Solid-phase synthesis method of Mg(BH4)2 hydrogen storage material
A technology of solid-phase synthesis and hydrogen storage materials, which is applied in the production of hydrogen, borane/diborane hydride, etc., can solve the problems of low purity of reaction products, high cost of materials, and complicated reaction process, etc. Easy to control, no organic pollution, and simple synthesis method
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
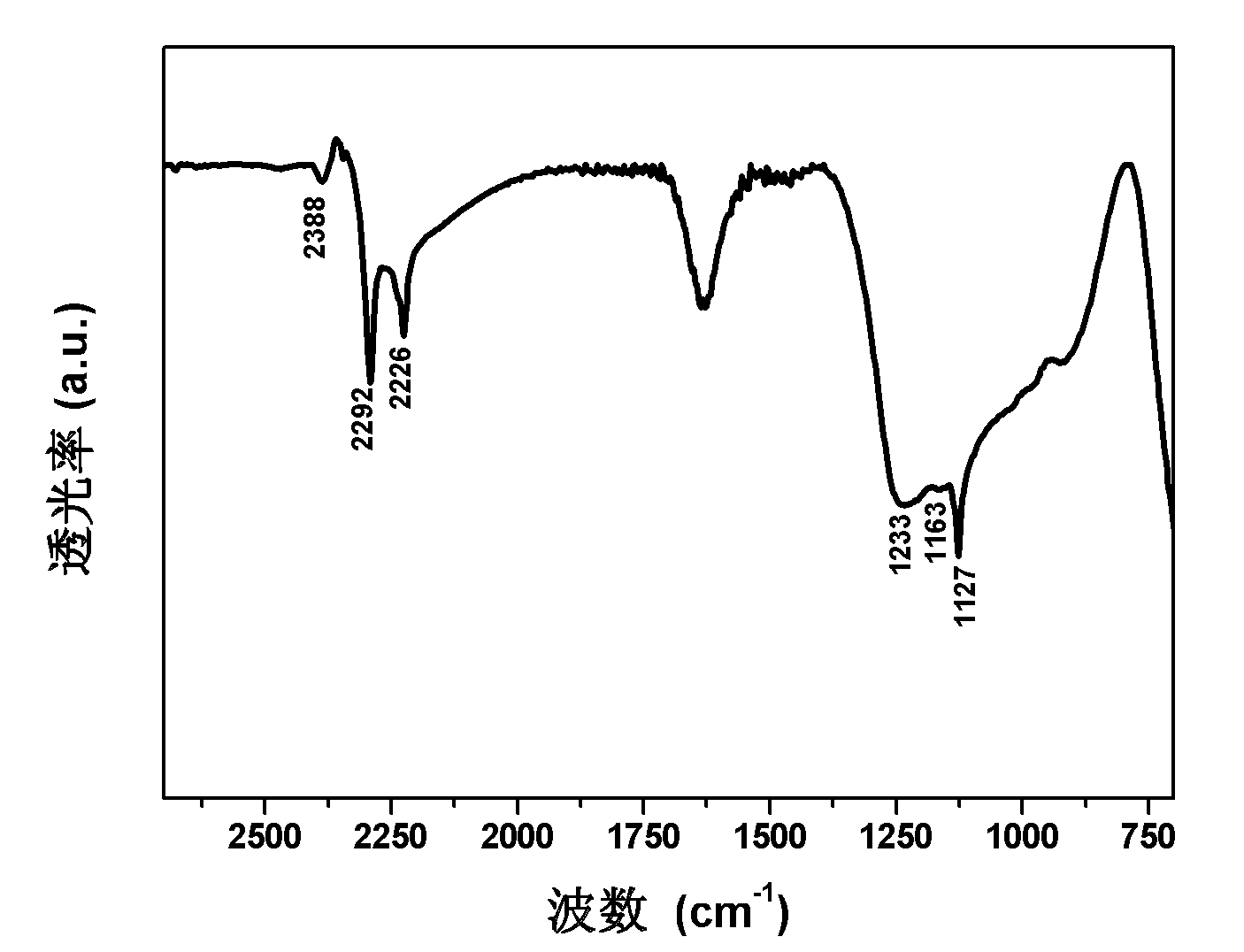
[0038] In a glove box filled with Ar gas, the MgB 2 Respectively with 1wt%, 2wt%, 3wt%, 5wt%, 8wt%, 10wt%, 12wt%, 15wt% of Sc, Ti, V, Fe, Co, Ni, Zr, Nb, Mo, Ru, Pd, Pt, Mix Rh and Hf powder, put it into a stainless steel ball mill tank that can be sealed and has an on-off valve. After the ball mill tank is evacuated, fill it with 80-100 atm hydrogen, and perform ball milling on a planetary ball mill under a high-pressure hydrogen atmosphere. The ratio is 50:1, the rotation speed is 500rpm, and the ball milling time is 24-36h. The samples after ball milling were tested by FTIR. The results showed that all ball milled products were at 1000-1400 and 2200-2400cm -1 Mg(BH 4 ) 2 The characteristic infrared absorption peak of Mg(BH 4 ) 2 Formation. figure 1 MgB shown 2 - FTIR spectrum of the product after high pressure ball milling of 12wt% Ni. Products at 1127, 1163, 1233, 2226, 2292, 2388cm -1 6 Mg(BH 4 ) 2 characteristic infrared absorption peaks.
Embodiment 2
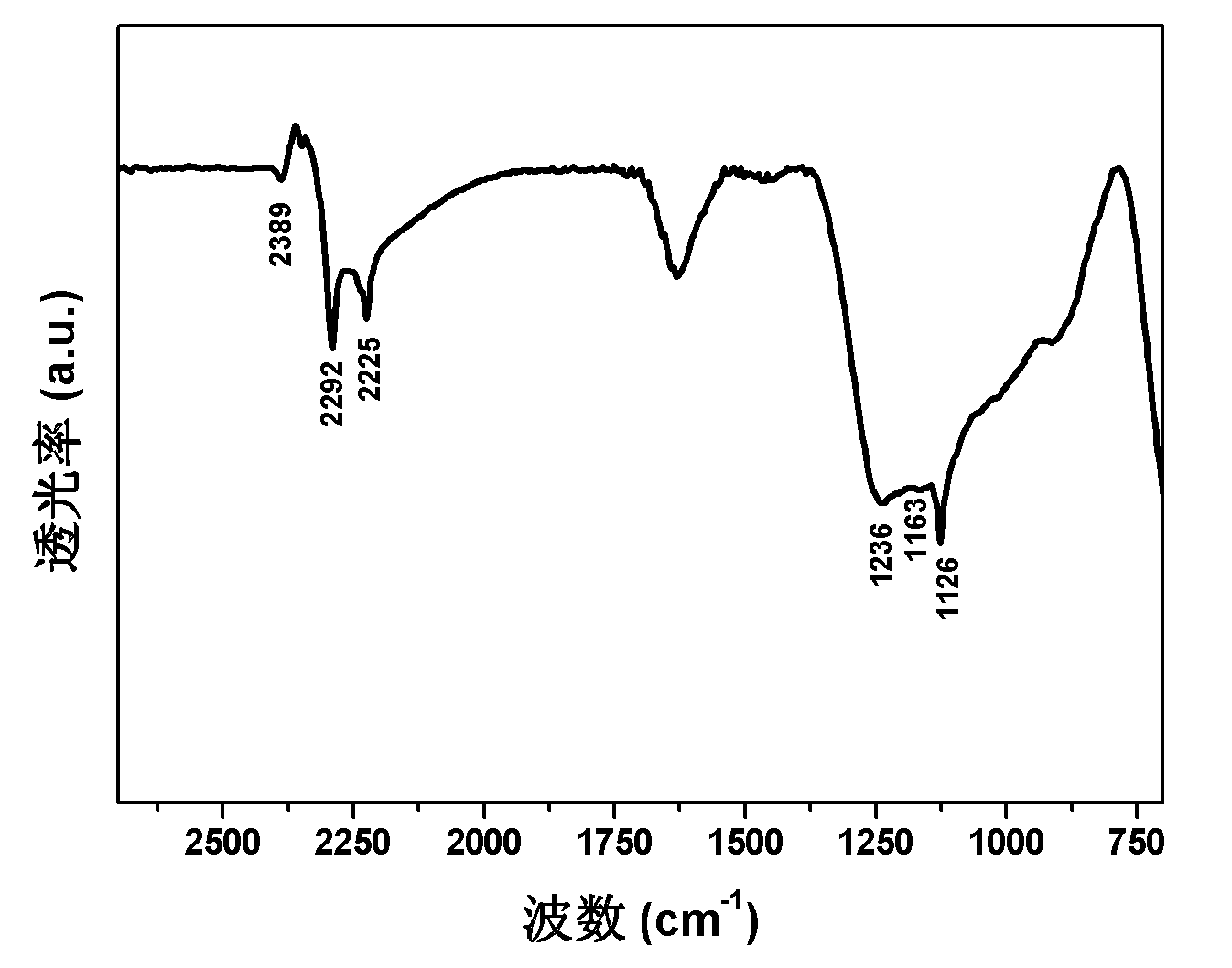
[0040] In a glove box filled with Ar gas, the MgB 2 Respectively with 1wt% Sc-2wt% Ti, 2wt% Sc-1wt% Ti, 2wt% Ti-3wt% V, 2wt% V-1wt% Co, 3wt% Fe-1wt% Ni, 3wt% Zr-4wt% Nb, 4wt%Mo-1wt%Ti, 5wt%Ru-2wt%Pd, 1wt%Pd-2wt%Pt, 6wt%Rh-2wt%Hf, 1wt%Sc-2wt%Ti-3wtV, 2wt%Fe-3wt%Co- 2wt%Ni, 3wt%Zr-2wt%Nb-5wt%Mo, 2wt%Ru-3wt%Pd-4wt%Pt, 3wt%Rh-2wt%Hf-6wt%V powder mixed, can be sealed, with a switch The stainless steel ball milling tank of the valve, after the ball milling tank is evacuated, is filled with 85 atm of hydrogen, and under the high-pressure hydrogen atmosphere, ball milling is carried out on a planetary ball mill with a ball-to-material ratio of 75:1, a rotating speed of 480 rpm, and a ball milling time of 32 hours. The samples after ball milling were tested by FTIR, and the results are listed in Table 1. It can be seen from the data in the table that all ball milling products are at 1000-1400 and 2200-2400cm -1 Mg(BH 4 ) 2 The characteristic infrared absorption peak of Mg(BH 4 ) ...
Embodiment 3
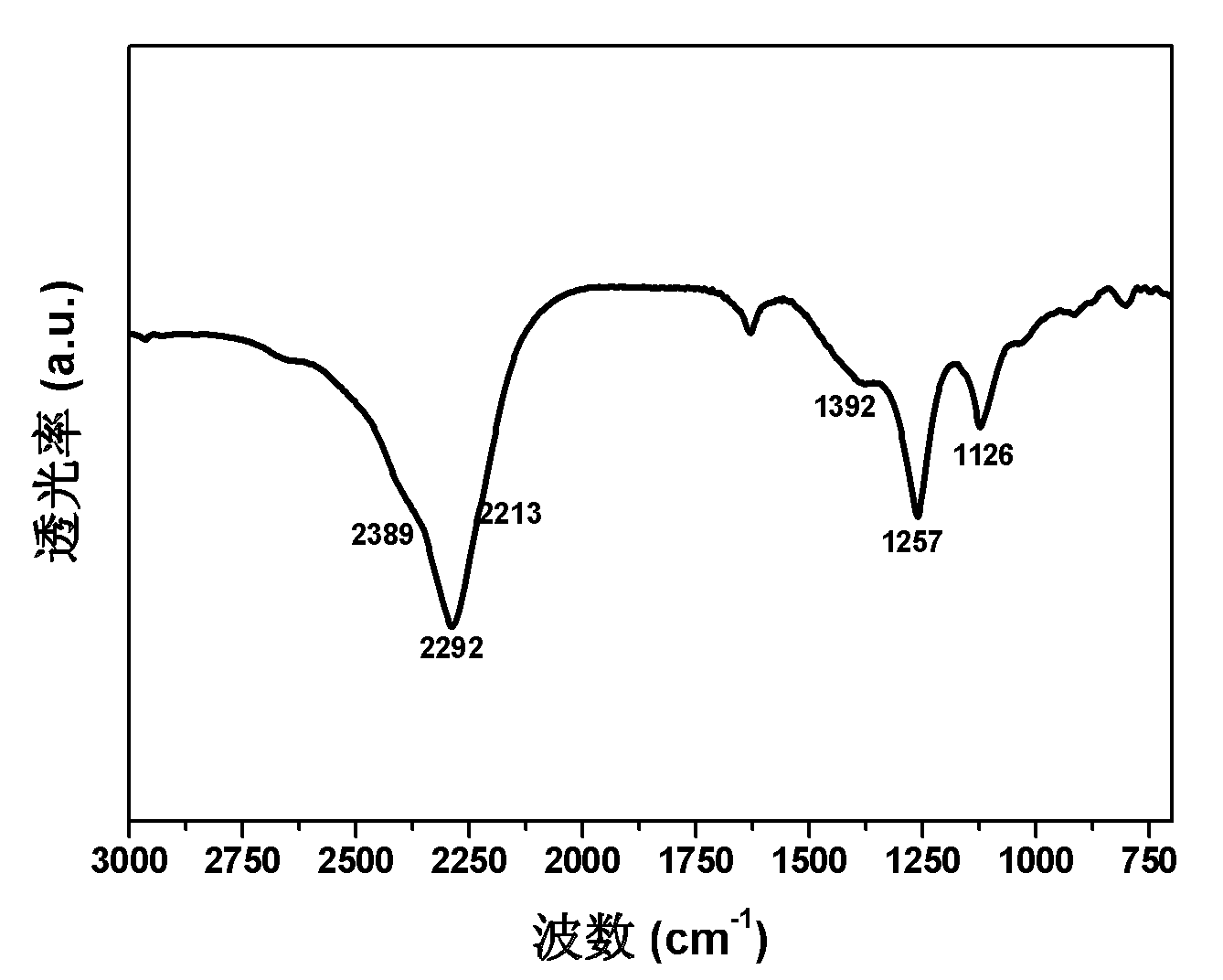
[0044] In a glove box filled with Ar gas, the MgB 2 -MgH 2 The mixture of 2wt%, 4wt%, 6wt%, 8wt%, 10wt% of ScF 3 , ScCl 3 、ScI 3 、TiF 3 、TiF 4 、TiCl 3 、TiCl 4 、TiI 4 , VF 3 , VF 4 , VCl 3 , VCl 4 , VBr 3 、CoCl 2 , FeCl 3 、NiCl 2 , YCl 3 , ZrCl 4 , NbCl 5 、MoCl 3 、MoCl 5 、MoF 6 、RuBr 3 、RuI 3 , RhCl 3 , HfCl 4 , PdCl 2 , PtCl 2 , PtCl 4 、LaCl 3 , CeCl 4 Mix and put it into a stainless steel ball mill tank that can be sealed and has a switch valve. After the ball mill tank is evacuated, it is filled with 30-80 atm of hydrogen respectively. : 1, the rotating speed is 450rpm, and the ball milling time is 36-48h. The products after ball milling were subjected to FTIR and volumetric hydrogen release tests respectively. The results showed that all the ball milled products were -1 The characteristic infrared absorption peaks of Mg(BH4)2 appear in the wavenumber range, showing that Mg(BH4) 4 ) 2 The hydrogen release of all samples occurred in the range...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com