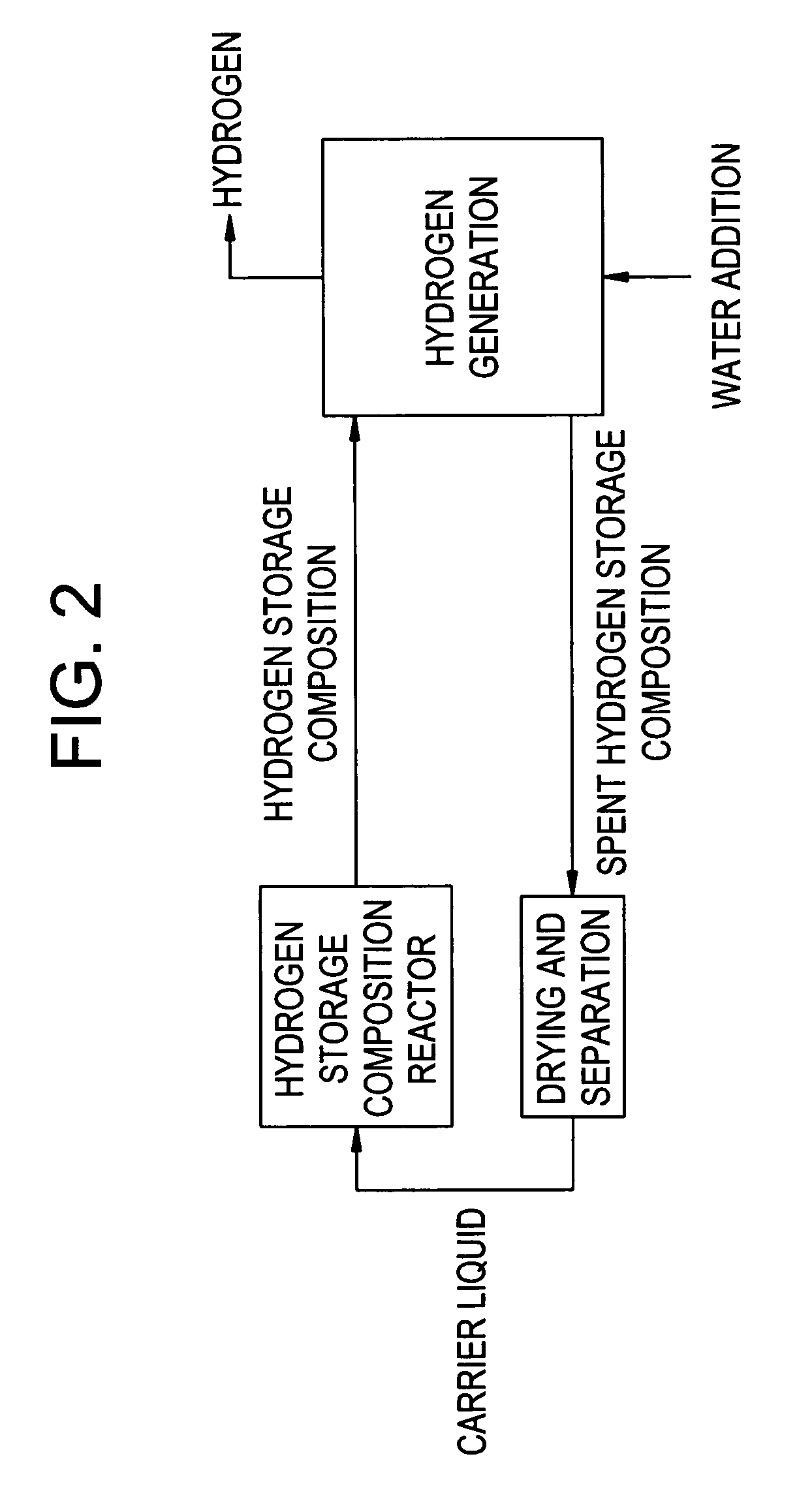
Compositions and methods for hydrogen storage and recovery
InactiveUS7175826B2Alkali/alkaline-earth/beryllium/magnesium hydridesReversible hydrogen uptakeIridiumRhenium
Disclosed herein is a hydrogen storage composition comprising a catalyst composition disposed upon a storage composition; wherein the catalyst composition comprises an alloy of calcium, barium, platinum, palladium, nickel, titanium, chromium, manganese, iron, cobalt, copper, silicon, germanium, rhodium, rhodium, ruthenium, molybdenum, niobium, zirconium, yttrium, barium, lanthanum, hafnium, tungsten, rhenium, osmium, iridium, or a combination comprising at least one of the foregoing metals.
Owner:GENERAL ELECTRIC CO
Method of controlled alcoholysis and regeneration of a borohydride
InactiveUS20050255024A1Efficient procedureEffective protocolMonoborane/diborane hydridesBoron/boridesSodium borohydrideBoric acid
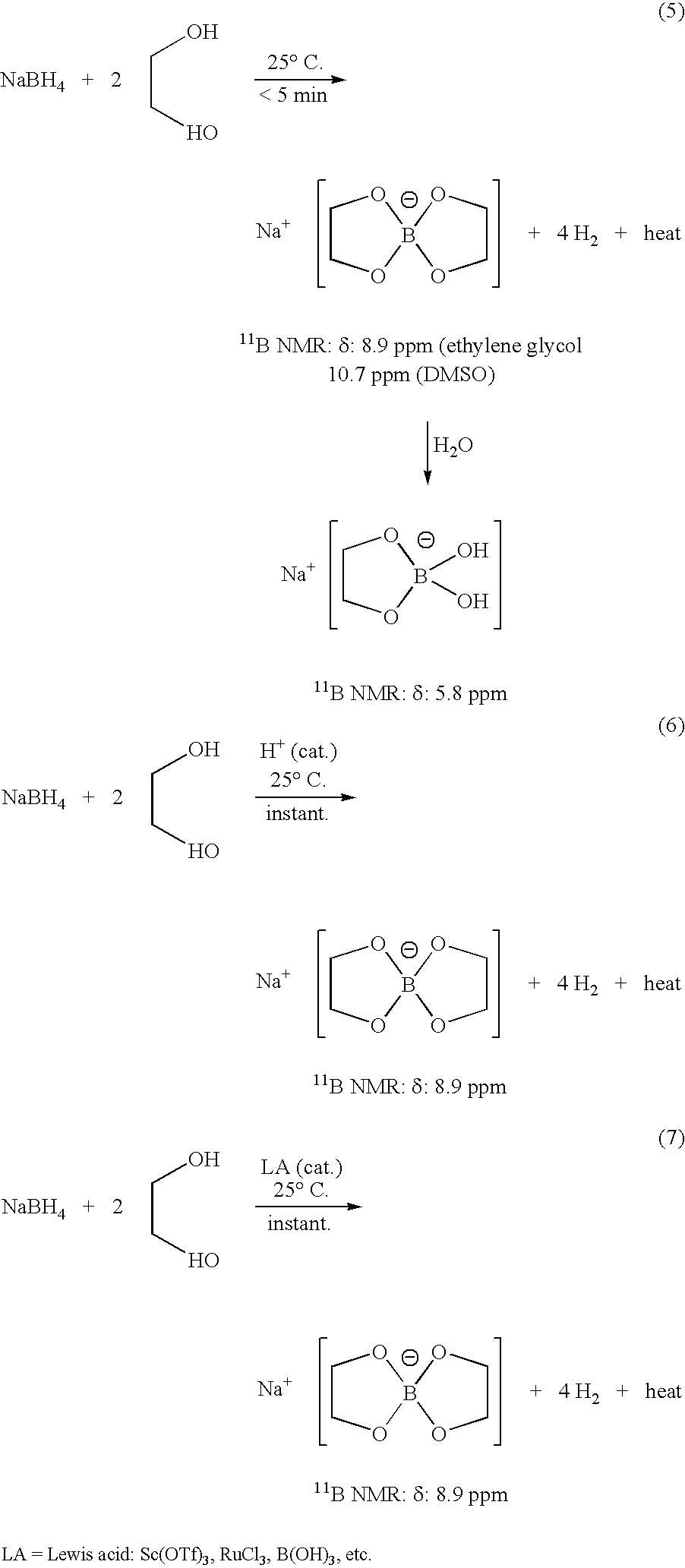
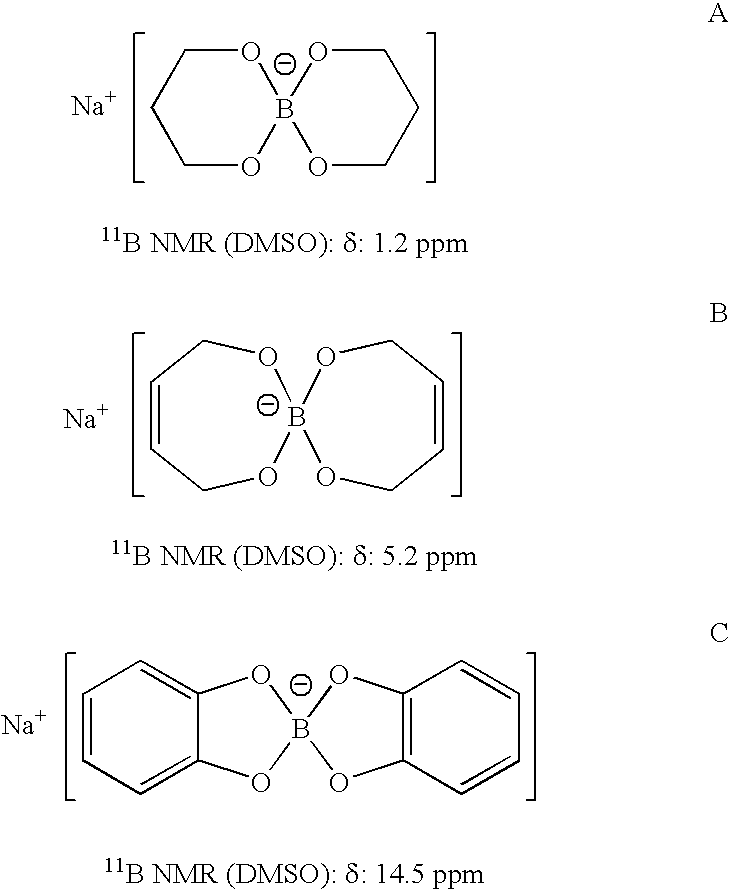
Methods of controlled hydrolysis / alcoholysis and regeneration of a borohydride are disclosed. Examples of the present invention show that hydrolysis of sodium borohydride or lithium borohydride with dilute acid provides simultaneous generation of H2 and boric acid for recycling. Other examples of the present invention show methods for regenerating a borohydride by reacting an aluminum hydride to a borate compound to provide a regenerated borohydride.
Owner:PURDUE RES FOUND INC
Process for production of sodium borohydride from sodium aluminum hydride with recycle of byproducts
A process for production of sodium borohydride. The process comprises the steps of: (a) combining a boric acid ester, B(OR)3 and sodium aluminum hydride to produce sodium borohydride and Al(OR)3; and (b) combining Al(OR)3 and sulfuric acid to produce alum and ROH.
Owner:ROHM & HAAS CO
Methods of forming nano-structured materials including compounds capable of storing and releasing hydrogen
InactiveUS20090068051A1Easy to paintOther chemical processesMonoborane/diborane hydridesNanostructured materialsNanostructure
Methods of forming materials that contain hydrogen storage materials and nano-structured matrices are described. In one embodiment, the hydrogen storage material is a complex hydride. In another embodiment, the method includes melting at least one compound capable of storing and releasing hydrogen, obtaining an aluminum-containing nano-structured matrix having a melting point higher than the temperature of the at least one compound, and contacting the molten at least one compound with the nano-structured matrix to facilitate the coating of the nano-structured material with the molten at least one compound. The matrix may undergo mechanical working to further modify the nano-structure. In yet another embodiment, the method includes forming a powder including a gas-atomized aluminum-containing powder, and pressing or sintering the powder to form a matrix, such that the matrix has nano-meter scale pores.
Owner:GROSS KARL
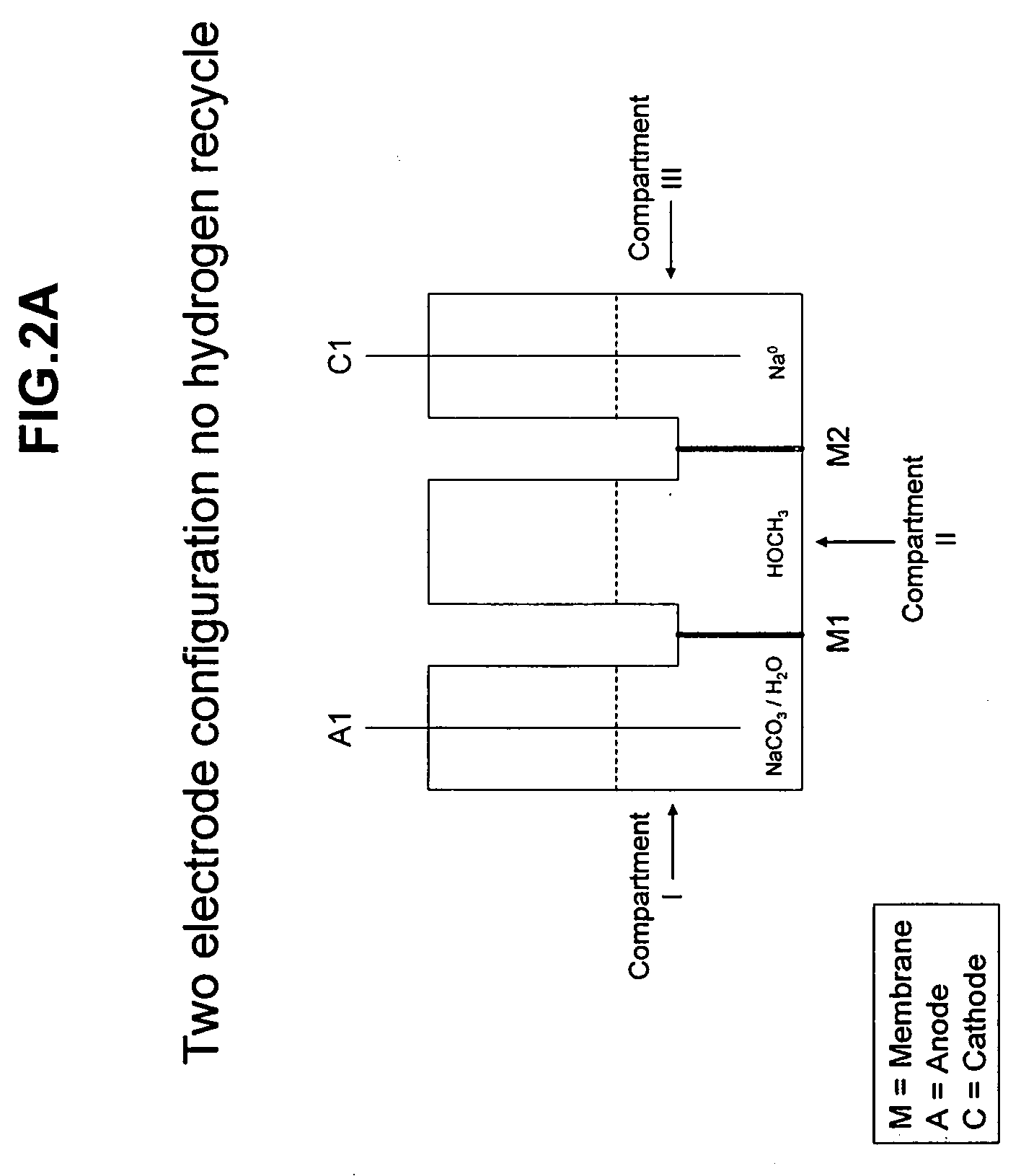
Processes and reactors for alkali metal production
Electrochemical processes and apparatus for obtaining metals from metal salts, including for separating alkali metal and alcohols from alkali metal alkoxide compounds, are disclosed. Aqueous solutions of metal alkoxides or metal carbonates are converted to metals by electrochemical processes which may also be integrated into processes for the production of borohydrides, such as sodium borohydride.
Owner:MILLENNIUM CELL
Processes for synthesizing alkali metal borohydride compounds
Processes for synthesizing borohydride compounds with reduced energy requirements and high efficiency are disclosed. The processes include the reaction of a base with a borane complex or diborane to produce a borohydride compound of formula YBH4, where Y is a monovalent cationic moiety.
Owner:MILLENNIUM CELL
Solid-phase synthesis method of Mg(BH4)2 hydrogen storage material
InactiveCN102730639ANo pollution in the processEasy to operateMonoborane/diborane hydridesHydrogen productionHigh pressure hydrogenHigh pressure
The invention discloses a solid-phase synthesis method of a Mg(BH4)2 hydrogen storage material. Magnesium boride or a mixture of magnesium boride and magnesium hydride is used as a starting material to carry out a mechanochemical reaction in high pressure hydrogen atmosphere, with a catalyst selected from one or more of a transition metal, a transition metal halide, a transition metal hydride and a transition metal oxide, so as to prepare the Mg(BH4)2. The preparation method of the Mg(BH4)2 hydrogen storage material of the invention has advantages of simple synthetic method, easily controlled reaction process, low cost and no organic pollution.
Owner:ZHEJIANG UNIV
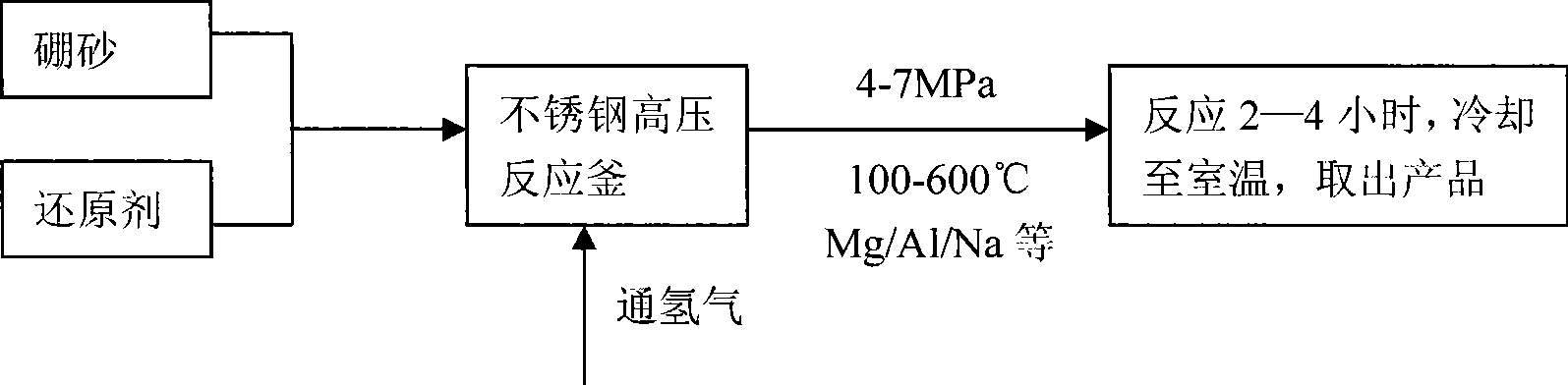
Method for preparing sodium borohydride
InactiveCN101269793AAvoid strict requirementsHigh yieldMonoborane/diborane hydridesHydrogen pressureSolid reaction
The invention belongs to the technical field of compound preparation, in particular relates to a preparation method of sodium borohydride. The invention is different from the traditional gas-solid heterogeneous reaction, and carries out solid-state reaction by a metal hydride and a boron-containing compound, and then the sodium borohydride is prepared. The reaction in the invention is carried out completely at the inner part of solid-state materials, so the sodium borohydride can be obtained under lower hydrogen pressure even in an atmosphere of inert gases, thus, high-pressure hydrogen avoids to be used and the requirement to equipment is reduced. The preparation method has the advantages of simple technical process and easy realization of scale production.
Owner:FUDAN UNIV
Graphene-metal or semimetal shell-core structure composite material and preparation method thereof
ActiveCN106045794AImprove performanceSolve application barriersAlkali/alkaline-earth/beryllium/magnesium hydridesCarbon compoundsSemimetalOrganic solvent
The invention relates to a graphene-metal or semimetal shell-core structure composite material and a preparation method thereof. The method comprises the steps that obtained modified graphene oxide is taken as a base to be concentrated and dried by evaporation, and then organic solvent displacement is conducted to obtain an organic solution of the modified graphene oxide; the surface of metal or semimetal is coated with the modified graphene oxide through a liquid-phase self-assembly method to form a graphene-metal or semimetal coating solution; after filtering and drying are conducted, the graphene-metal or semimetal shell-core composite material is obtained. According to the method, a conventional organic matter and inorganic matter coating process is improved, the influences of the water solvent and high temperature on the activity of some metals and semimetals with the high reaction activity are reduced, process realization of a coating method is expanded, and application barriers of the graphene and the active metal or semimetal in an energy-containing material are solved.
Owner:HUBEI INST OF AEROSPACE CHEMOTECHNOLOGY
Devices and methods for hydrogen storage and generation
InactiveUS7029517B2Reversible hydrogen uptakeMultiple metal hydridesChemisorptionMicrowave irradiation
Disclosed herein is a method for the storage of hydrogen comprising contacting a hydrogen storage composition with a gaseous mixture comprising hydrogen; and irradiating the hydrogen storage composition with radio frequency radiation or microwave radiation in an amount effective to facilitate the absorption, adsorption and / or chemisorption of hydrogen into the hydrogen storage composition.
Owner:GENERAL ELECTRIC CO
Direct elemental synthesis of sodium borohydride
Owner:ROHM & HAAS CO
Method for preparing sodium borohydride by chemical mechanical mechanics method
InactiveCN101519188ALow costSimple and safe processMonoborane/diborane hydridesChemical reactionHydrogen pressure
The invention relates to a method for preparing alkali borohydride, in particular to a method for preparing sodium borohydride by a chemical mechanical mechanics method and solving the problems of environmental pollution, severe operation condition and the like during the preparation process of the sodium borohdride in the prior art. The method takes magnesium hydride as a hydrogen source, takes borax as a boron source and prepares the sodium borohydride by combining mechanical grinding with chemical reaction. In the hydrogen atmosphere, under a certain temperature and pressure, the magnesium powder is mechanically grinded and generates hydrogenation reaction with the hydrogen, thus preparing magnesium hydride; subsequently, the borax, sodium carbonate and magnesium hydride are arranged in a ball mill with a certain proportion; certain hydrogen pressure, ball material ratio and grinding time are kept, thus obtaining the sodium borohydride. The purity of the sodium borohydride prepared by the method can achieve more than 97%. The chemical mechanical mechanics method is adopted to prepare the sodium borohydride, and compared with the currently general Schlesinger method and Baeyer method, the method has the advantages of low raw material cost, simple, convenient and safe process, no pollution and the like.
Owner:INST OF METAL RESEARCH - CHINESE ACAD OF SCI
Hydrogen storage compositions and methods of manufacture thereof
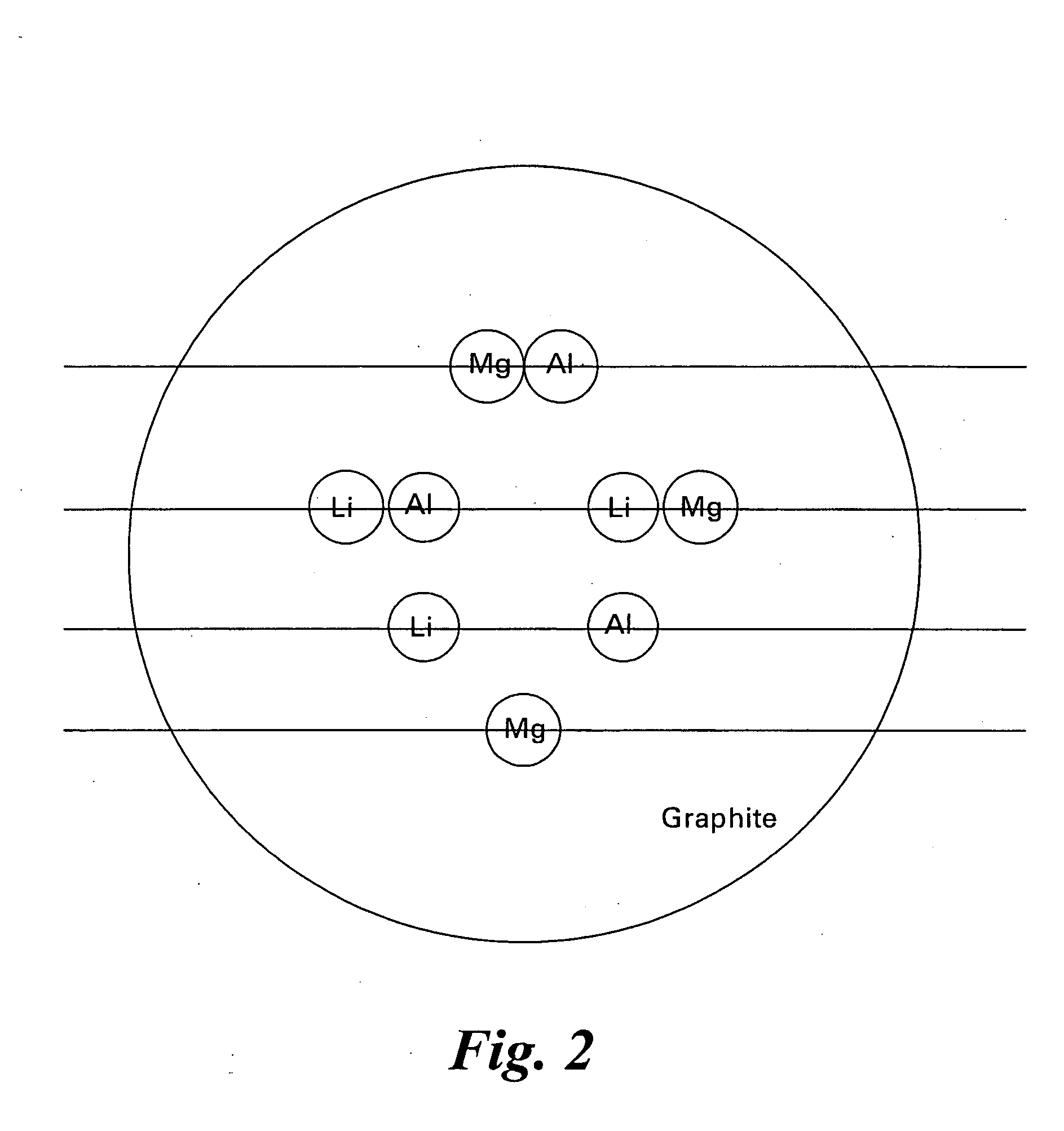
Disclosed herein is a method for making a combinatorial library comprising disposing on a substrate comprising silicon, silicon nitride, silicon carbide or silicon boride at least one reactant, wherein the reactants are lithium, magnesium, sodium, potassium, calcium, aluminum or a combination comprising at least one of the foregoing reactants; heat treating the substrate to create a diffusion multiple having at least two phases; contacting the diffusion multiple with hydrogen; detecting any absorption of hydrogen; and / or detecting any desorption of hydrogen.
Owner:GENERAL ELECTRIC CO
Hydrogen storage compositions and methods of manufacture thereof
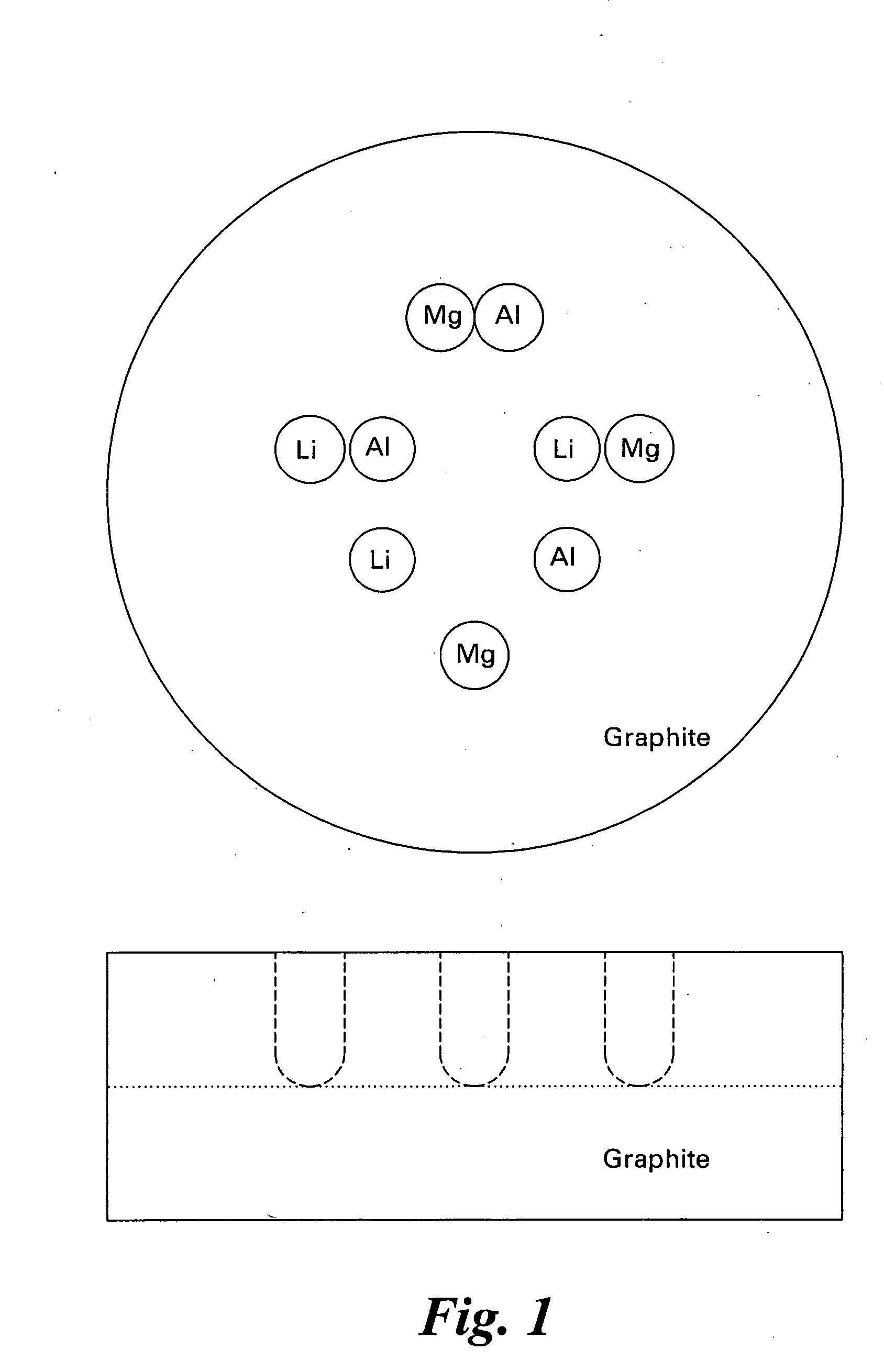
Disclosed herein is a method for making and screening a combinatorial library comprising disposing in a substrate comprising graphite or boron carbide at least one reactant, wherein the reactants are lithium, magnesium, sodium, potassium, calcium, aluminum or a combination comprising at least one of the foregoing reactants; heat treating the substrate to create a diffusion multiple; contacting the diffusion multiple with hydrogen having at least two phases; detecting any absorption of hydrogen; and / or detecting any desorption of hydrogen.
Owner:GENERAL ELECTRIC CO
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com