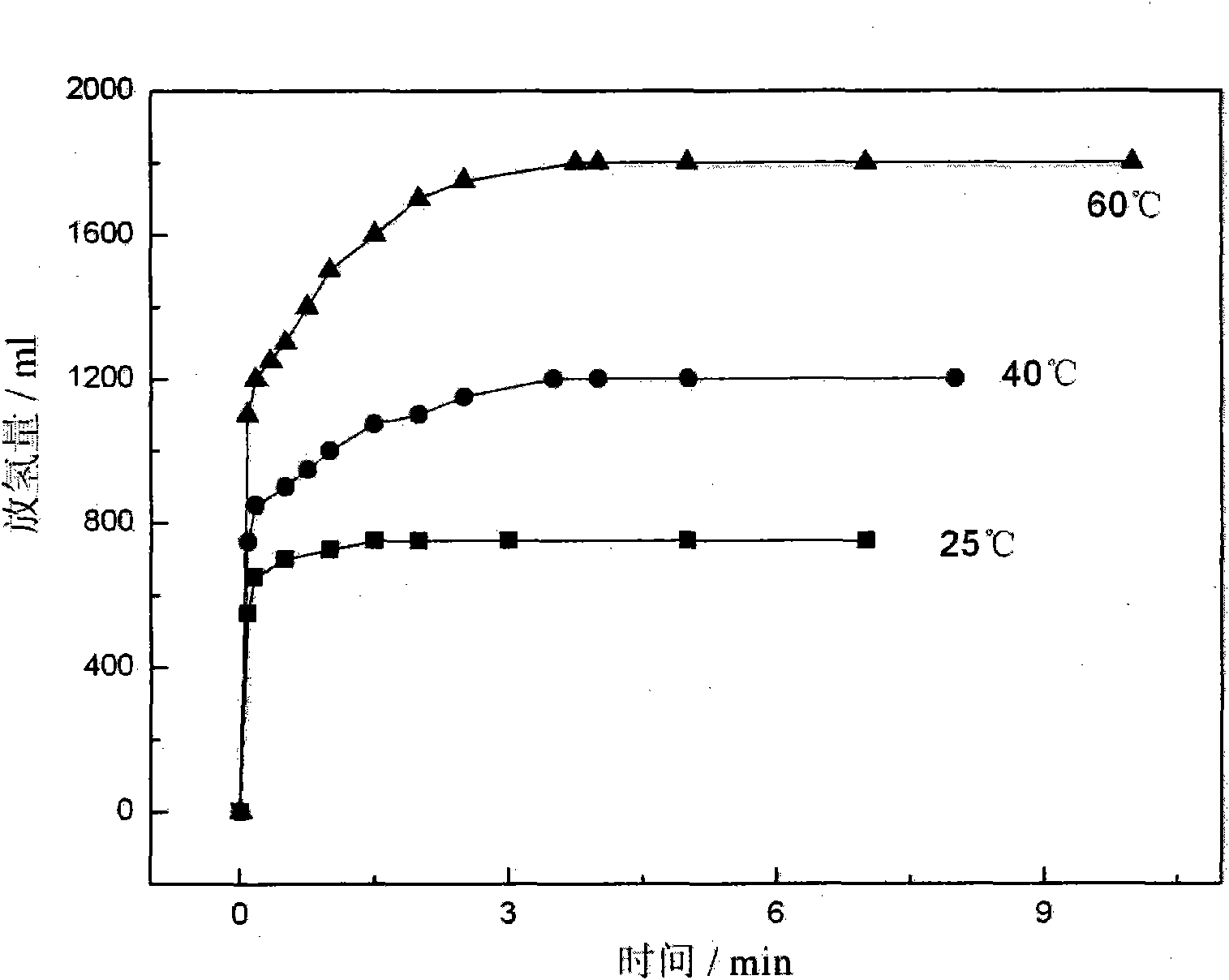
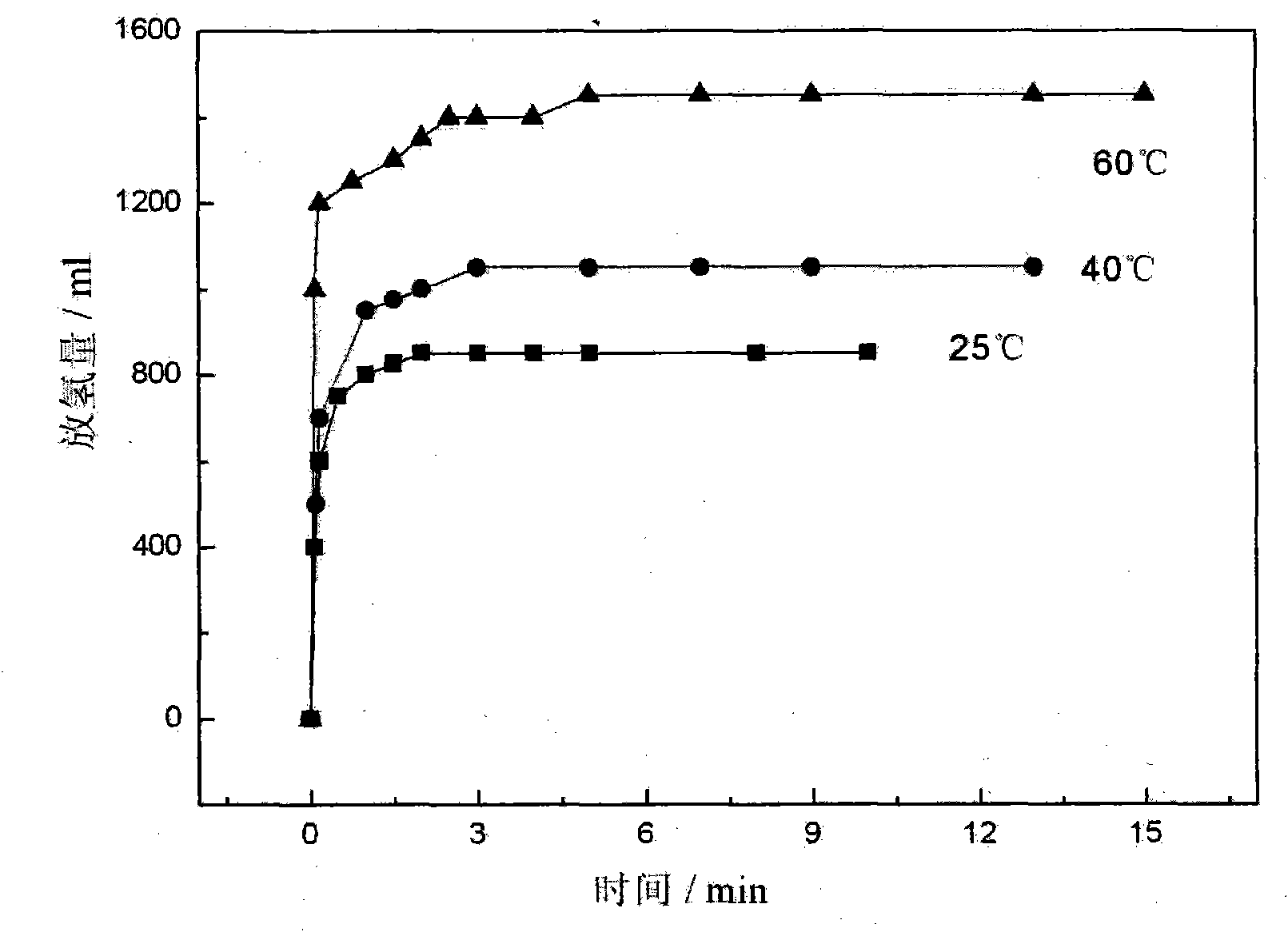
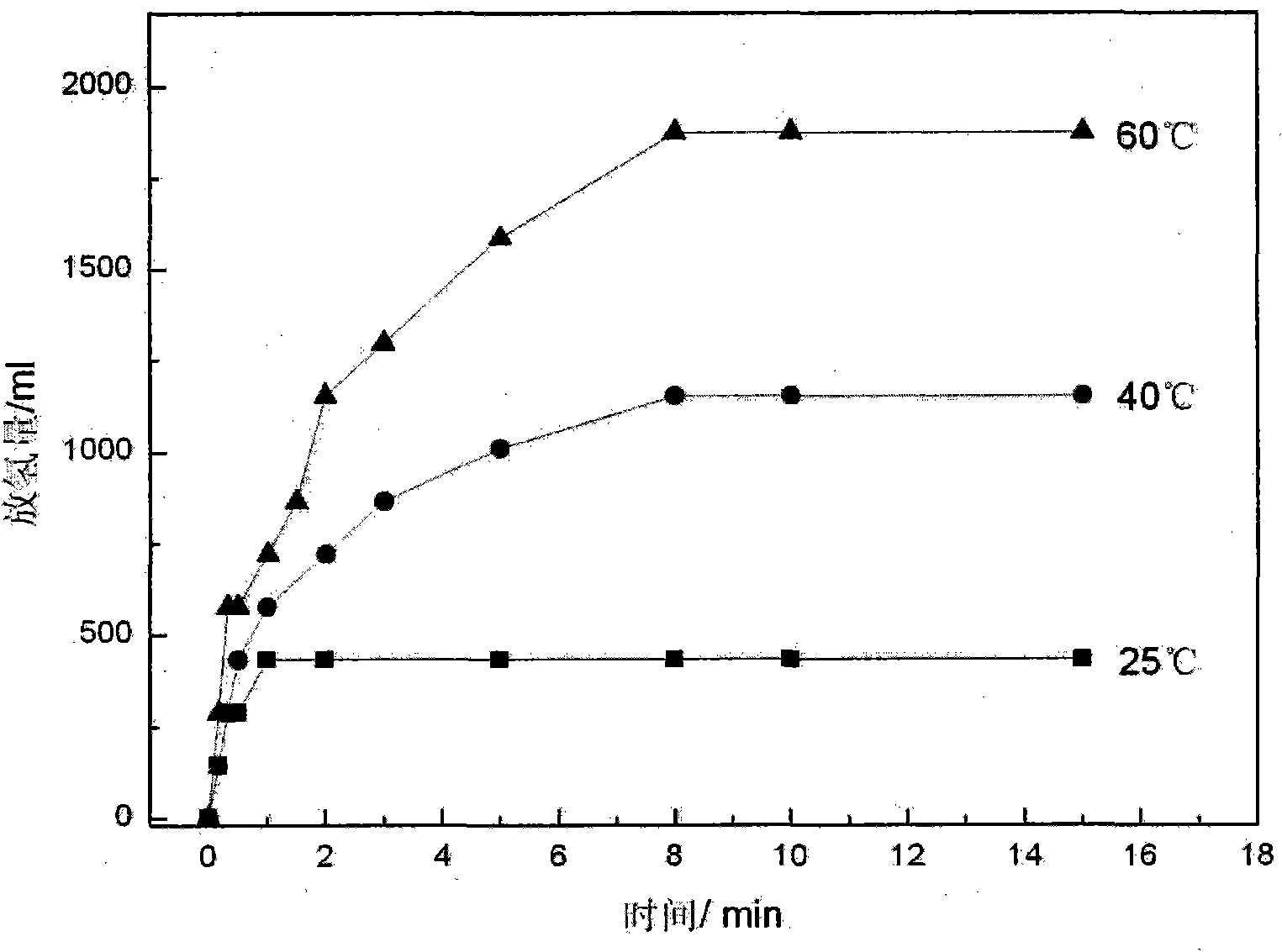
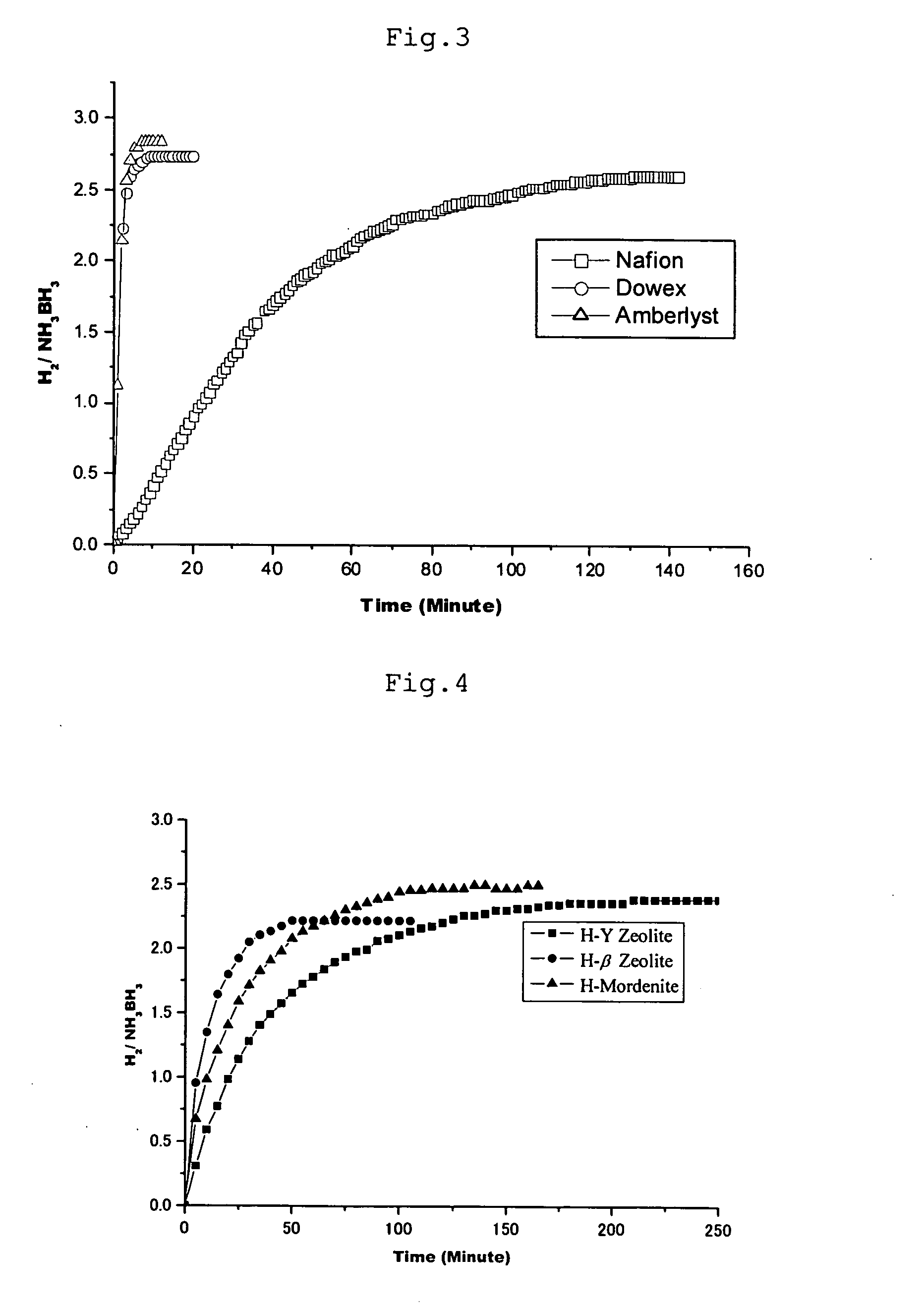
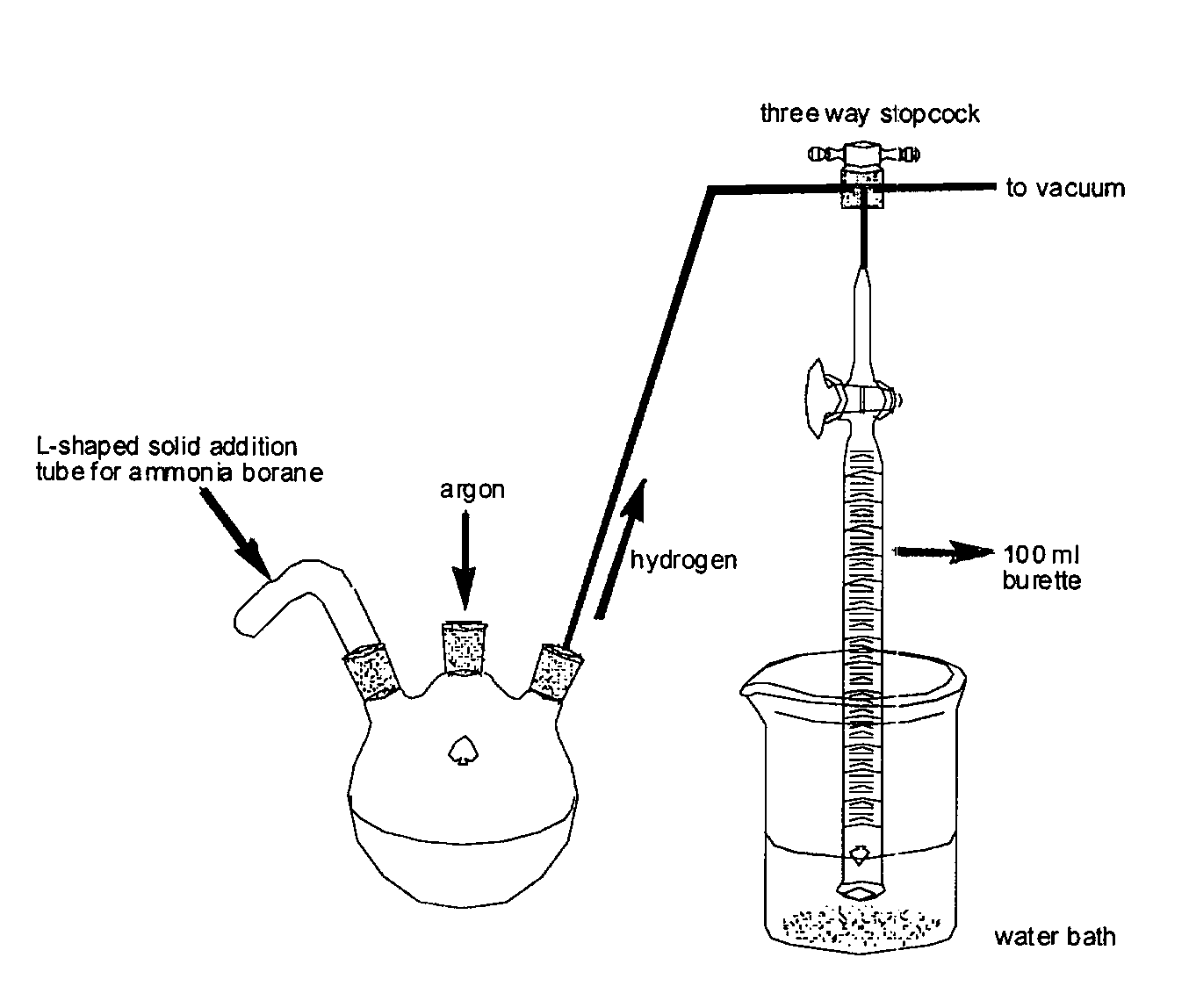
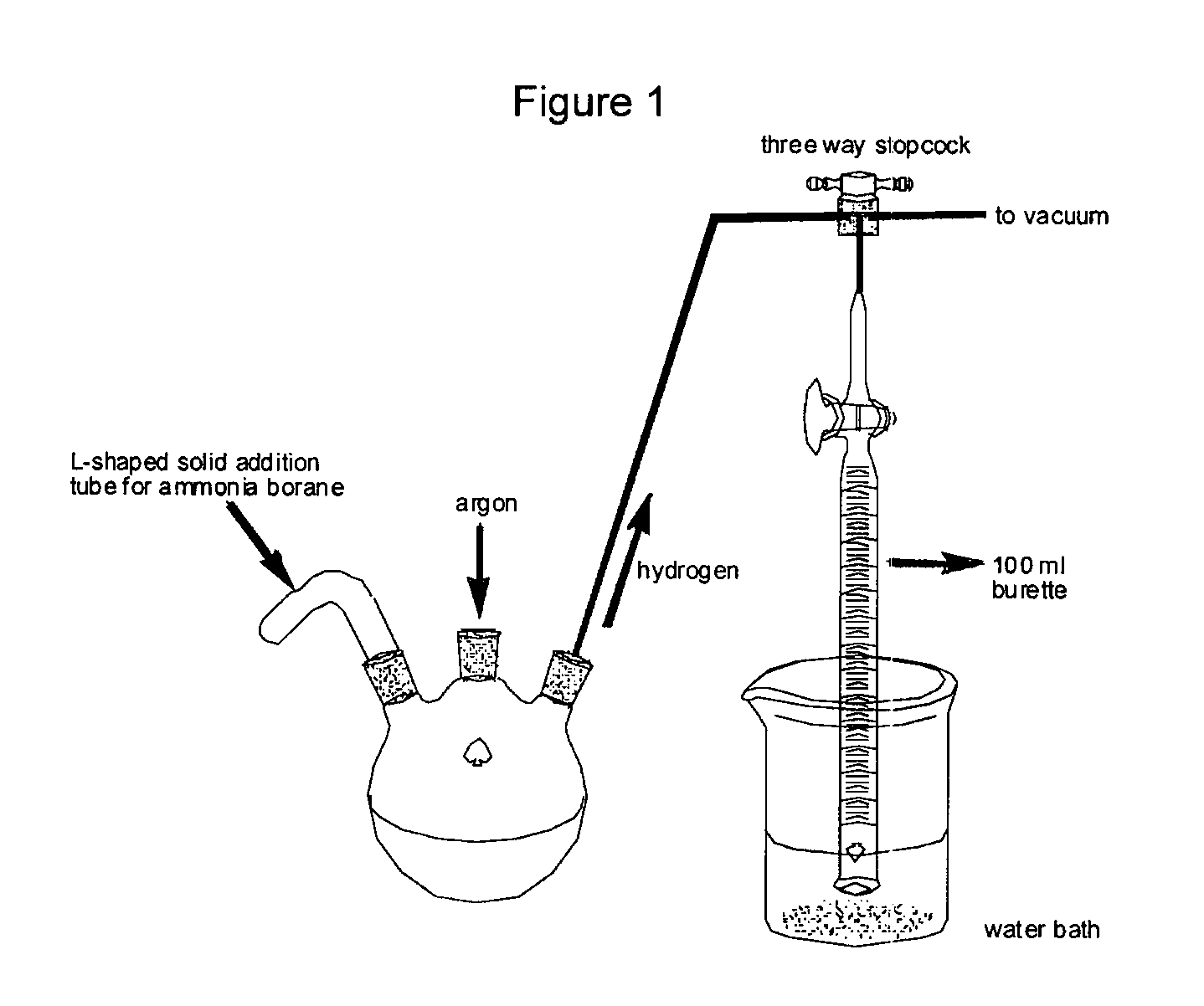
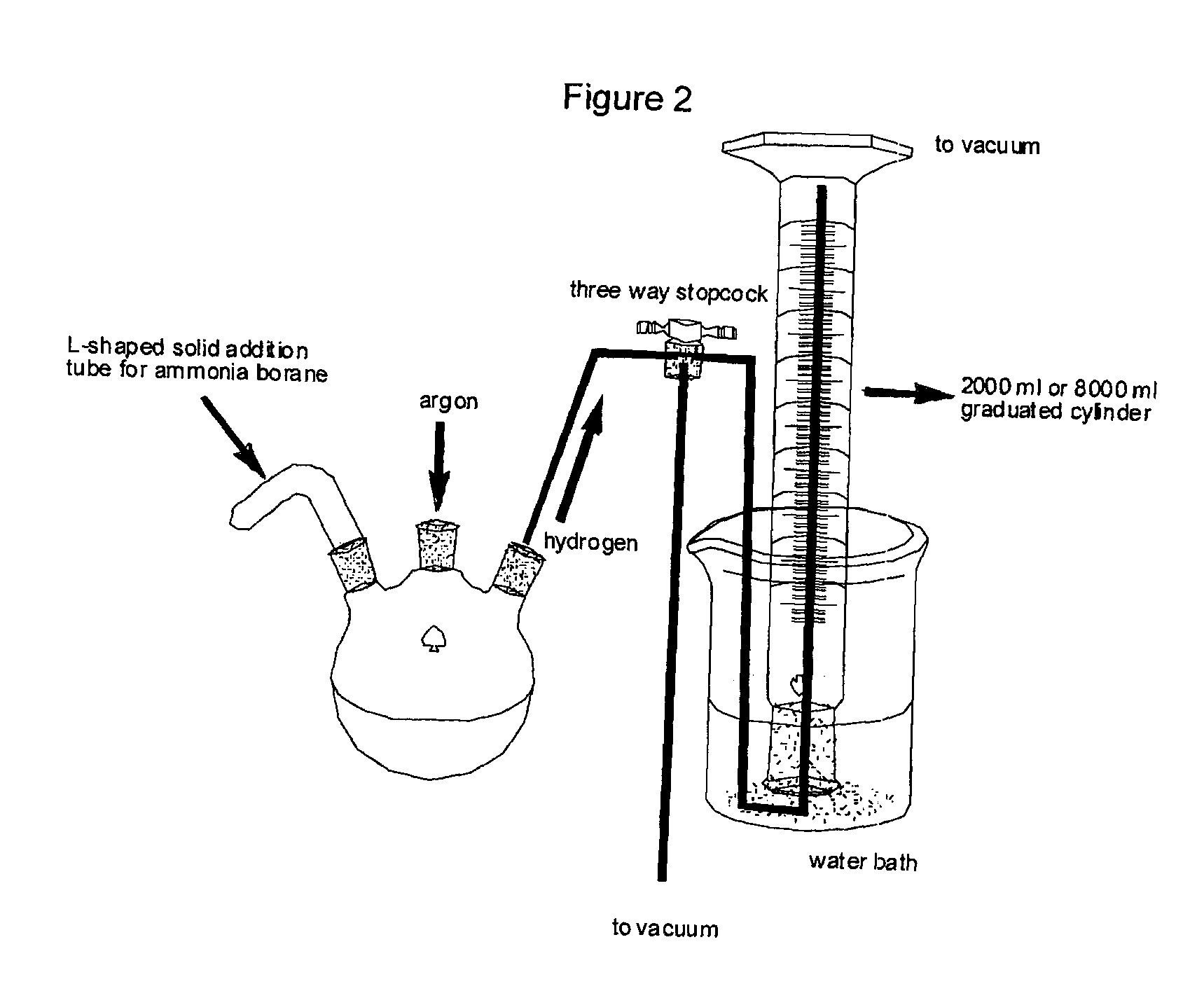
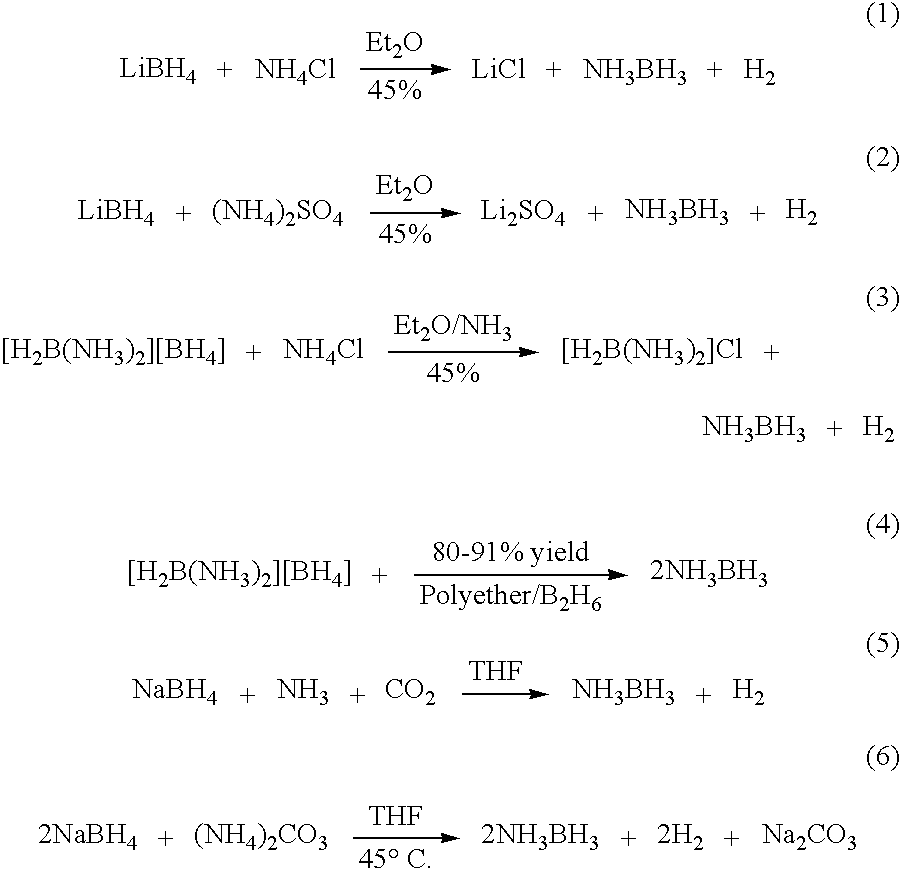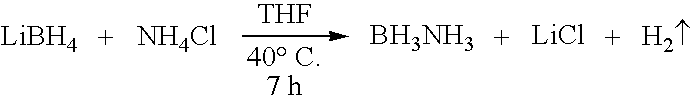Patents
Literature
394 results about "Ammonia borane" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
Ammonia borane (also systematically named amminetrihydridoboron), also called borazane, is the chemical compound with the formula H₃NBH₃. The colourless or white solid is the simplest molecular boron-nitrogen-hydride compound. It has attracted attention as a source of hydrogen fuel, but is otherwise primarily of academic interest.
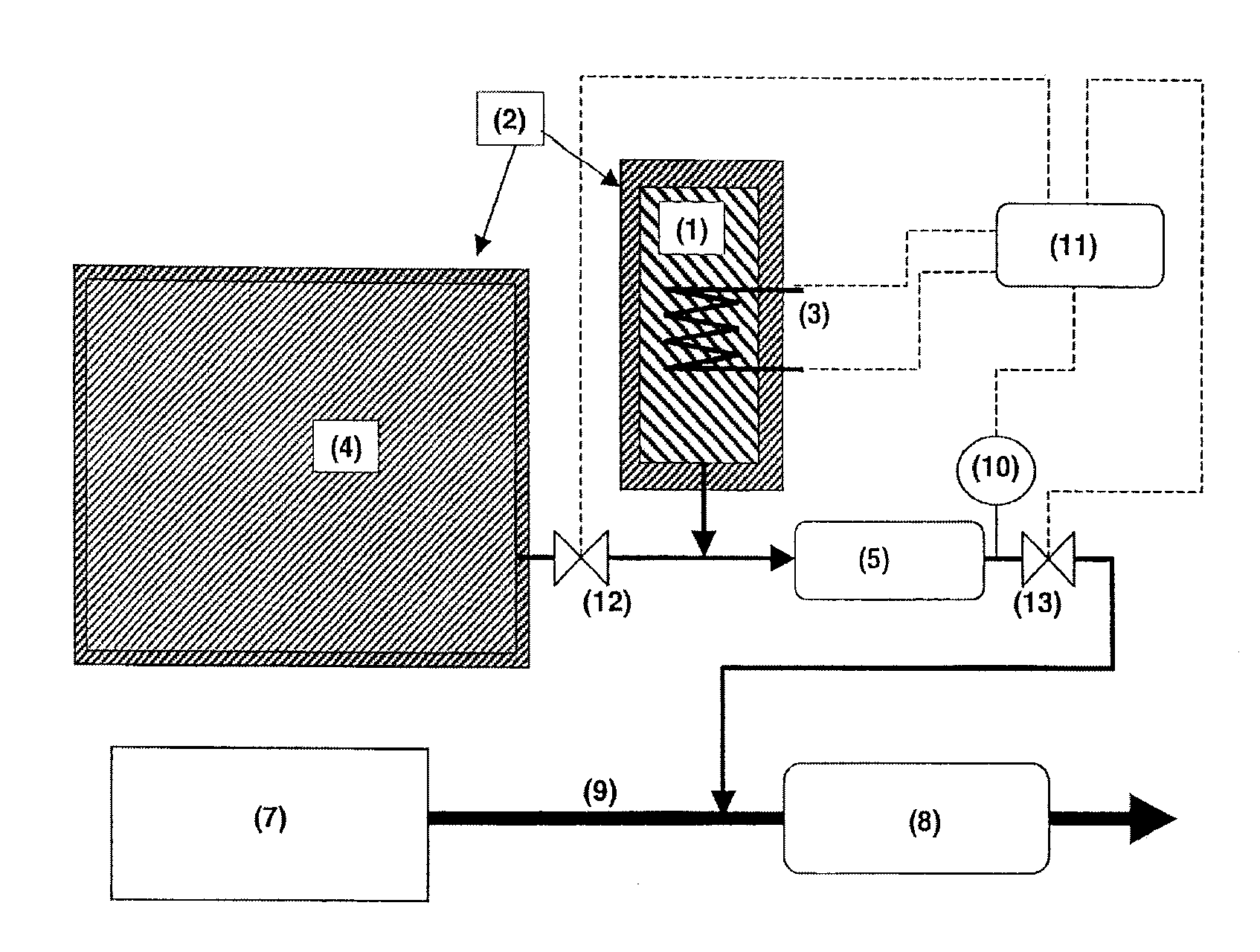
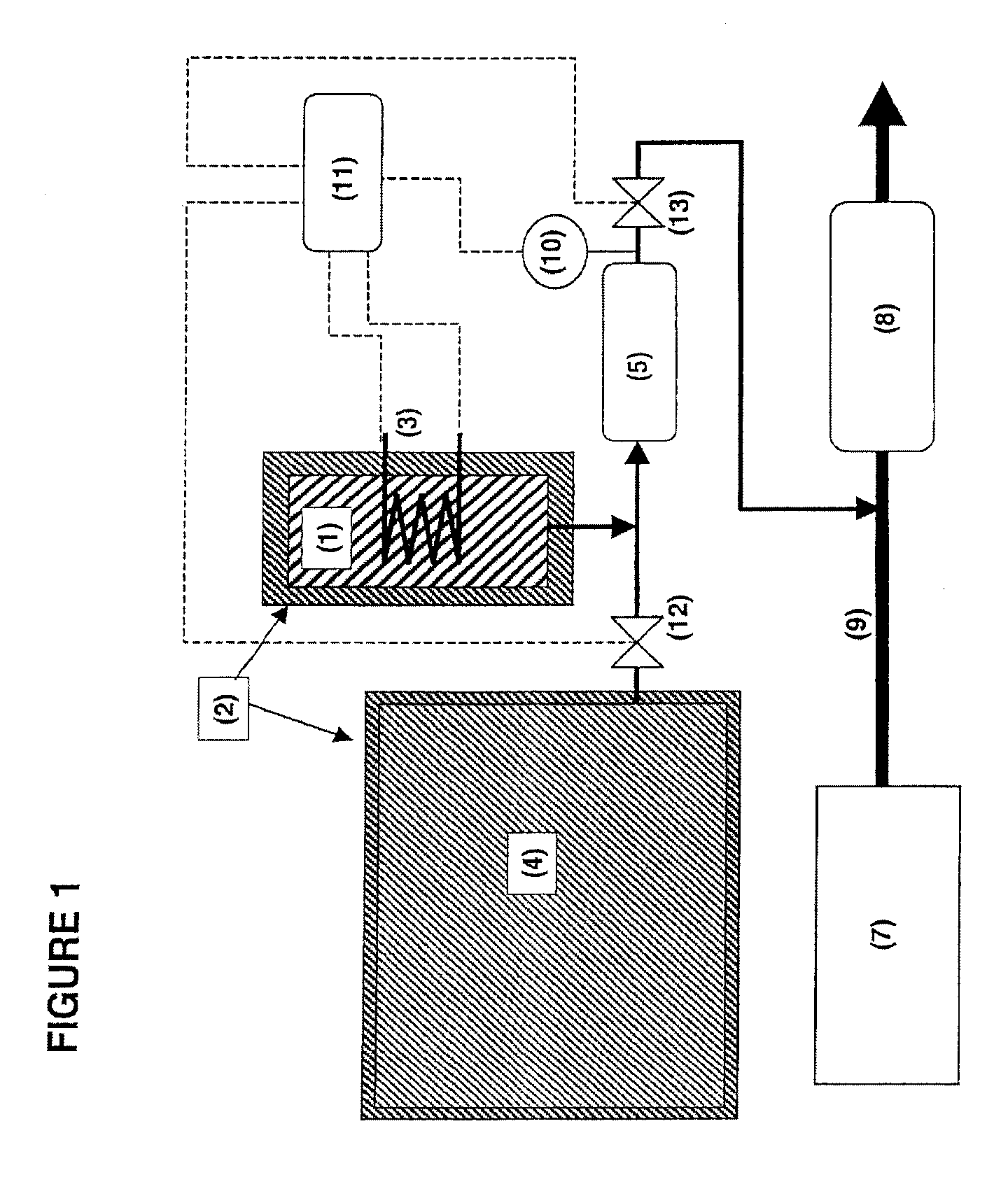
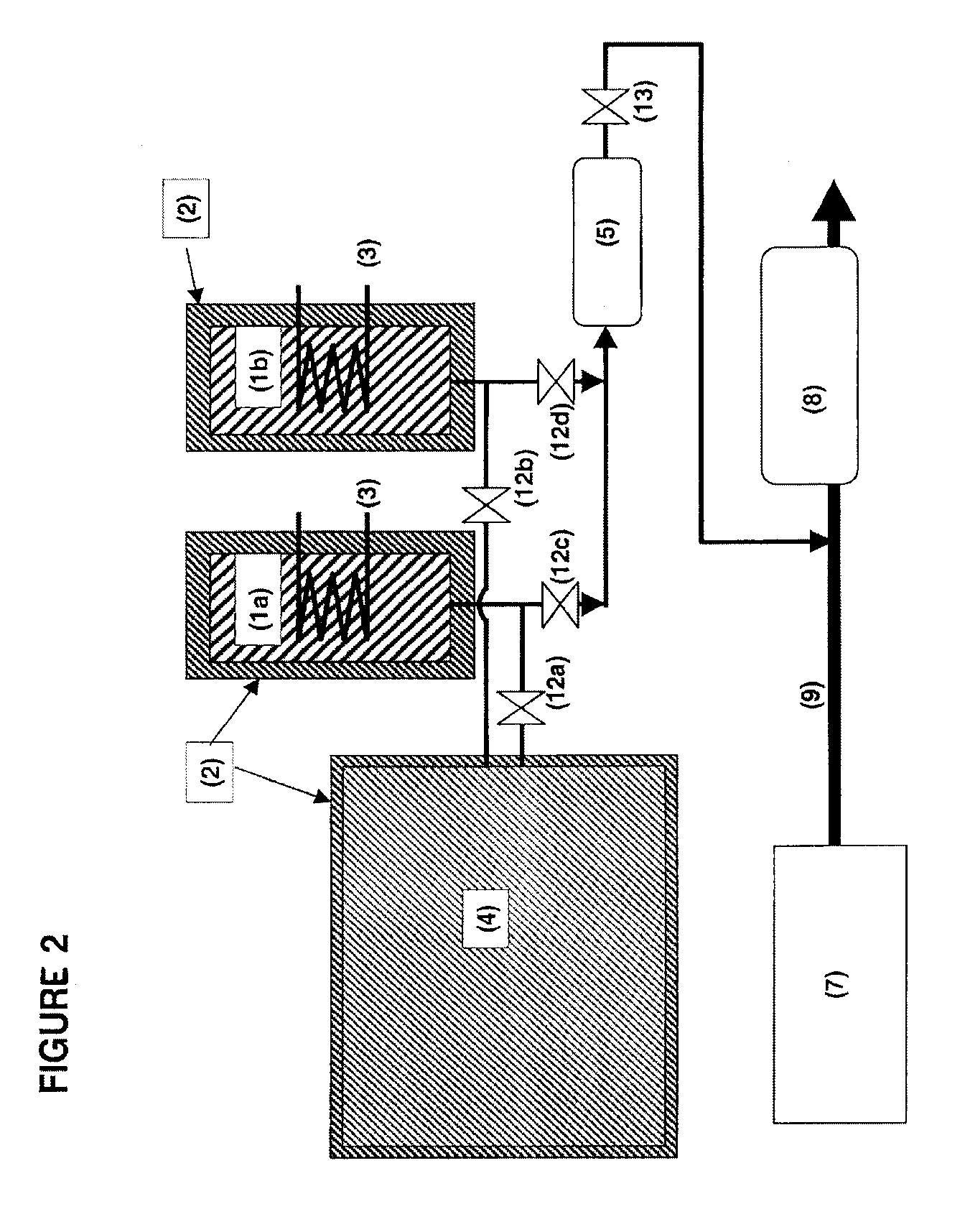
Adsorption based ammonia storage and regeneration system
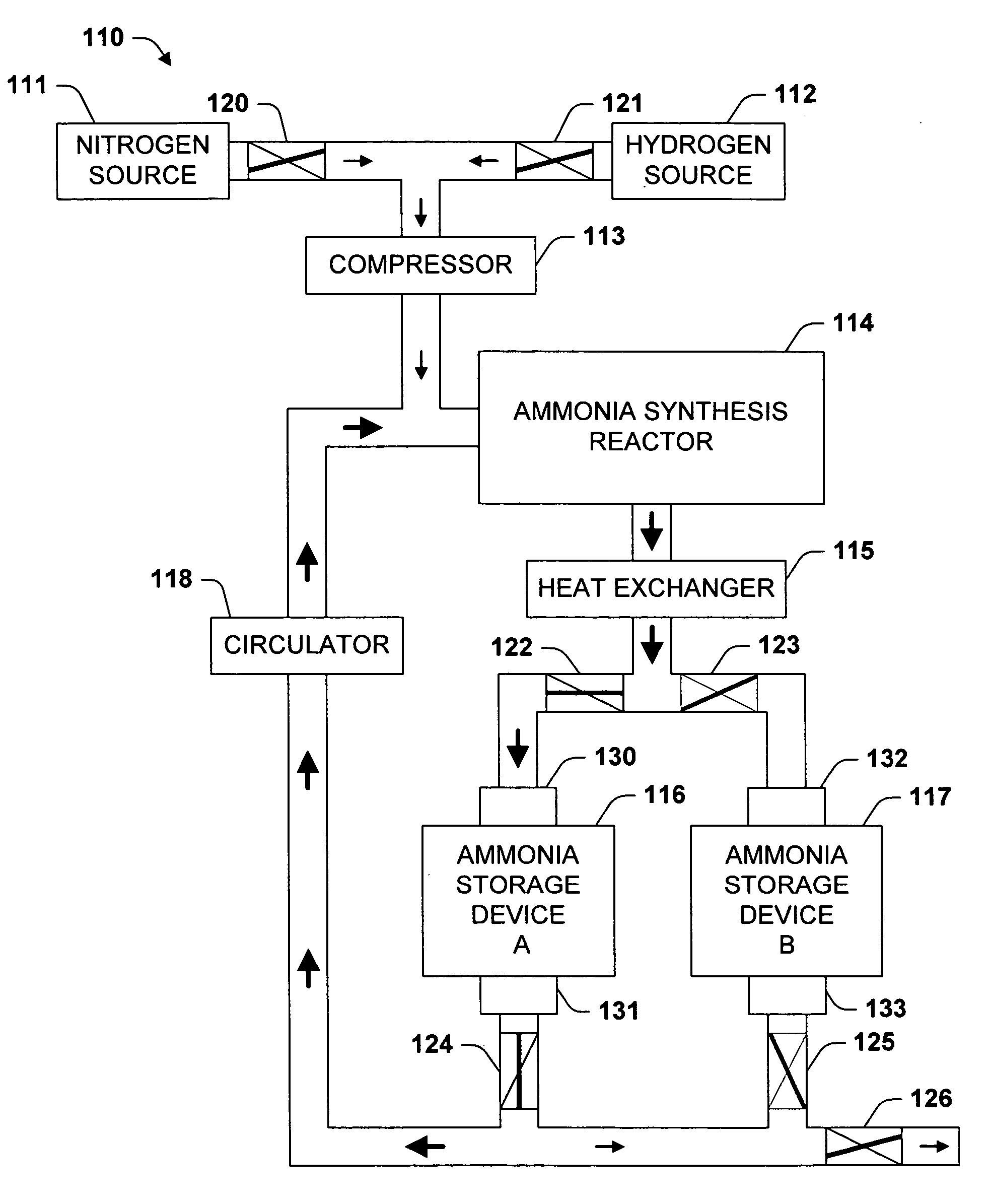
One aspect of the invention relates to a device for storing ammonia for use in SCR on board a vehicle. The device comprises an adsorption bed with a high capacity for storing ammonia. The device can be designed to hold a long-lasting charge of ammonia comparable to a urea tank, but will not release substantial amounts of ammonia into the environment even if the device is accidentally ruptured. In one embodiment, the devices are charged at stationary locations. In another embodiment, the devices are charged by vehicle-mounted ammonia synthesis plants. The device facilitate the use of small ammonia synthesis plants that operate at low pressures and give low conversions. Preferably, the devices are operated through temperature swing adsorption.
Owner:EATON CORP
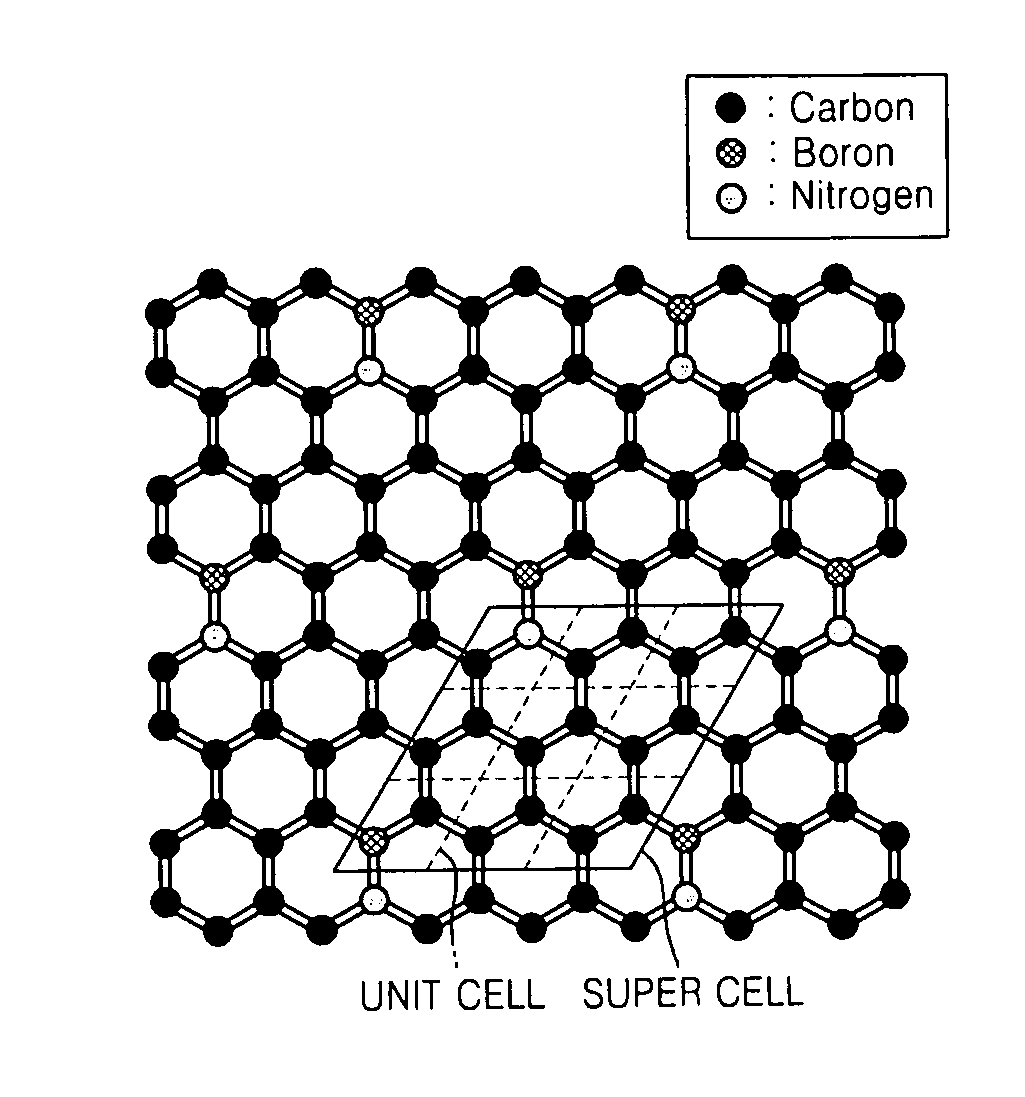
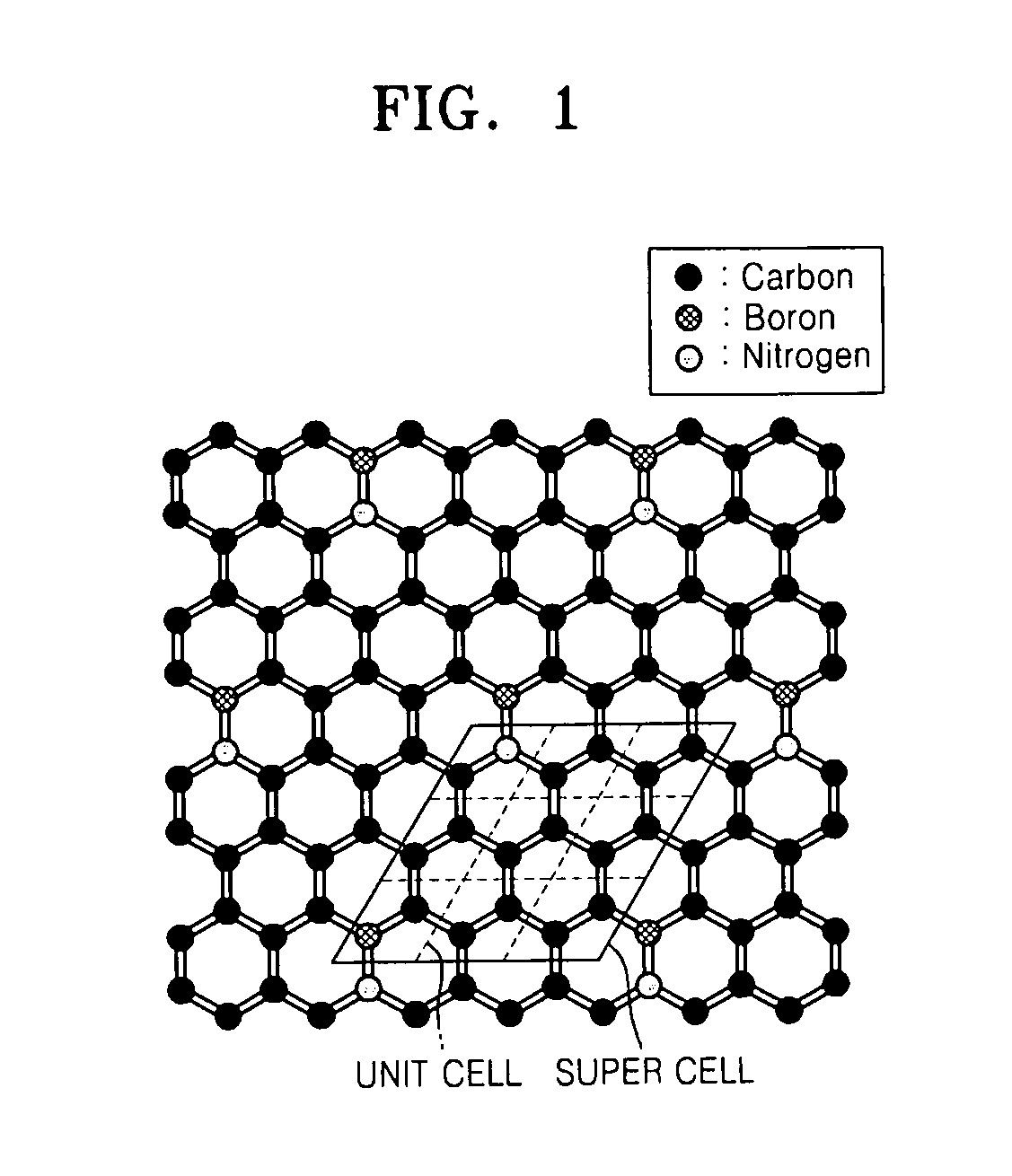
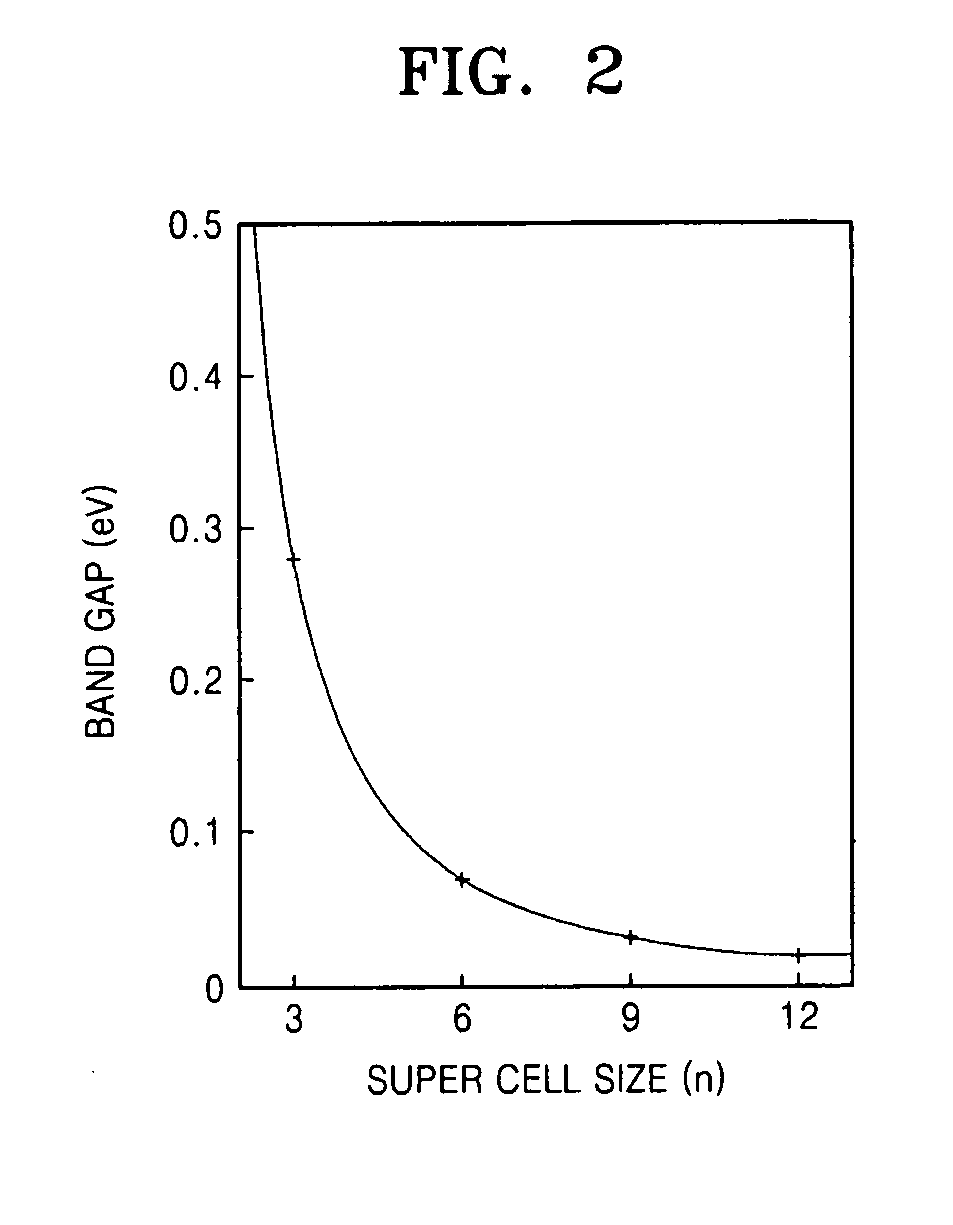
Graphene substituted with boron and nitrogen , method of fabricating the same, and transistor having the same
Graphene, a method of fabricating the same, and a transistor having the graphene are provided, the graphene includes a structure of carbon (C) atoms partially substituted with boron (B) atoms and nitrogen (N) atoms. The graphene has a band gap. The graphene substituted with boron and nitrogen may be used as a channel of a field effect transistor. The graphene may be formed by performing chemical vapor deposition (CVD) method using borazine or ammonia borane as a boron nitride (B—N) precursor.
Owner:SAMSUNG ELECTRONICS CO LTD
Method and device for ammonia storage and delivery using in situ re-saturation of a delivery unit
InactiveUS20100062296A1Partially be recoveredInternal combustion piston enginesExhaust apparatusAmmonia storageAbsorbent material
Disclosed is a method for storing and delivering ammonia, wherein a first ammonia adsorbing / absorbing material having a higher vapor pressure at a given temperature than a second ammonia adsorbing / absorbing material is used as an ammonia source for said second ammonia adsorbing / absorbing material when said second adsorbing / absorbing material is depleted of ammonia by consumption, and a device for performing the method.
Owner:AMMINEX
Preparation method of boron/nitrogen-doped microporous carbon material
InactiveCN103508434AGood hydrogen storage performanceStrong selective adsorption performanceOther chemical processesCarbon preparation/purificationArgon atmosphereNitrogen gas
The invention relates to a preparation method and gas adsorption properties of a boron / nitrogen-doped microporous carbon material, particularly a boron / nitrogen-doped microporous carbon material prepared by using metal organic framework ZIF-8 (zeolitic imidazolate framework-8) and boron nitrogen compounds as precursors by a high-temperature sintering method and gas adsorption properties of the boron / nitrogen-doped microporous carbon material for hydrogen, carbon dioxide, nitrogen and the like. The preparation method comprises the following steps: 1) preparing the porous metal organic framework ZIF-8; 2) limiting the boron nitrogen compounds (such as ammonia borane) to the inside of the pores of the metal organic framework ZIF-8 by a solution impregnating method; and 3) carrying out high-temperature sintering on the composite material in an argon atmosphere to obtain the boron / nitrogen-doped microporous carbon material. The preparation technique is simple; and the prepared carbon material implements simultaneous doping of boron and nitrogen and centralized distribution of micropore sizes, and has favorable adsorption property for hydrogen and selective adsorption property for carbon dioxide.
Owner:DALIAN INST OF CHEM PHYSICS CHINESE ACAD OF SCI
Ammonia borane or hydrazine hydrate catalytic hydrolysis hydrogen release system containing nano-metal phosphide MxPy catalyst and application of catalytic hydrolysis hydrogen release system
InactiveCN105126884ALow costStable in naturePhysical/chemical process catalystsHydrogen productionHydrogenHydrazine compound
The invention discloses an ammonia borane or hydrazine hydrate catalytic hydrolysis hydrogen release system containing a nano-metal phosphide MxPy catalyst and an application of the catalytic hydrolysis hydrogen release system. The system comprises the nano-metal phosphide catalyst, ammonia borane or hydrazine hydrate, and water, wherein the nano-metal phosphide catalyst can be represented as MxPy, M is Fe, Co, Ni or Cu, x is larger than or equal to 1 and smaller than or equal to 20, and y is larger than or equal to 1 and smaller than or equal to 10. The nano-metal phosphide MxPy catalyst with the low cost is used for catalyzing hydrolysis of ammonia borane or hydrogen release of hydrazine hydrate, and the cost is low. The catalyst is stable and safe in property, the efficiency is high when the catalyst is applied to catalytic hydrogen release, raw materials required by preparation are cheap, and a preparation method is simple. The catalytic hydrogen release system adopts heterogeneous catalytic reactions, and recycling of the catalyst is facilitated.
Owner:YUNNAN NORMAL UNIV
Organic matter and ammonia borane compounded hydrogen storage material and preparation method thereof
ActiveCN102030313ALowering the temperature of thermally liberated hydrogenInhibitionMonoborane/diborane hydridesPolyethylene oxideSolvent
The invention relates to an organic matter and ammonia borane compounded hydrogen storage material. The hydrogen storage material is prepared by compounding the organic matter and the ammonia borane, wherein the organic matter is phthalic anhydride, polyethylene oxide, dextrose, mannitol or mannitol hexaacetic ester. The preparation method comprises the following steps: 1) adding the organic matter to the purified acetonitrile solvent, and stirring for dissolving; 2) dissolving the ammonia borane into the mixing solvent comprising acetonitrile and methanol, and stirring at the temperature of 20 to 70 DEG C to obtain a uniform solution; and 3) carrying out vacuum drying, and removing the solvent, thus obtaining the hydrogen storage material. The invention has the advantages that the ammonia borane and the organic matter are taken as raw materials to prepare the hydrogen storage material at the lower hydrogen discharge temperature; the thermal decomposition and hydrogen discharge temperature of the ammonia borane can be effectively reduced; the generation of harmful gas impurities of borazole, diborane, ammonia and the like is effectively inhibited; the hydrogen storage material has quicker hydrogen discharge kinetics; in addition, the heat discharge amount is less in the hydrogen discharge course; and the enthalpy change of a decomposition reaction approaches to thermal neutrality; and the hydrogen storage material is beneficial to realizing the regeneration of reaction products through a solid-gas reaction or a chemical process under the relatively mild condition.
Owner:NANKAI UNIV
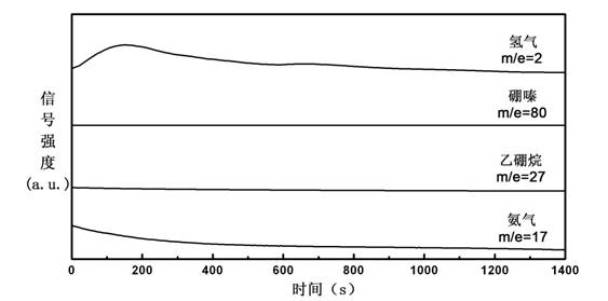
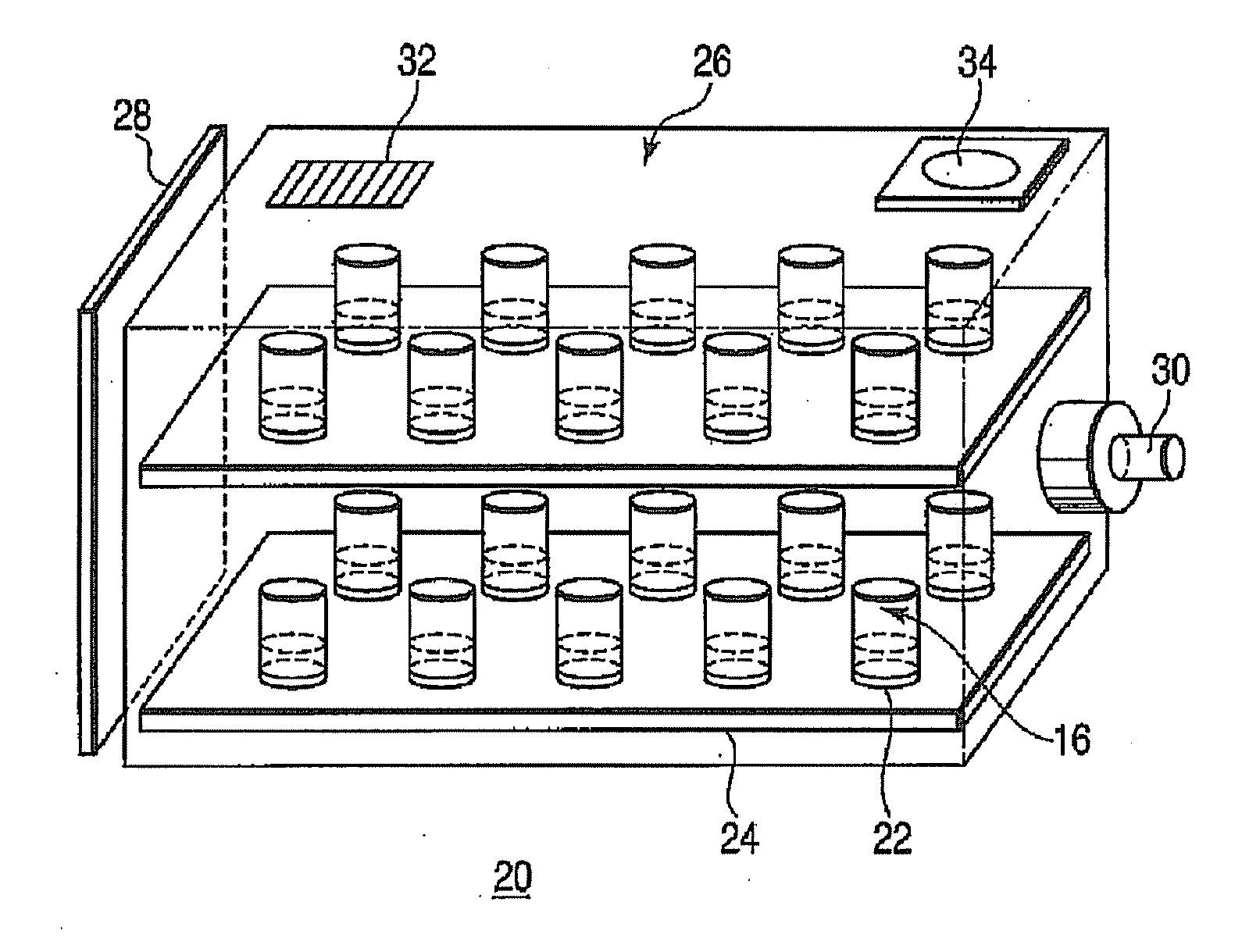
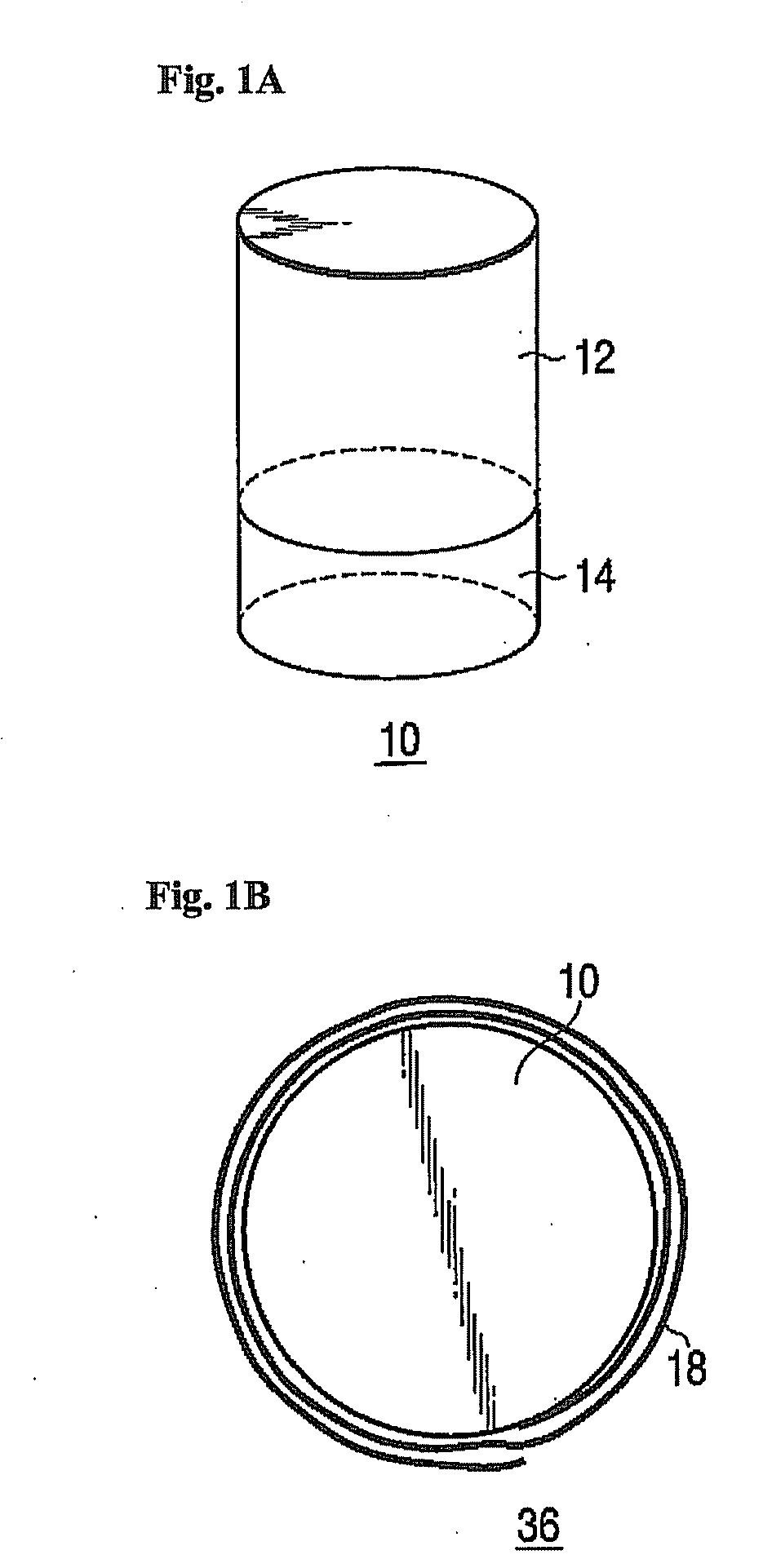
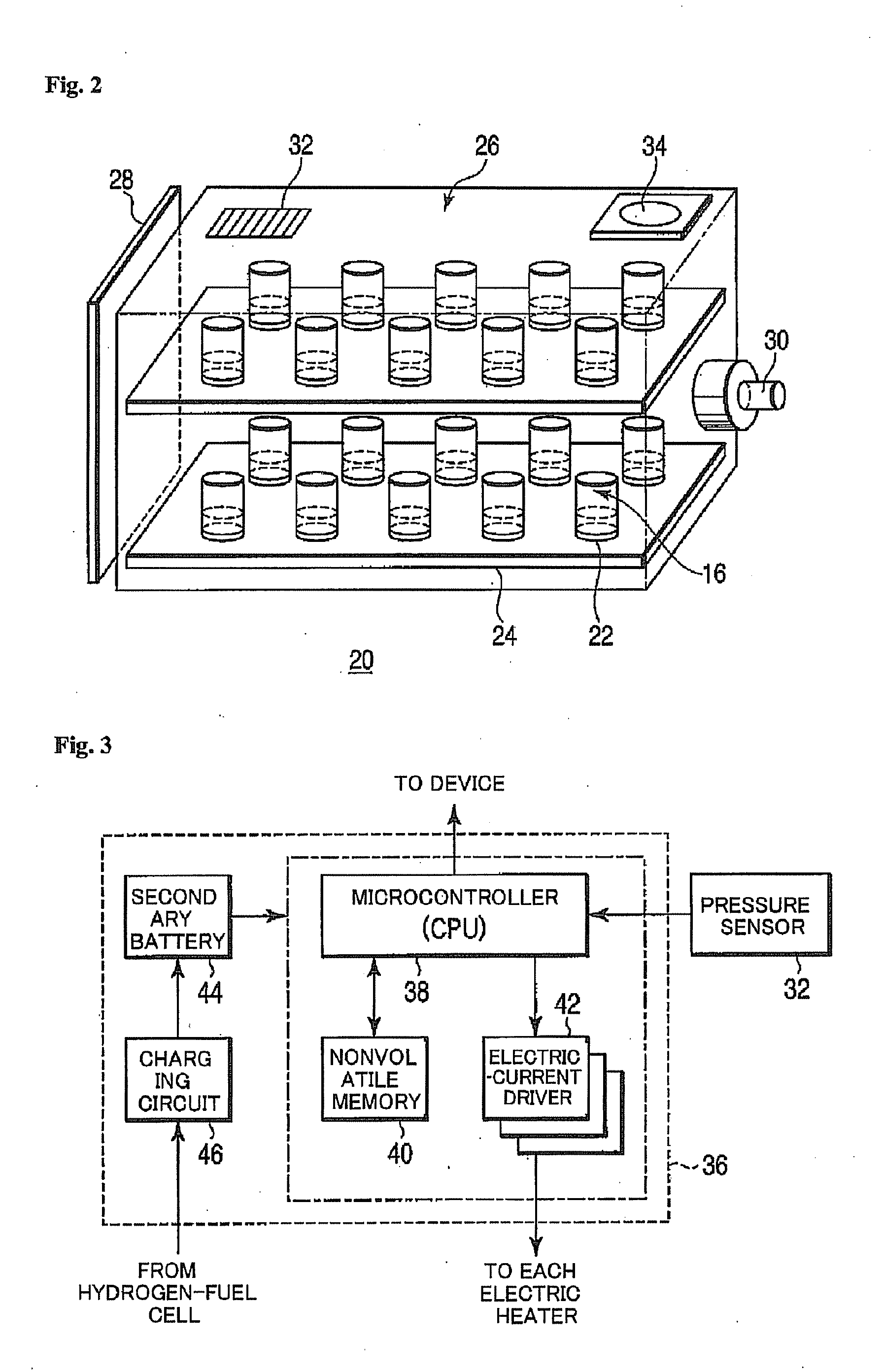
Hydrogen Generator and Fuel Pellet
InactiveUS20110033342A1Generate efficientlyIncrease power outputPressurized chemical processSamplingChemical reactionHydrogen
A hydrogen generator comprising a plurality of a fuel pellets (10) containing a hydrogen-generating compound such as ammonia borane, a case serving as a pressure-resistant container for containing the fuel pellets, and a controller for controlling hydrogen generation from the fuel pellets. This hydrogen generator generates hydrogen from the hydrogen-generating compound by a chemical reaction. The periphery of the fuel pellet is surrounded by a member including a thin plate of metal aluminum such as aluminum foil (18) on its surface.
Owner:QINETIQ LTD
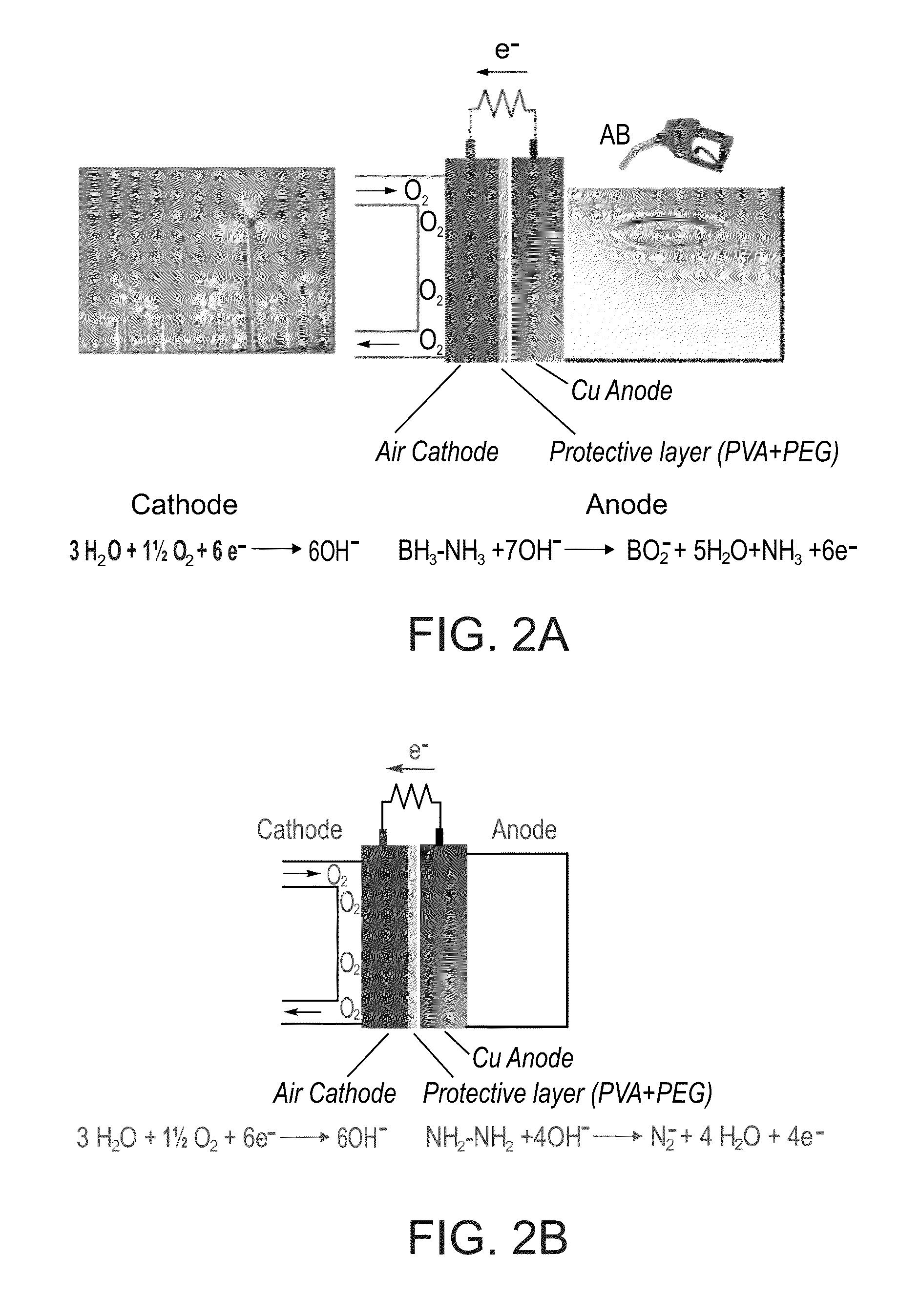
Direct liquid fuel cell having ammonia borane, hydrazine, derivatives thereof or/and mixtures thereof as fuel
A fuel cell system comprising an anode compartment which comprises an anode having a copper catalyst layer, a cathode configured as an air cathode and a separator interposed between said anode and said cathode, operable by an amine-derived fuel and oxygen (or air) is disclosed. Further disclosed are fuel cell systems comprising an anode compartment which comprises an anode having a copper catalyst layer, a cathode and a separator interposed between said anode and said cathode, which are operable by a mixture of two types of amine-derived compounds (e.g., ammonia borane, hydrazine and derivatives thereof). Also disclosed are methods of producing electric energy by, and electric-consuming devices containing and operable by, the disclosed fuel cell systems.
Owner:RAMOT AT TEL AVIV UNIV LTD
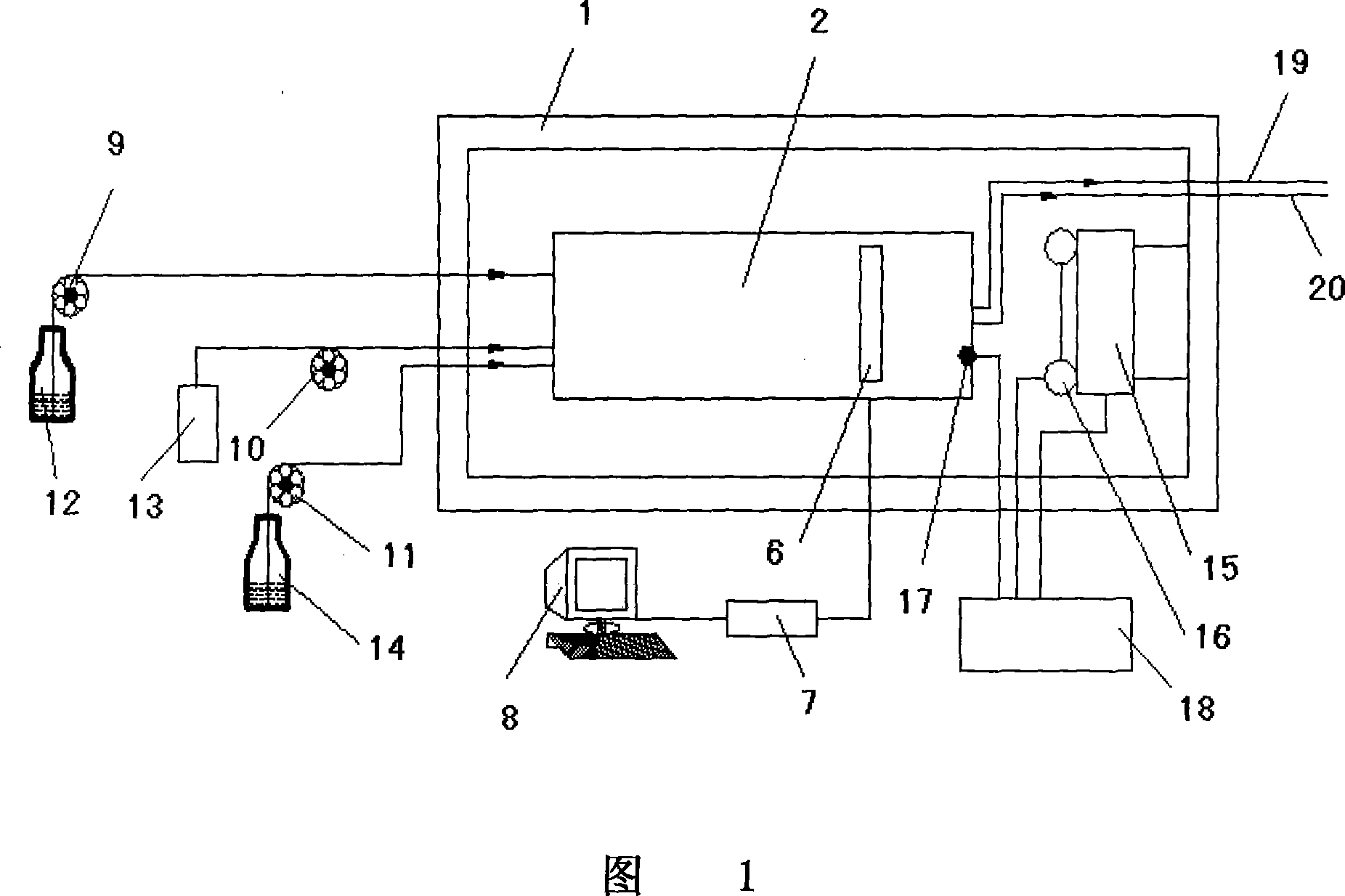
Ammonia test method and device
The invention relates to an ammonia check method and a relative device, used in the water solution which pH is not lower than 12. 3. Based on the theory that all ammonia ions can be converted into ammonia gas, the invention reacts the ammonia ion solution with alkali to generate ammonia gas, uses the secondary water at another side of a semi-permeable membrane to catch and absorb the ammonia gas, and measures the conductivity change of the secondary water to realize the test on original ammonia ion density. The inventive device comprises a controllable constant-temperature box, a check module in the constant-temperature box, an electrode connected with the check module, a signal collector connected with the electrode, and a computer connected with the signal collector. The check module comprises upper and lower modules connected together via bolts. The invention is based on atmospheric environment, which can respectively measure the ammonia density of gas and aerosol, with the application in water condition and online measurement.
Owner:PEKING UNIV
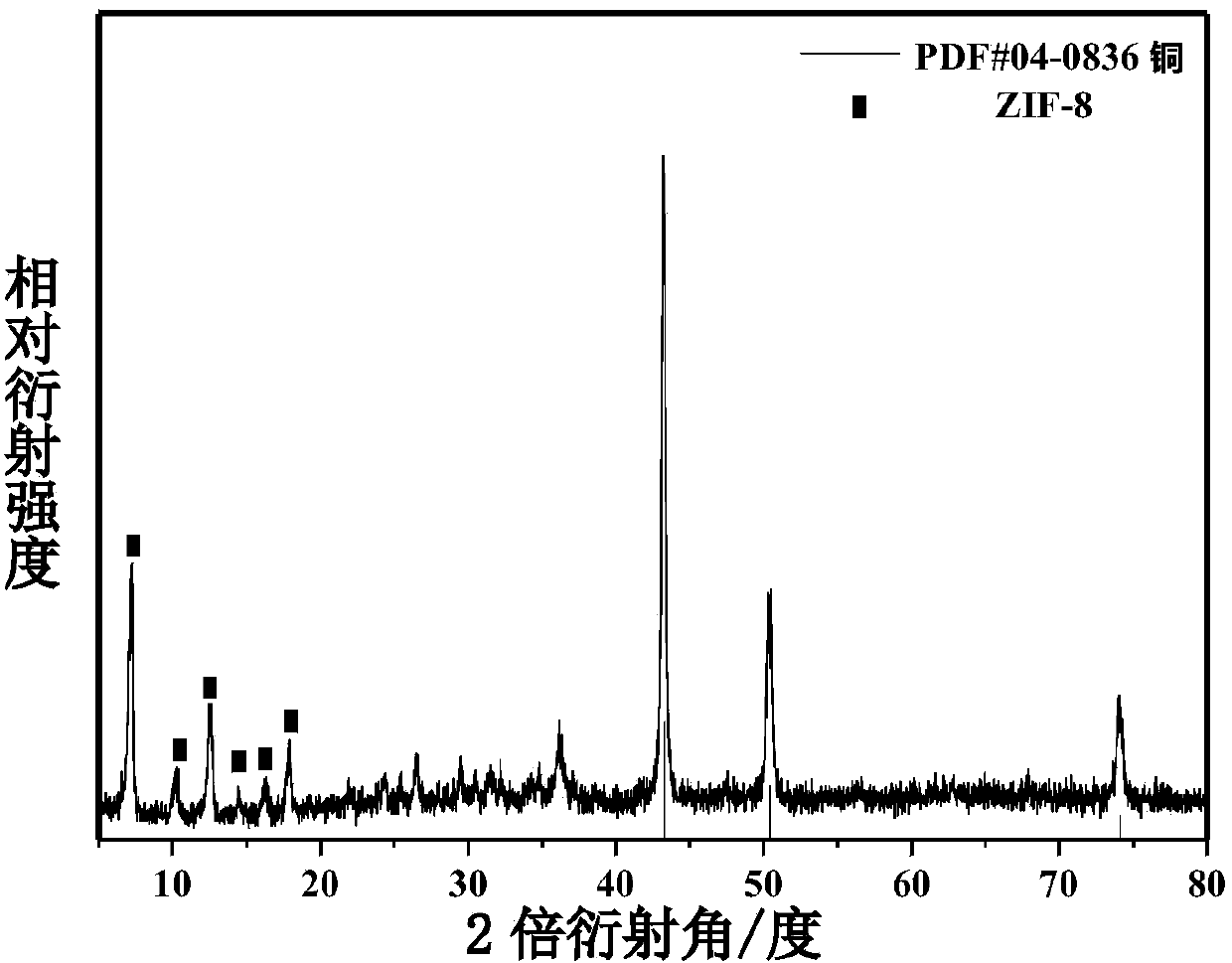
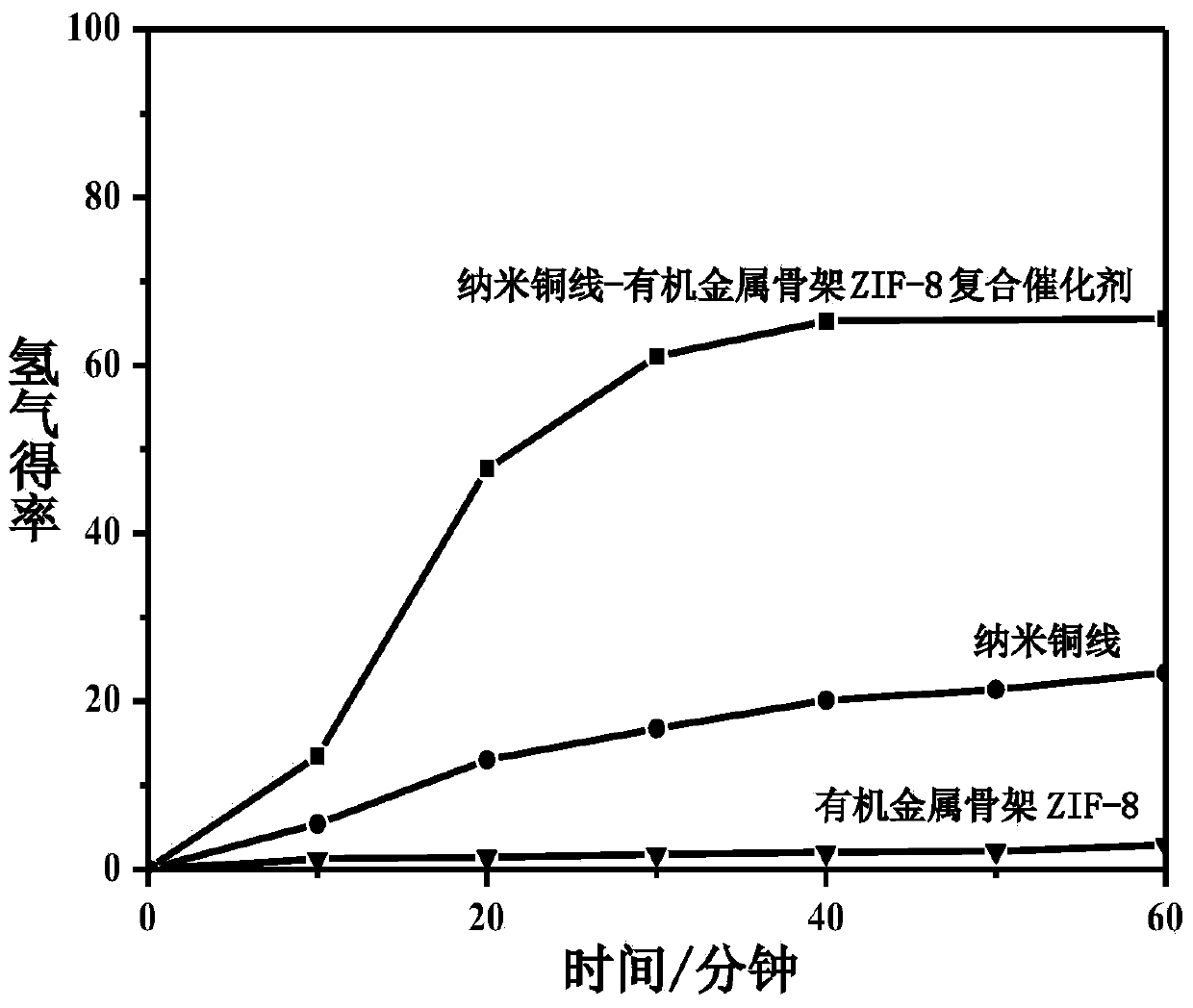
Preparation method and application of environment-friendly core shell one-dimensional nanometer copper wire-organic metallic framework ZIF-8 composite catalyst
InactiveCN104001547AImprove stabilityHigh activityOrganic-compounds/hydrides/coordination-complexes catalystsHydrogen productionN dimethylformamideSynthesis methods
The invention discloses an environment-friendly core shell one-dimensional nanometer copper wire-organic metallic framework ZIF-8 composite catalyst and a preparation method and application of the environment-friendly core shell one-dimensional nanometer copper wire-organic metallic framework ZIF-8 composite catalyst. A microwave induction coring heating synthesis method is adopted, N, N-dimethylformamide is used as a solvent, polyvinylpyrrolidone is used as a trapping agent, a copper wire is used as a one-dimensional structure loading carrier, the environment-friendly core shell one-dimensional nanometer copper wire-organic metallic framework ZIF-8 composite catalyst is synthesized fast, the decomposition rate of the catalyst on ammonia borane in an ammonia borane decomposition catalytic reaction is 71 percent, and the good catalytic activity is shown. The preparation method of the catalyst is simple, and environmental pollution is small. An organic metallic framework ZIF-8 is combined with a non-precious metal copper nanometer wire with the good conductivity and certain catalytic performance, and the catalytic performance of the material of this kind is greatly improved. The material is potentially applied to energy storage, pollution gas absorption, sewage treatment, new energy development and other fields.
Owner:SHANGHAI NORMAL UNIVERSITY
Three-dimensional boron nitride foam and preparation method thereof
ActiveCN103232027AImprove performanceLow densityNitrogen compoundsPtru catalystHexagonal boron nitride
The invention relates to three-dimensional boron nitride foam and a preparation method of the three-dimensional boron nitride foam. The preparation method comprises the following steps of: heating a borane ammonia complex serving as a solid-state source in an independent container to reach a specific temperature in order to decompose the solid-state source into a gas-state source through the chemical vapor deposition process; dispersing to the surface of a metal bubble template; cracking and depositing to form a three-dimensional boron nitride film network structure; cooling the three-dimensional boron nitride film network structure; continuously dispensing a high-molecular polymer layer; removing the metal template through a corrosive liquid; and removing the high-molecular polymer layer at a high temperature, thus obtaining three-dimensional boron nitride foam. The method is simple and convenient in operation, low in requirement on equipment and high in yield; the prepared boron nitride foam is in form of a net shaped structure in which hollow hexagonal boron nitride thin-wall tubes are interconnected; and the prepared boron nitride foam has outstanding characteristics of low density, high thermal stability, elastic recovery after compressing, low Young modulus and the like, therefore, the foundation is provided for the boron nitride foam to be applied in the fields of high-temperature environment, catalyst carriers, mechanics sensing, insulation and the like.
Owner:NANJING UNIV OF AERONAUTICS & ASTRONAUTICS
Preparation method and application of nitrogen-doped porous carbon-loaded cobalt catalyst
InactiveCN105498823ASimple methodIncreased Cobalt LoadingPhysical/chemical process catalystsHydrogen productionPorous carbonSynthesis methods
The invention discloses a preparation method of a nitrogen-doped porous carbon-loaded cobalt catalyst. The preparation method includes: adding N, N-bis(salicylidene) ethylene dimino cobalt (II) or bi(3-methoxysalicylaldehyde) ethylenediamine cobalt chloride into a crucible, placing into a tubular furnace, and heating to 400-900 DEG C for calcining for 1-10 h in a hydrogen-argon mixed atmosphere; cooling to room temperature to obtain the nitrogen-doped porous carbon-loaded cobalt catalyst which is used for hydrolytic dehydrogenation of ammonia borane. The preparation method has the advantages that the catalyst is prepared by adopting a one-step pyrolysis synthesis method, the preparation method is simple, cobalt loading quantity is greatly increased, and industrial production is facilitated; the catalyst is used for catalyzing hydrolytic dehydrogenation of ammonia borane, maximum dehydrogenation rate reaches 1383 mL H2min-1gCo-1, and activation energy is 31.0kJ / mol; especially, after cobalt nanoparticles are embedded in nitrogen-doped porous carbon, circulating stability is improved greatly.
Owner:NANKAI UNIV
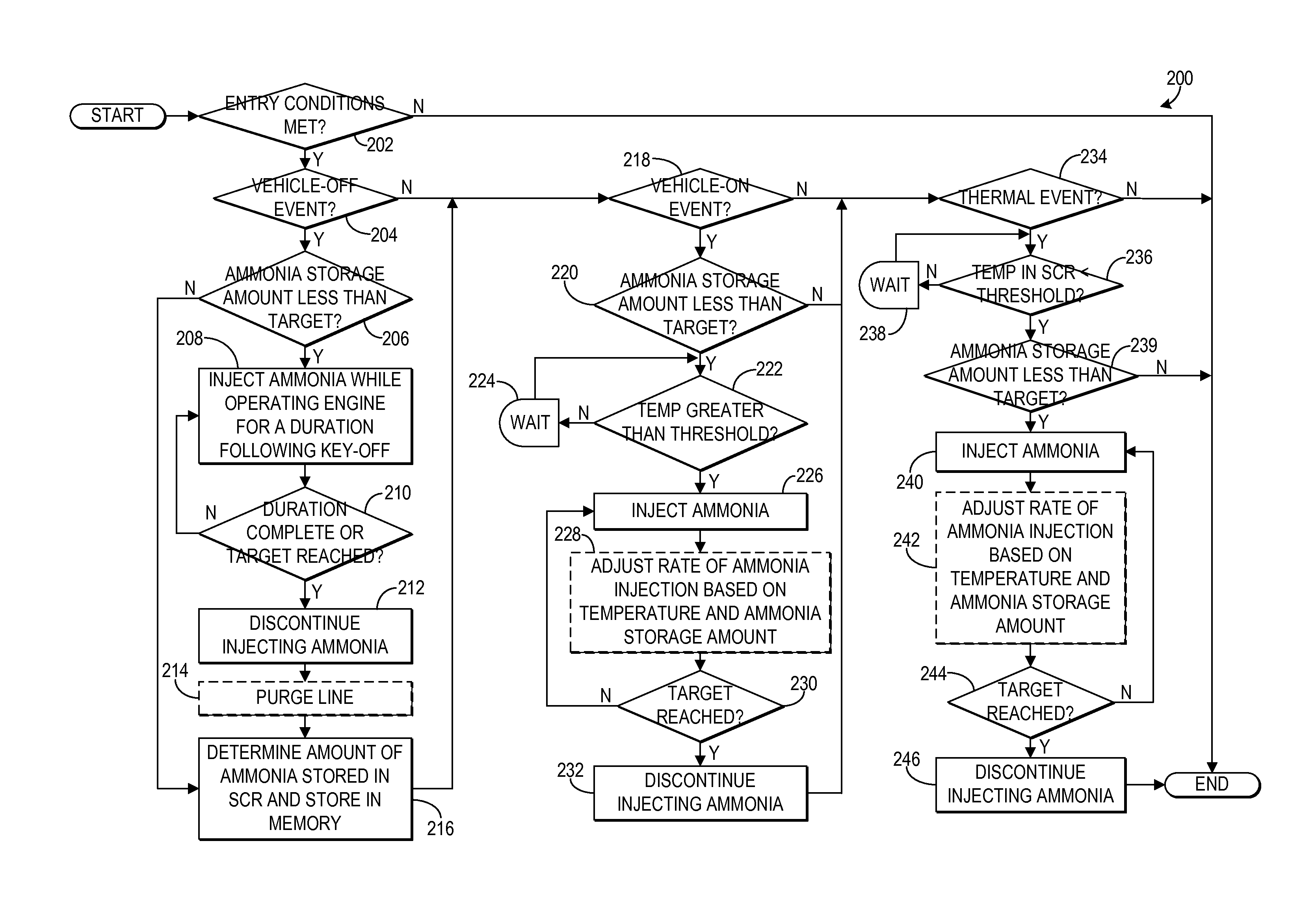
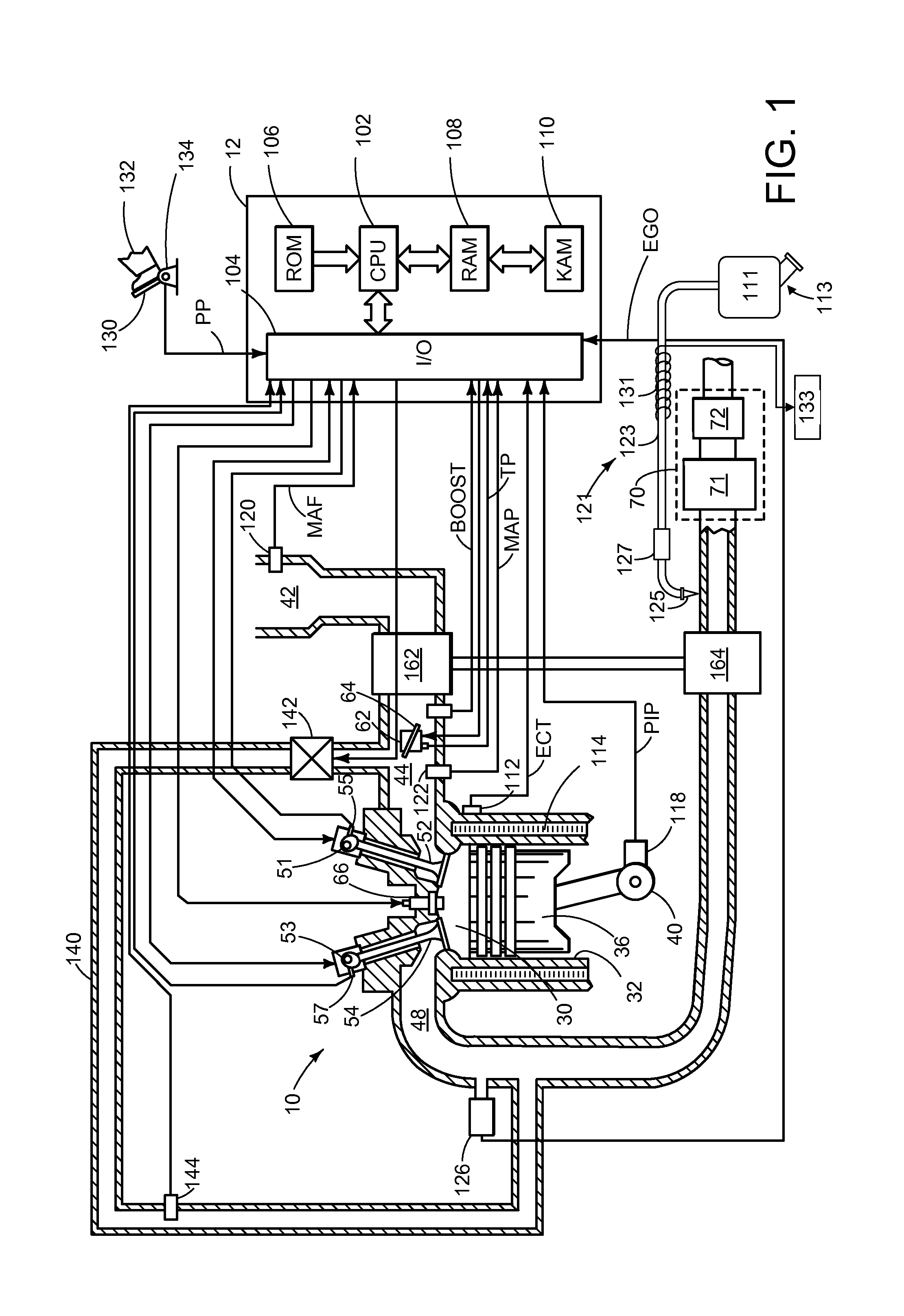
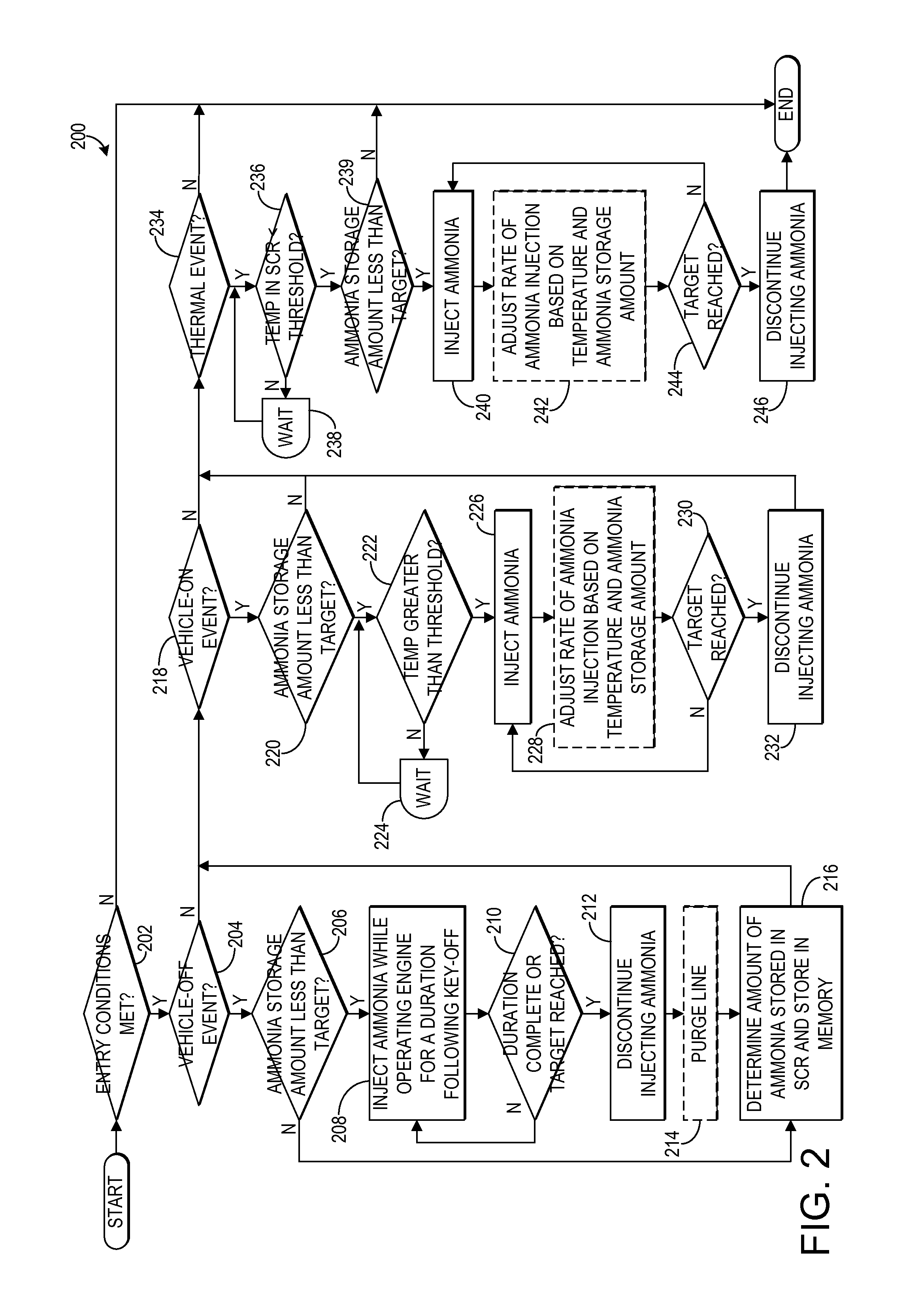
Ammonia storage management for scr catalyst
ActiveUS20150013309A1Emission reductionMinimize an anticipated storage deficitPower operated startersInternal combustion piston enginesAmmonia storageAmmonia borane
Various systems and methods are described for managing ammonia storage in an SCR catalyst. In one example approach, a method comprises, in response to a vehicle-off event, injecting ammonia during a final exhaust blowdown until a predetermined value of ammonia is stored in the SCR catalyst; and in response to a subsequent vehicle-on event when an amount of ammonia stored in the SCR catalyst is less than the predetermined value, injecting ammonia until the predetermined value of ammonia is stored in the SCR catalyst.
Owner:FORD GLOBAL TECH LLC
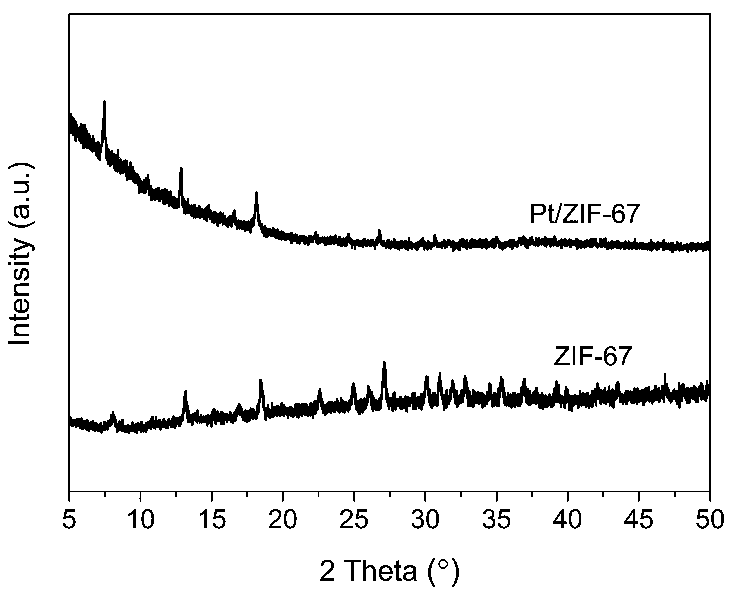
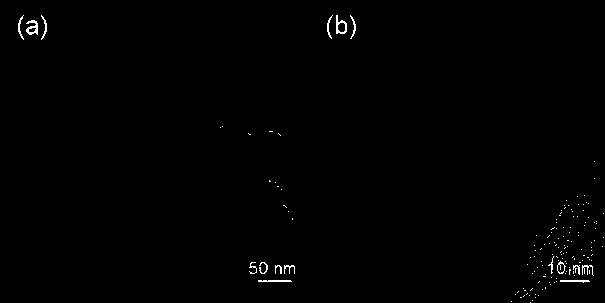
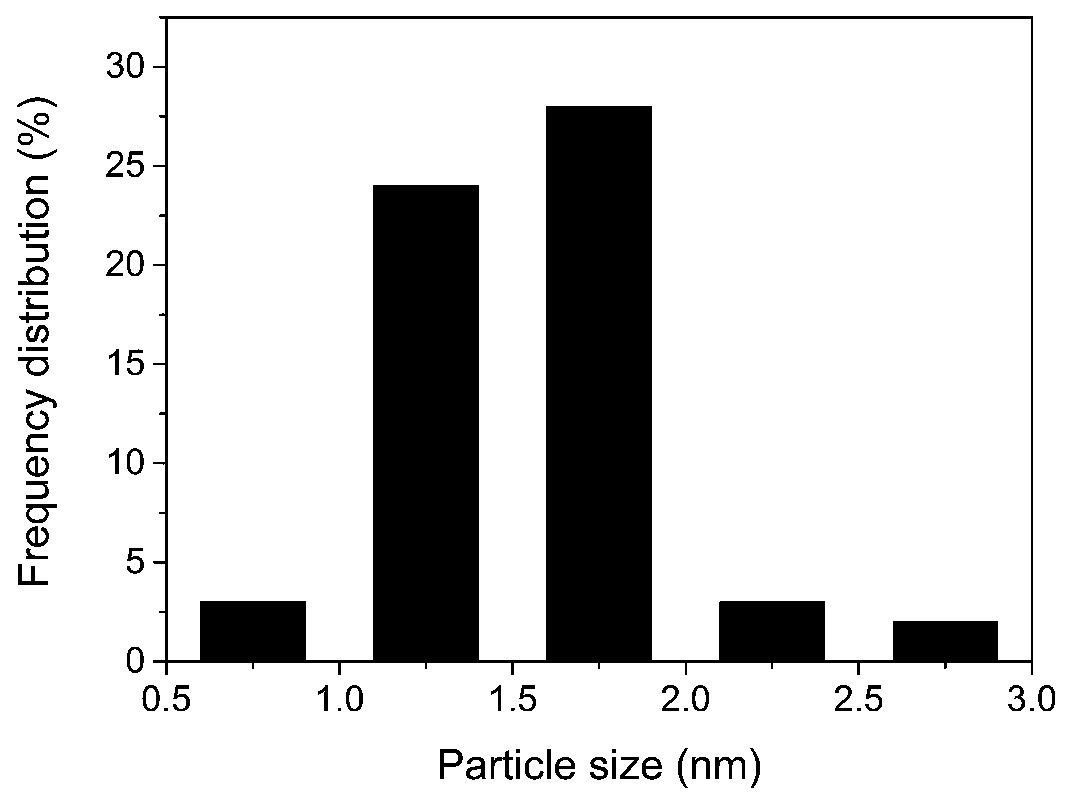
Pt/ZIF-67 composite used for catalyzing hydrolysis of ammonia borane for hydrogen production
InactiveCN107930697AImproved hydrogen release performanceHigh TOF valueOrganic-compounds/hydrides/coordination-complexes catalystsHydrogen productionReaction rateMetal-organic framework
The invention discloses a Pt / ZIF-67 composite used for catalyzing hydrolysis of ammonia borane for hydrogen production. The Pt / ZIF-67 composite is prepared by mixing chloroplatinic acid with a metal organic framework ZIF-67 and then carrying out one-step reduction. The structure of the Pt / ZIF-67 composite retains the frame structure of ZIF-67; the average size of Pt nanoparticles is 1-2 nm, the Ptnanoparticles are uniformly distributed, and no obvious Pt metal characteristic diffraction peak occurs according to XRD detection results. A preparation method comprises the following steps: step 1)preparation and activation of the ZIF-67; and step 2) loading of the Pt nanoparticles: adding the activated ZIF-67 into water for ultrasonic dispersion, adding chloroplatinic acid, and then adding anaqueous NaBH4 solution drop by drop, and subjecting a product to filtering, washing and drying. As the Pt / ZIF-67 composite is applied to catalysis of the hydrolysis of ammonia borane for hydrogen production, the turn over frequency (TOF) of a reaction rate reaches 70-100 mol H2 min<-1> Pt mol<-1>, and activation energy is 30-40 kJ mol<-1>. The synergistic effect of the ZIF-67 and the Pt nanoparticles brings in better catalytic performance. Therefore, the Pt / ZIF-67 composite has good application prospects in the field of hydrogen production.
Owner:GUILIN UNIV OF ELECTRONIC TECH
Double Metal-Carbon Nanotube Hybrid Catalyst and Method for Preparation Thereof
InactiveUS20110034328A1High speed hydrogen generationHigh-speed generationMaterial nanotechnologyCatalyst activation/preparationHydrogenCarbon nanotube
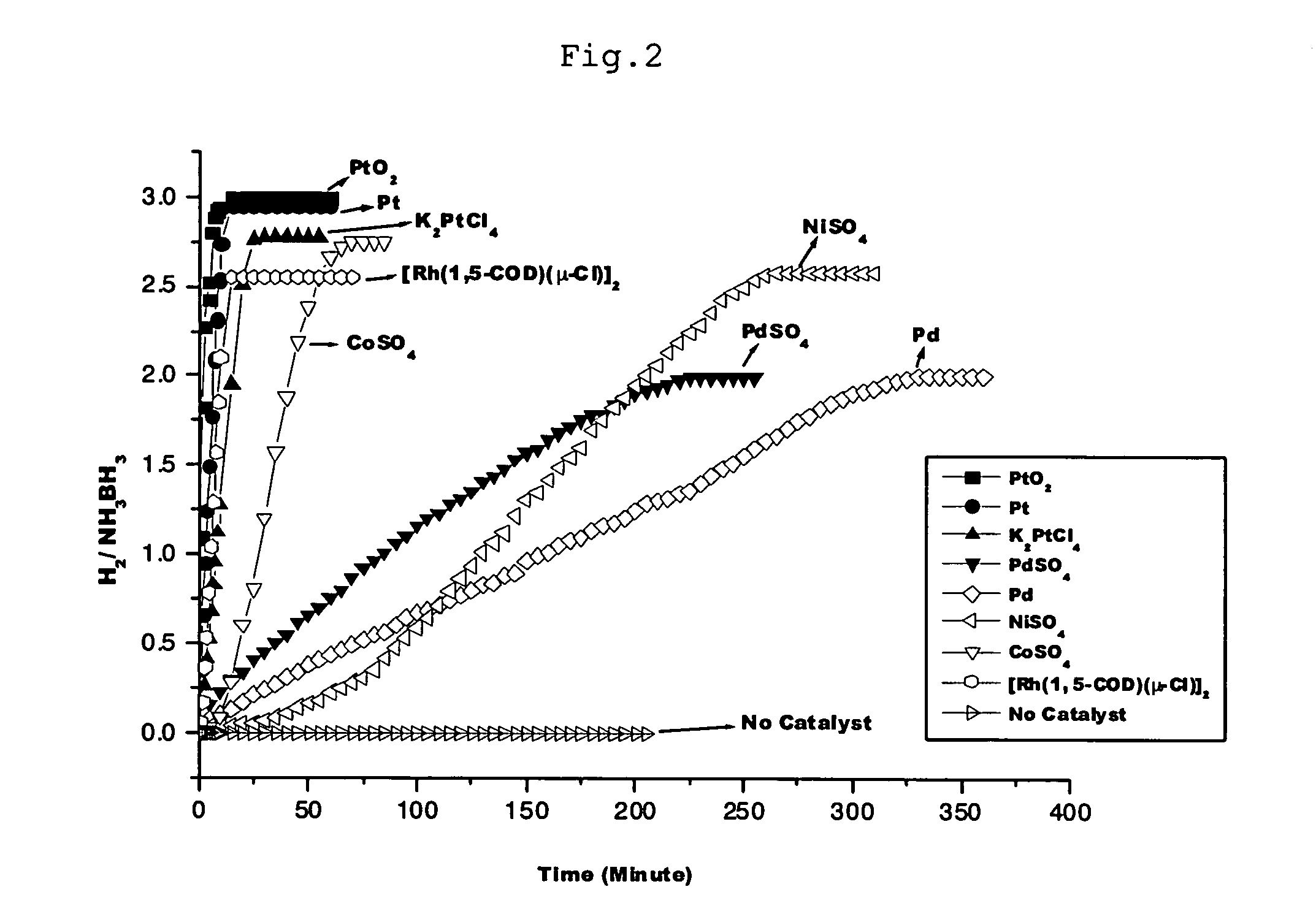
Disclosed are a double metal-carbon nanotube hybrid catalyst comprising at least two of transition metals selected from a group consisting of Mn, Fe, Co, Ni, Cu, Mo, Tc, Ru, Rh, Pd, Ag, Re, Os, Ir and Pt which are distributed in the catalyst. The double metal-carbon nanotube hybrid catalyst contains at least two different transition metals with high catalytic activity and may generate hydrogen from an aqueous ammonia-borane (NH3BH3) solution at a high speed and a method for preparation of a double metal-carbon nanotube hybrid catalyst.
Owner:KOREA ADVANCED INST OF SCI & TECH
Hydrogen storage material decomposing and hydrogen release system
ActiveCN109225284ASave raw materialsIncrease catalytic rateReversible hydrogen uptakeChemical recyclingOrganic solventSolvent
The invention discloses a hydrogen storage material decomposing and hydrogen release system. The system comprises a hydrogen storage material, a catalyst and a solvent, wherein the catalyst is a mixture of two or more of metal compounds mixed in any proportion. The invention provides the cheap and stable catalytic hydrogen storage material decomposing and hydrogen release system, and the raw materials for catalyst preparation are cheap. The catalyst has stable properties and high hydrogen release efficiency when applied to catalysis of a hydrogen storage material; the catalytic hydrogen release system is heterogeneous catalytic reaction, and the catalyst is convenient to recycle; if the catalytic hydrogen release system is conducted in an organic solvent, the catalytic reaction can be conducted at a temperature of 273 K or lower; in the catalytic hydrogen release system, when the hydrogen storage material is ammonia borane and methanol is used as a solvent, ammonia borane can be obtained again from NH4B(OCH3)4 obtained after alcoholysis under certain conditions.
Owner:TECHNICAL INST OF PHYSICS & CHEMISTRY - CHINESE ACAD OF SCI
Porous carbon material loaded with ruthenium nanoparticles, and preparation method and application thereof
InactiveCN108160073ALow costConducive to the realization of standardized productionCatalyst activation/preparationHydrogen productionPorous carbonHydrolysis
The invention discloses a porous carbon material loaded with ruthenium nanoparticles; with glucose as a carbon source, a certain amount of nitrogen-containing compound is added, then a nitrogen-containing precursor is prepared by a hydrothermal method and then is calcined and treated to obtain a porous structural carbon material, and then the porous carbon material is loaded with metal ruthenium by an in-situ reduction method to obtain the porous carbon material loaded with the ruthenium nanoparticle material, wherein the specific surface area is in a range of 1800-2000 m<2>*g<-1>, the specific surface area of micropores is 1000-1100 m<2>*g<-1>, the micropore content is 52-55%, the pore size distribution is uniform, and the pore size distribution is 1.68-2.30 nm. A preparation method includes the following steps: 1) preparation of the nitrogen-containing precursor; 2) preparation of the porous structural carbon material; and 3) loading of the ruthenium nanoparticles and obtaining of the porous carbon material loaded with the ruthenium nanoparticles. As a catalyst for hydrolysis and hydrogen release of ammonia borane, hydrogen release is completed in 75-100 s, and the hydrogen release rate reaches 2000-3300 ml*s<-1>*g<-1>; the porous carbon material loaded with the ruthenium nanoparticles can be recycled, and the hydrogen release amount can be kept at 99-100%; the porous carbonmaterial loaded with the ruthenium nanoparticles has broad application prospects in hydrogen production materials, fuel cells and other fields.
Owner:GUILIN UNIV OF ELECTRONIC TECH
Novel ammonia borane composite material for hydrolysis hydrogen production
InactiveCN101837953AImprove the kinetic performance of hydrolysis hydrogen releaseSmall particle sizeHydrogen productionMetal chlorideAlkaline earth metal
The invention relates to a novel ammonia borane composite material, in particular to the novel ammonia borane (NH3BH3) composite material used for inducing the hydrolysis of an ammonia borane compound to produce hydrogen under a mild condition. The novel ammonia borane composite material comprises hydrogen storage materials which have micron, submicron and nanometer particle sizes and are obtained by using an inducer (metal hydride MHx or a salt) and an ammonia borane mixture as initial raw materials. The metal hydride MHx comprises one of or a combination of a plurality of types of alkali metal hydride, alkaline earth metal hydride, transition metal hydride and rear earth metal hydride; and the salt comprises one of or a combination of a plurality of types of metal chloride and sulfate. The initiative phase component molar ratio of the inducer to the ammonia borane (NH3BH3) is (2-0.02):1. The novel ammonia borane composite material greatly improves the dynamics performance of hydrolysis hydrogen discharge of the ammonia borane, and has a high hydrogen discharge amount and a high hydrogen production rate when hydrolyzed under the mild condition.
Owner:SICHUAN UNIV
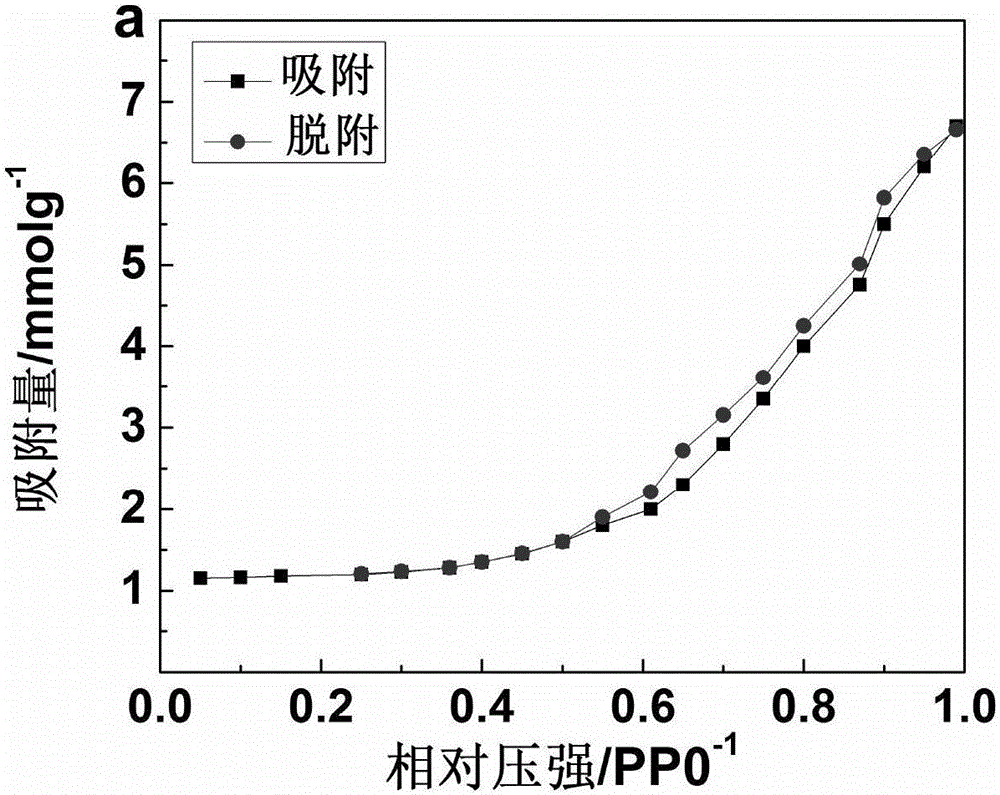
Preparation method of high-adsorbability graphene aerogel
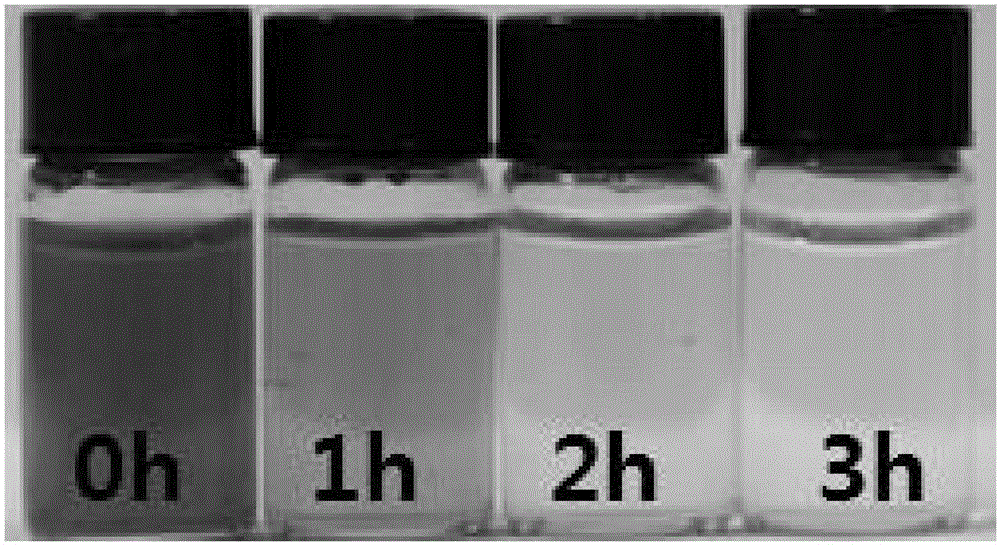
InactiveCN106006616ARestore fastSimple and fast operationOther chemical processesWater contaminantsFreeze-dryingAdsorption effect
The invention discloses a preparation method of graphene airgel with high adsorption performance, which comprises the following preparation steps: (1) adding graphene oxide into deionized water to prepare graphene oxide aqueous solution; (2) adding ammonia borane Mix the aqueous solution, ferrous sulfate aqueous solution and graphene oxide aqueous solution evenly; (3) prepare the graphene hydrogel by subjecting the mixed aqueous solution to constant temperature hydrothermal reaction; (4) soak the graphene hydrogel, pre-freeze, and then freeze After drying, graphene airgel with high adsorption performance can be obtained. The invention uses ammonia borane and ferrous sulfate to reduce graphene oxide, accelerates the reduction speed of constant temperature hydrothermal reaction, and has simple operation and simple preparation method; the prepared graphene airgel has an adsorption effect on rhodamine B dye Efficient, the adsorption can be basically completed in 3 hours.
Owner:JIANGSU UNIV OF SCI & TECH
Hydrogen generation method
InactiveUS20070151153A1Generate efficientlyControl generationHydrogenGranular/pulverulent flues gasificationFuel cellsHydrogen
The invention provides a hydrogen generation method comprising the step of bringing, in the presence of water, ammonia borane represented by the chemical formula: NH3BH3 into contact with (1) a catalyst comprising as an active ingredient at least one member selected from the group consisting of metal catalysts and metal compound catalysts; (2) a solid acid; or (3) carbon dioxide. The method of the invention can generate hydrogen gas for use as fuel for fuel cells, etc., under controllable conditions without heating a starting material at high-temperature, and moreover can efficiently generate hydrogen gas at low cost.
Owner:NAT INST OF ADVANCED IND SCI & TECH
Method for the production of hydrogen from ammonia borane
InactiveUS20110070152A1Rapid generation of hydrogenGaseous chemical processesOrganic-compounds/hydrides/coordination-complexes catalystsHydrogenMetal catalyst
The present disclosure relates to processes and methods of generating hydrogen via the hydrolysis or solvolyis of a compound of the formula (I), R1R2HNBHR3R4, using ligand-stabilized homogeneous metal catalysts.
Owner:KANATA CHEM TECH
Ru-Co bimetallic nano supported catalyst for hydrolytic hydrogen release of ammonia borane and preparation method of Ru-Co bimetallic nano supported catalyst
InactiveCN107376996AEvenly dispersedGive full play to the synergistic catalytic effectMaterial nanotechnologyOrganic-compounds/hydrides/coordination-complexes catalystsMetal-organic frameworkAlloy
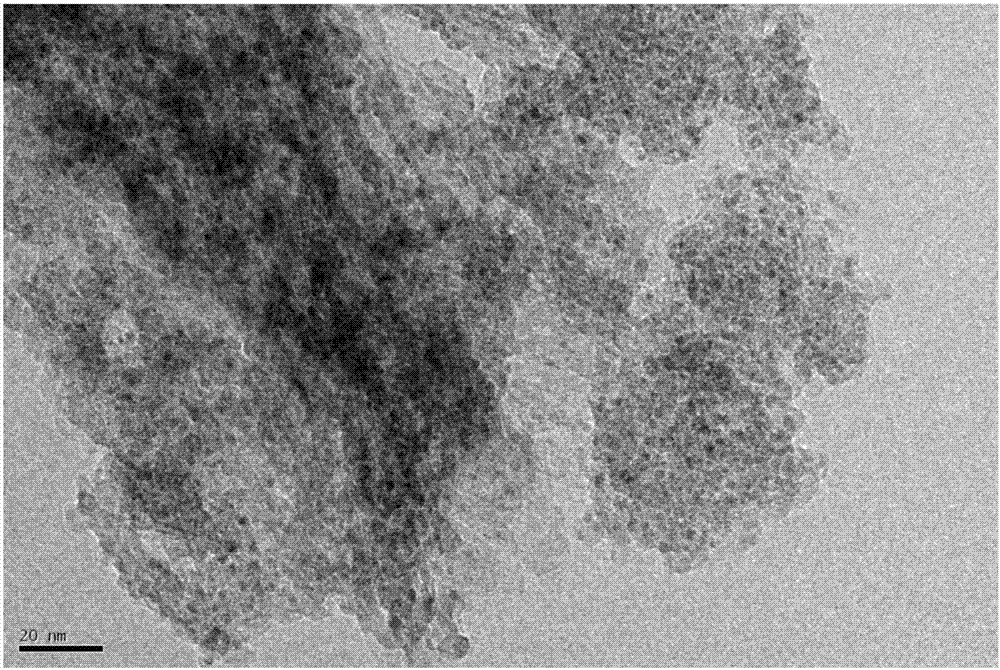
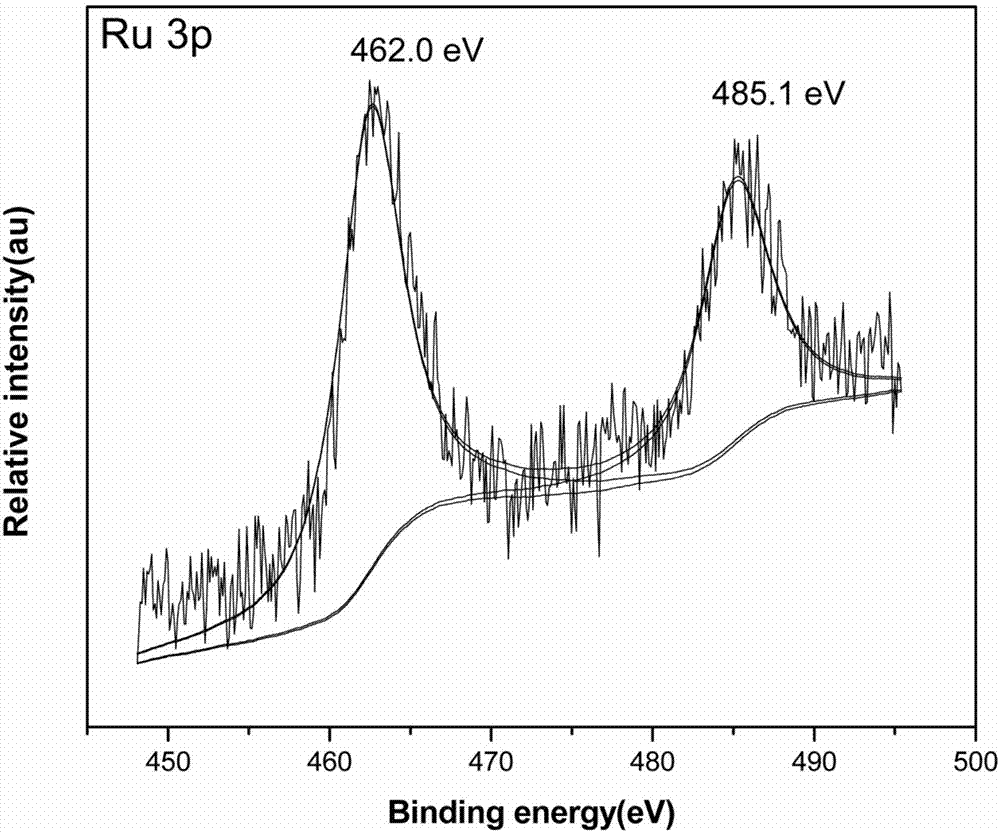
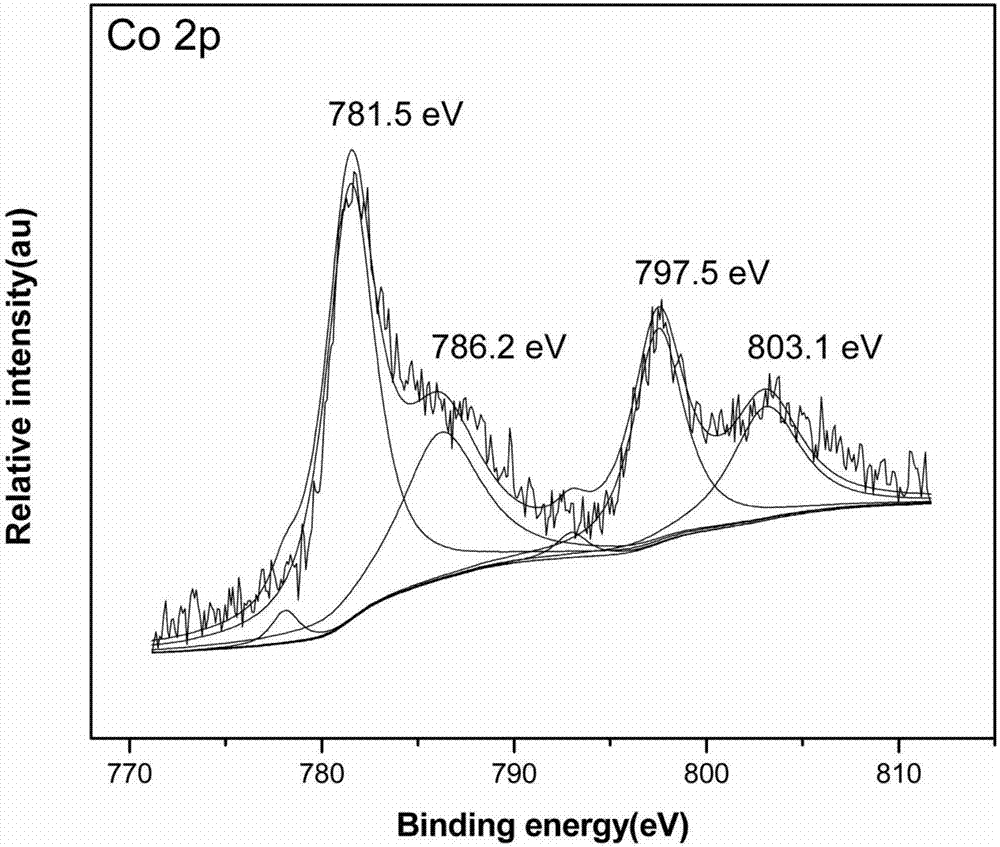
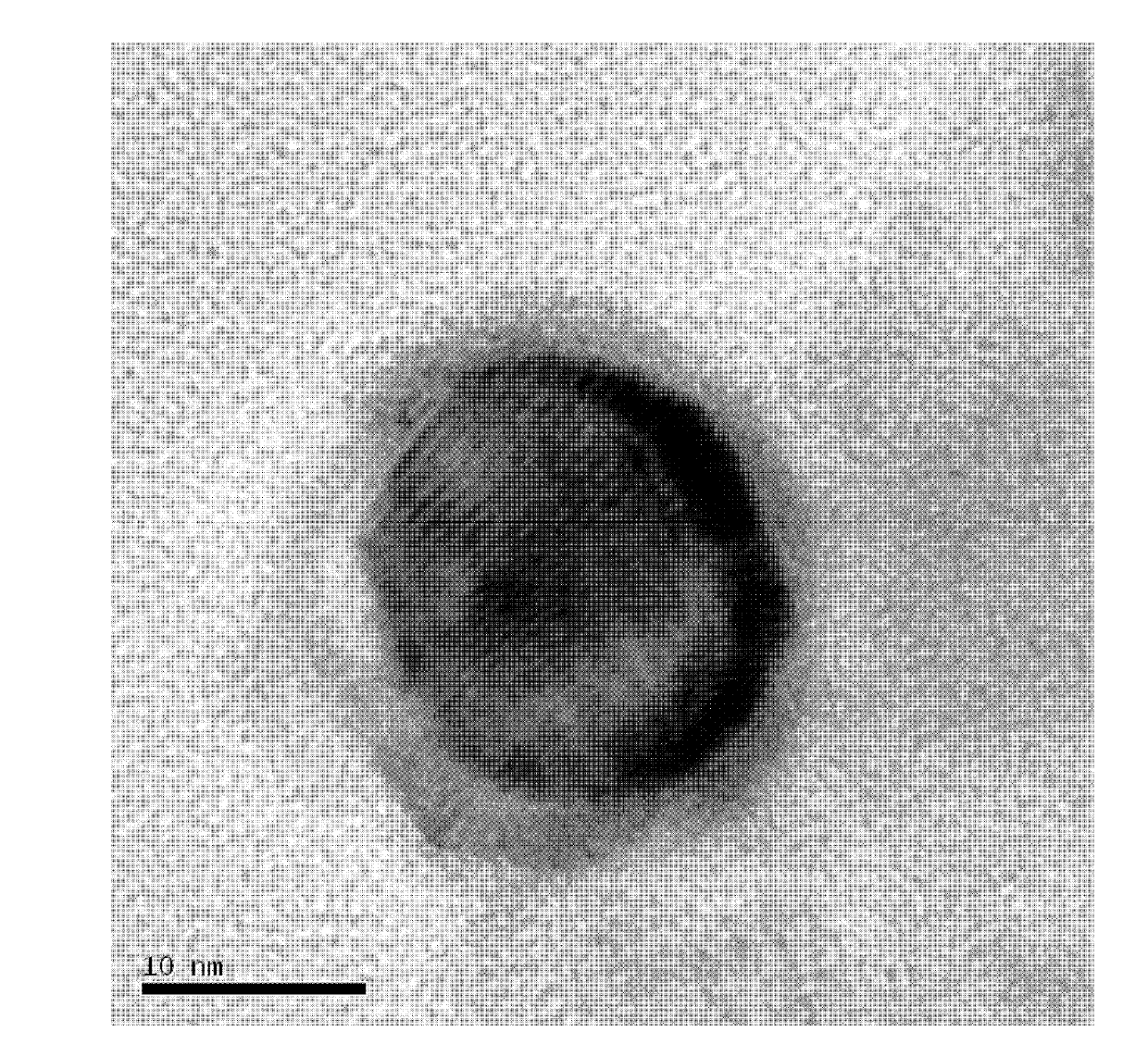
The invention provides a Ru-Co bimetallic nano supported catalyst for hydrolytic hydrogen release of ammonia borane and a preparation method of the Ru-Co bimetallic nano supported catalyst. Ru and Co are taken as active components, a metal organic framework MIL-110 is taken as a carrier, NaBH4 is taken as a reductant, and precursors (ruthenium salt and cobalt salt) are reduced into RuCo to be supported on MIL-110 to obtain the Ru-Co bimetallic nano supported catalyst RuCo@MIL-110. RuCo alloy particles have the average grain size of about 2.3 nm. At the room temperature, the Ru-Co bimetallic nano supported catalyst shows high catalytic activity in catalysis of ammonia borane for hydrolytic hydrogen release, the reaction activation energy (Ea) is 31.7kJ mol<-1>, and the conversion frequency (TOF) is 533.2 mol H2 / min<-1> (mol / Ru)<-1>. The catalyst still maintains 79.0% of catalytic activity after being recycled for five times, and has extremely high toxin immunity and cyclic stability. Compared with mono-metallic supported and bimetallic unsupported catalysts, the Ru-Co bimetallic nano supported catalyst shows higher catalytic activity. Compared with a traditional noble metal catalyst, the Ru-Co bimetallic nano supported catalyst is low in cost, simple to prepare, easily accessible in raw material, suitable for industrial production and wide in application prospect.
Owner:HUBEI UNIV
Ternary transition-metal catalyst for ammonia borane hydrolysis and preparation method thereof
InactiveCN102513125AImprove catalytic performanceReduce manufacturing costHydrogen productionMetal/metal-oxides/metal-hydroxide catalystsHydrogen desorptionTernary operation
The invention discloses a ternary transition-metal catalyst for ammonia borane hydrolysis and a preparation method thereof. The catalyst provided by the invention is a Ag0.04@CoxNi0.96-x(x=0-0.96) catalyst with a core-shell structure. According to the invention, ammonia borane is used as a reducing agent to directly reduce a mixed solution of silver nitrate, cobalt salt and nickel salt at different proportions to obtain the catalyst which is directly used for catalyzing ammonia borane hydrolysis. Due to the core-shell structure, the series of catalysts have high catalytic activity. By the adoption of the series of the catalysts for catalyzing ammonia borane hydrolysis at room temperature, the maximum hydrogen desorption rate can reach 1627.3 mlmin<-1>g<-1> and activation energy of the reaction is 28.54 kJmol<-1>. The core-shell structured ternary transition-metal catalyst has characteristics of small particle size, large specific surface area, many catalytic active sites and the like, is beneficial to catalytic hydrolysis of ammonia borane, has advantages of rich resources, low production cost and the like in comparison with a traditional noble metal catalyst, and is a promising catalyst.
Owner:天津天环光伏太阳能有限公司
Porous active charcoal material supporting cobalt nanoparticle material, preparation method, and application thereof
InactiveCN107159214ALow costConducive to the realization of standardized productionHydrogen productionMetal/metal-oxides/metal-hydroxide catalystsFuel cellsPorous carbon
The invention discloses a porous active charcoal material supporting cobalt nanoparticle material, which is prepared by performing hydrothermal treatment and subsequent treatment to glucose and a nitrogen-containing compound to obtain a porous carbon material, and supporting cobalt particles onto the carbon material through an impregnating chemical reduction method. The material is 3026-3277 m<2> / g in specific surface area, is more than 95.18% in micropore content, and has uniform distribution in pore size, which is mainly distributed in 1.24-1.95 nm. The preparation method includes the steps of: 1) preparing a nitrogen-containing precursor; 2) preparing the porous carbon material; and 3) supporting the cobalt nanoparticles. The material, when being used as a catalyst for catalyzing hydrolytic hydrogen release of ammonia borane, can complete the hydrogen release within 10 min, hydrogen release rate reaching 865.2 ml*min<-1>*g<-1>. The material can be recycled, and after circulation for 4 times, the hydrogen release time can be maintained in 10-45 min and hydrogen release rate can be maintained at 208.2-865.2 ml*min<-1>*g<-1>. The preparation method is simple and has low production cost. The material can be recycled, has good practicability, and has wide application prospect in the fields of hydrogen production, fuel cells, etc.
Owner:GUILIN UNIV OF ELECTRONIC TECH
Preparation method of porous silica coated Co-N-C hollow nanotube material and application thereof
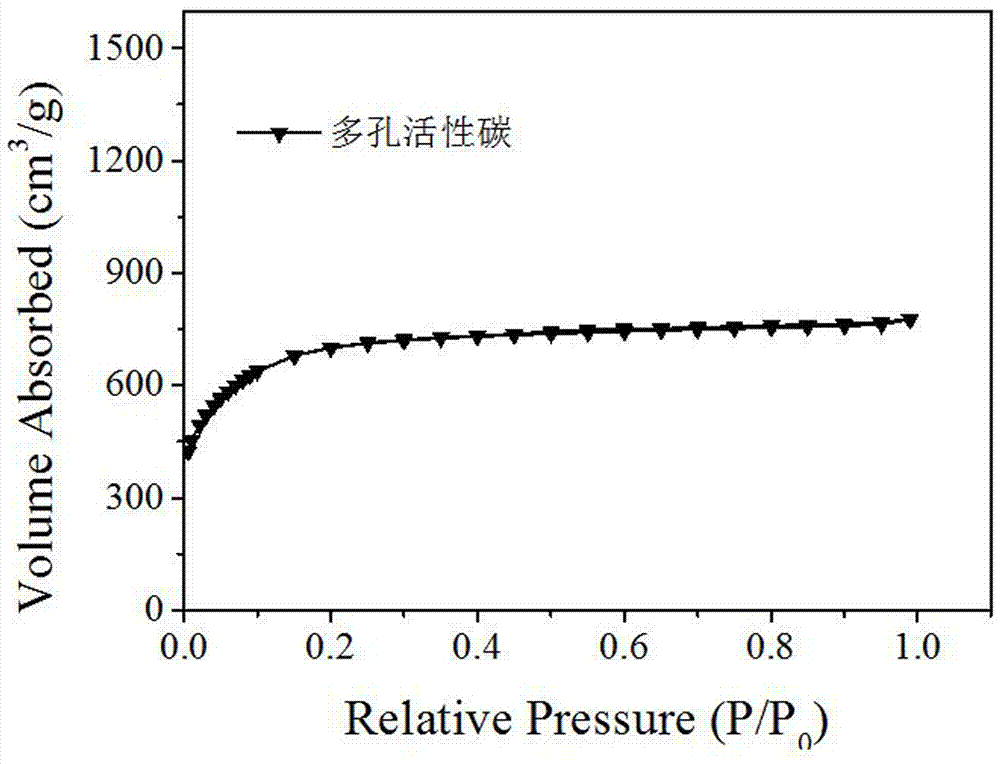
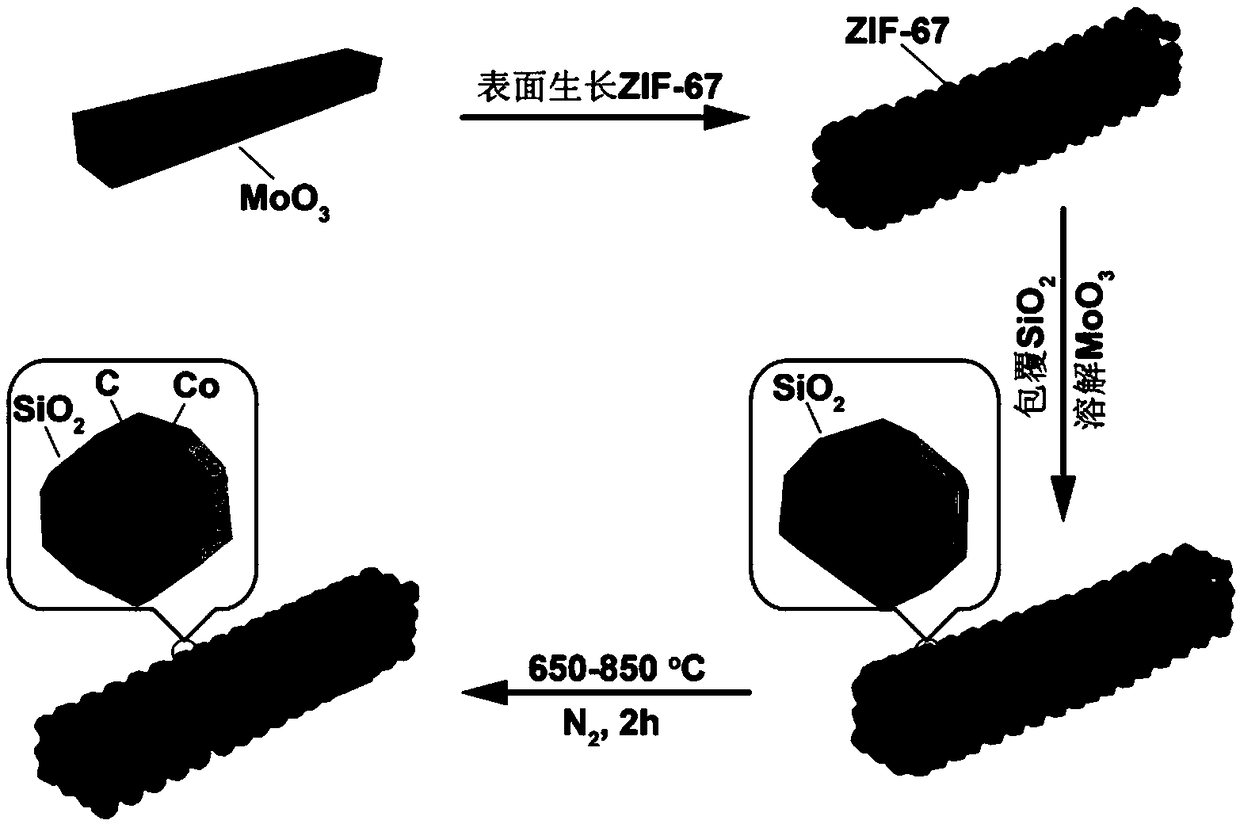
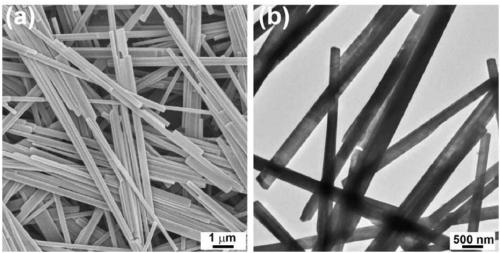
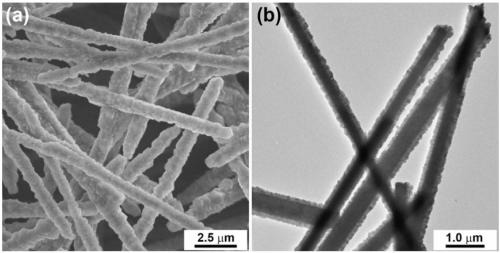
ActiveCN109174155AEasy transferSmall sizeCatalyst activation/preparationHydrogen productionHydrogenActivation energy
The invention discloses a preparation method of a porous silica coated Co-N-C hollow nanotube material and an application thereof. According to the invention, MoO3 is prepared as nanorod, the surfaceof MoO3 as the nanorod is loaded with ZIF-67, and MoO3@ZIF-67 nanorod is obtained; MoO3 is used as a self-sacrificial template is used, a 2-methylimidazole aqueous solution can provide the alkaline environment for simultaneously dissolving MoO3 and promoting the hydrolysis of a SiO2 precursor, and the ordered ZIF-67@SiO2 hollow nanorod can be obtained; and the porous Co-N-C@SiO2 nanotube can be obtained by high temperature calcination under nitrogen protection. and can be used as a non-precious metal catalyst for efficiently catalyzing the hydrolysis of ammonia borane. A TOF value can reach 8.4mol min<-1>mol<-1>(Co) at 298K, the material has low activation energy of 36.1kJ mol<-1>, and can achieve recyclability by more than ten times.
Owner:ANHUI NORMAL UNIV
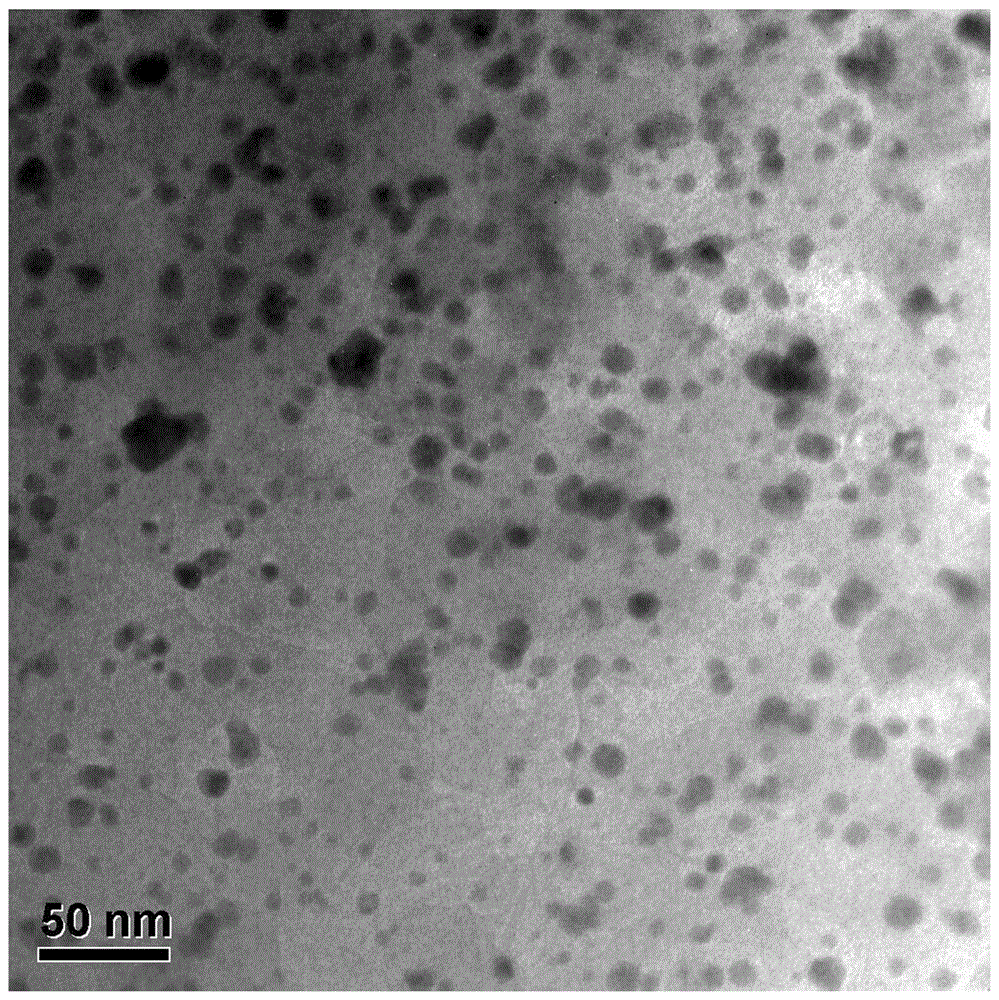
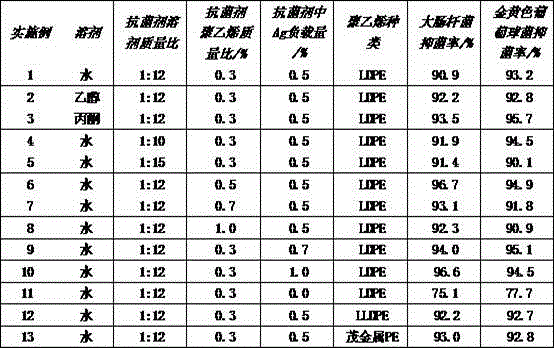
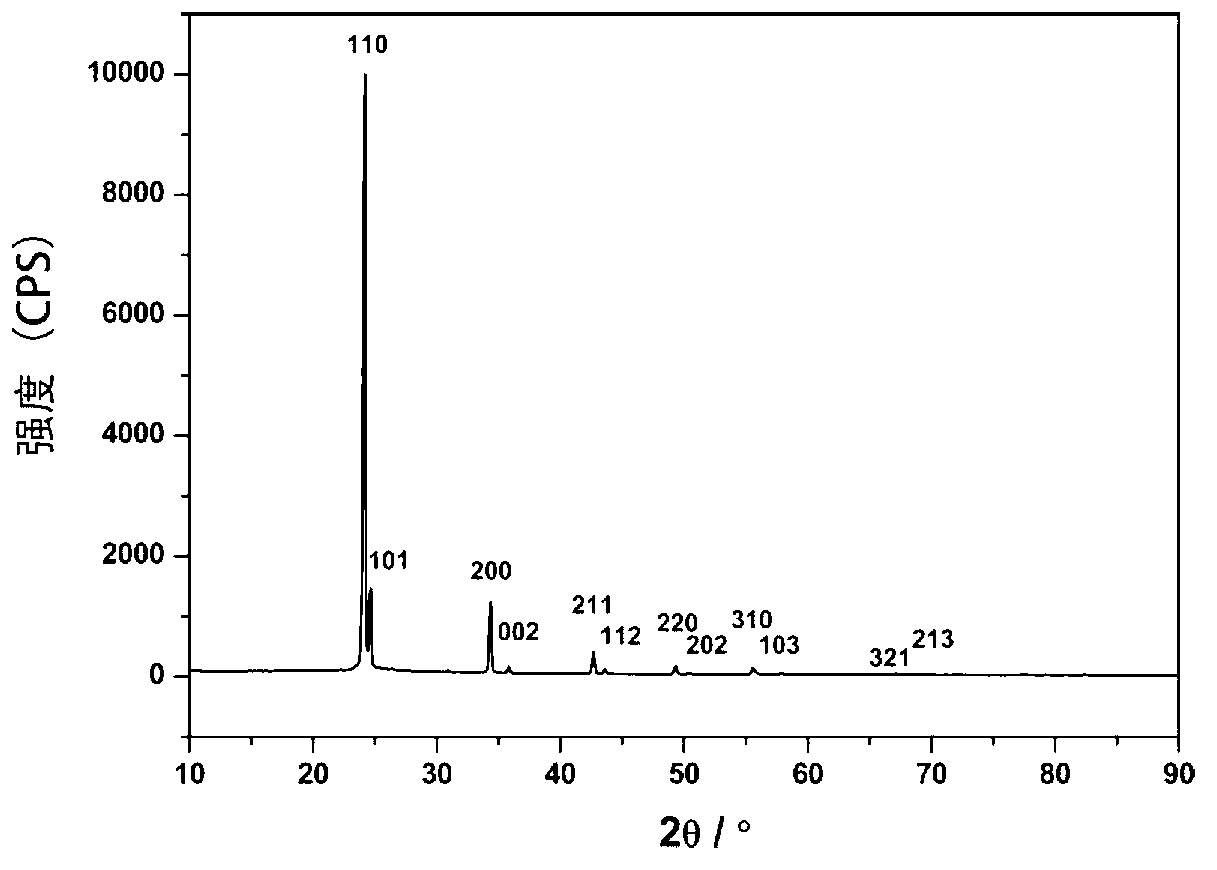
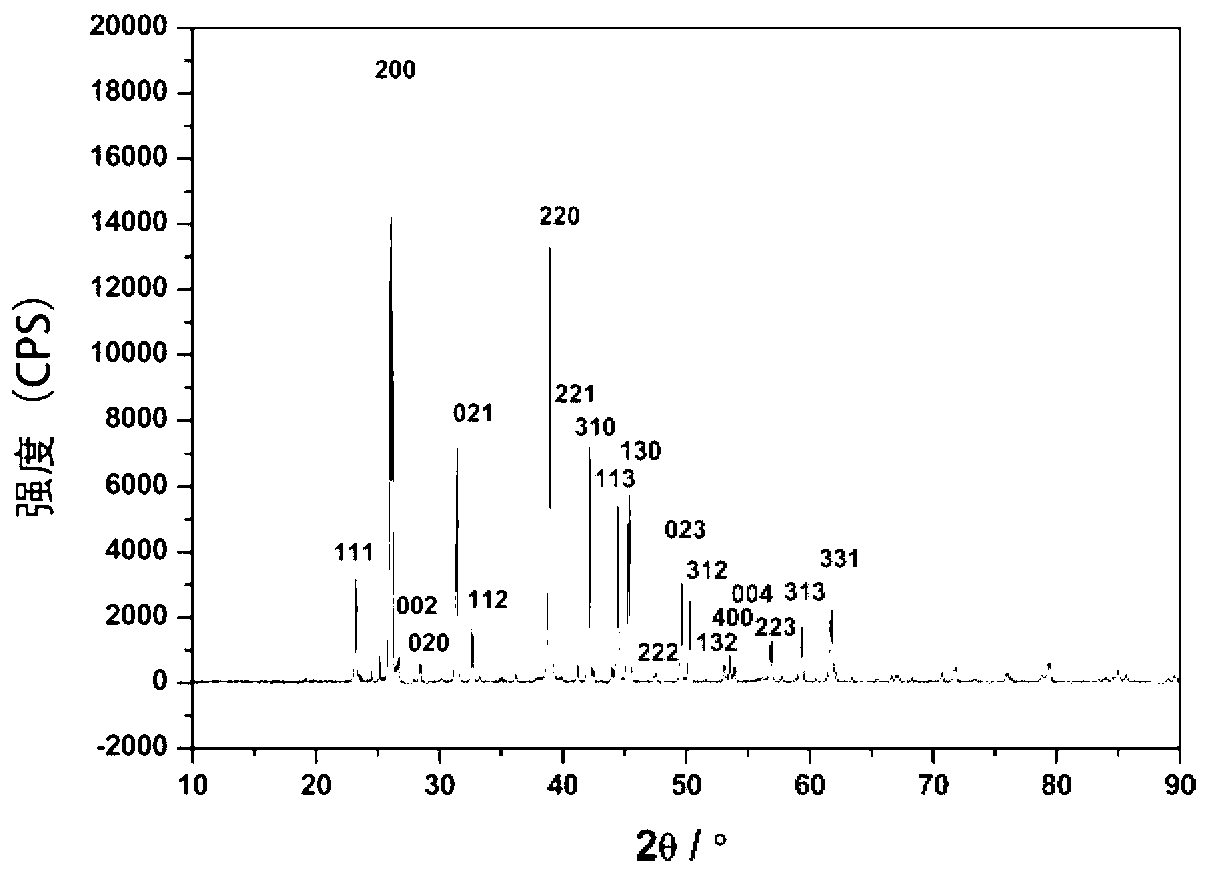
Preparation method of polyethylene antimicrobial packaging film
ActiveCN105968489AComposite uniformThe preparation process is simple and environmentally friendlyEscherichia coliEnvironmental resistance
The invention discloses a preparation method of a polyethylene antimicrobial packaging film. The preparation method is characterized by comprising the following steps of adding 0.3 to 1.0 part by weight of silver-loaded nano titania antimicrobial agent into 100 parts by weight of polyethylene resin; then adding 0.06 to 0.24 part of antioxidant 1076 and 0.04 to 0.16 part of auxiliary antioxidant 168; sufficiently mixing in a high-speed mixer, then granulating, drying and blowing film to prepare the polyethylene antimicrobial packaging film. Compared with the prior art, the preparation method disclosed by the invention has the beneficial effects that silver-ammonia ions are induced to form a strong interaction with the surface of titania by regulating a pH value; the silver-ammonia ions are reduced by using a water extract of a biomass loquat fruit, and further uniform compounding of silver and titania at the nanoscale is realized; no dispersing agent is needed, so that a preparation process is green and environmentally-friendly; the antibacterial rate of the prepared polyethylene antimicrobial packaging film to escherichia coli and staphylococcus aureus reaches 90 percent or above; the polyethylene antimicrobial packaging film is non-toxic, safe and sanitary.
Owner:SHANTOU HONGQIAO PACKAGING IND
Method for synthesizing ammonia borane
InactiveCN103303867ASufficient hydrogen release effectGood natureMetal hydridesRotary evaporatorSodium borohydride
The invention discloses a method for synthesizing ammonia borane. The method comprises the following two synthesizing steps of: 1, reacting a mixture of ammonium fluoroborate and sodium borohydride in an experimental container with a reflux unit in which a dioxane solvent is filled, thereby obtaining a dioxane solvent for dissolving ammonia borane; 2, performing heating and spin drying treatment on the solvent by utilizing a rotary evaporator, thereby obtaining the product ammonia borane. In the process, the dioxane recovered by utilizing the rotary evaporator can be repeatedly utilized to serve as the solvent in the ammonia borane preparation process. In the method, the ammonium fluoroborate serves as an ammonium source for the first time, the synthetic method is simple, the product yield is considerable, and the purity is high.
Owner:HEBEI UNIV OF TECH
Heat generating structures
A heat generating structure includes a substrate of a first material and a second material coating at least a portion and preferably all of the first material, where the second material is different from the first material. The structure also includes an additional material or compound such as ammonia borane that is impregnated or located within the structure. When the structure is thermally energized, the first and second materials react with each other in an exothermic and self-sustaining reaction that pyrolyzes the impregnated ammonia borane compound to create a target gas, for example, hydrogen from the ammonia borane. An additional material, for example, a thermite, may be interposed between the structure and the ammonia borane to facilitate the ignition of the ammonia borane.
Owner:ENSIGN BICKFORD AEROSPACE & DEFENSE
Mesoporous silica protected ultra-thin ferro-nickel nitride composite material and preparation thereof
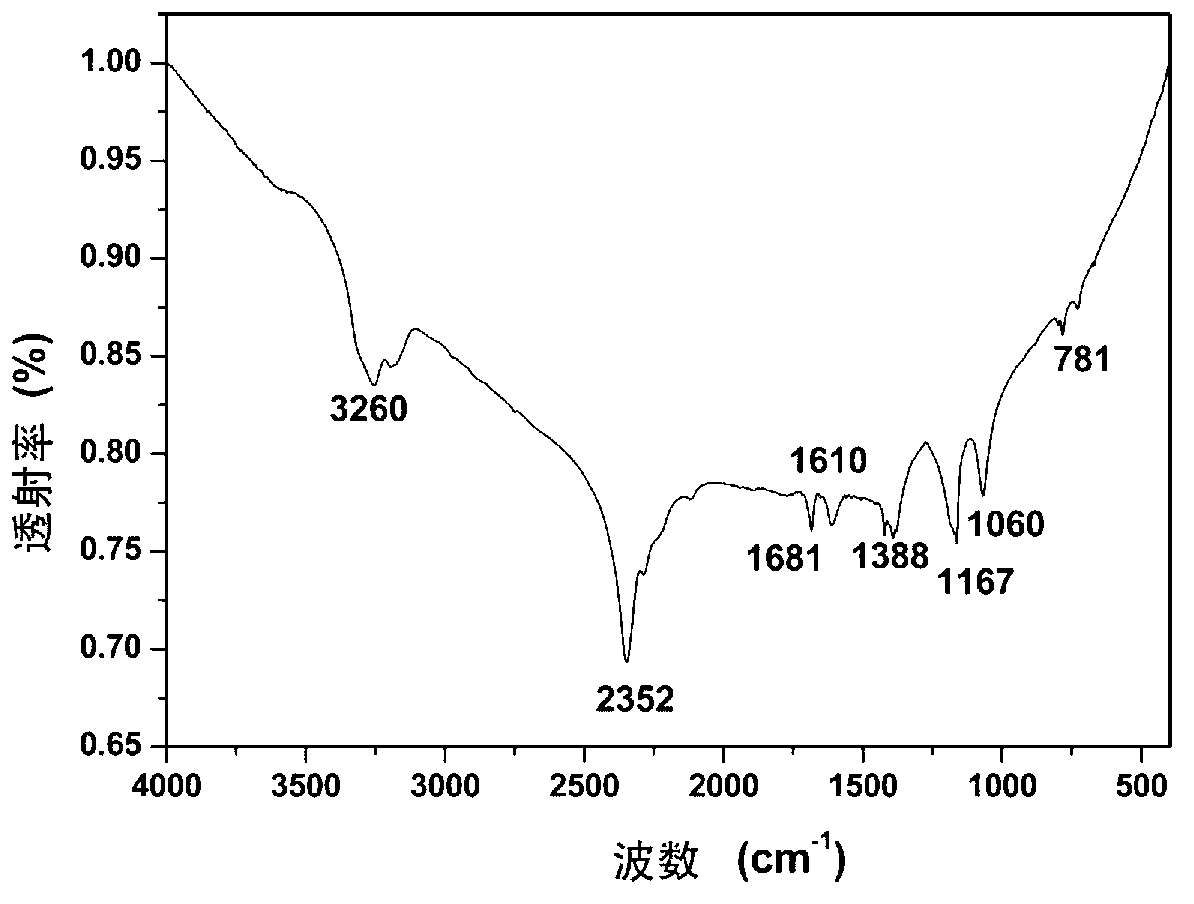
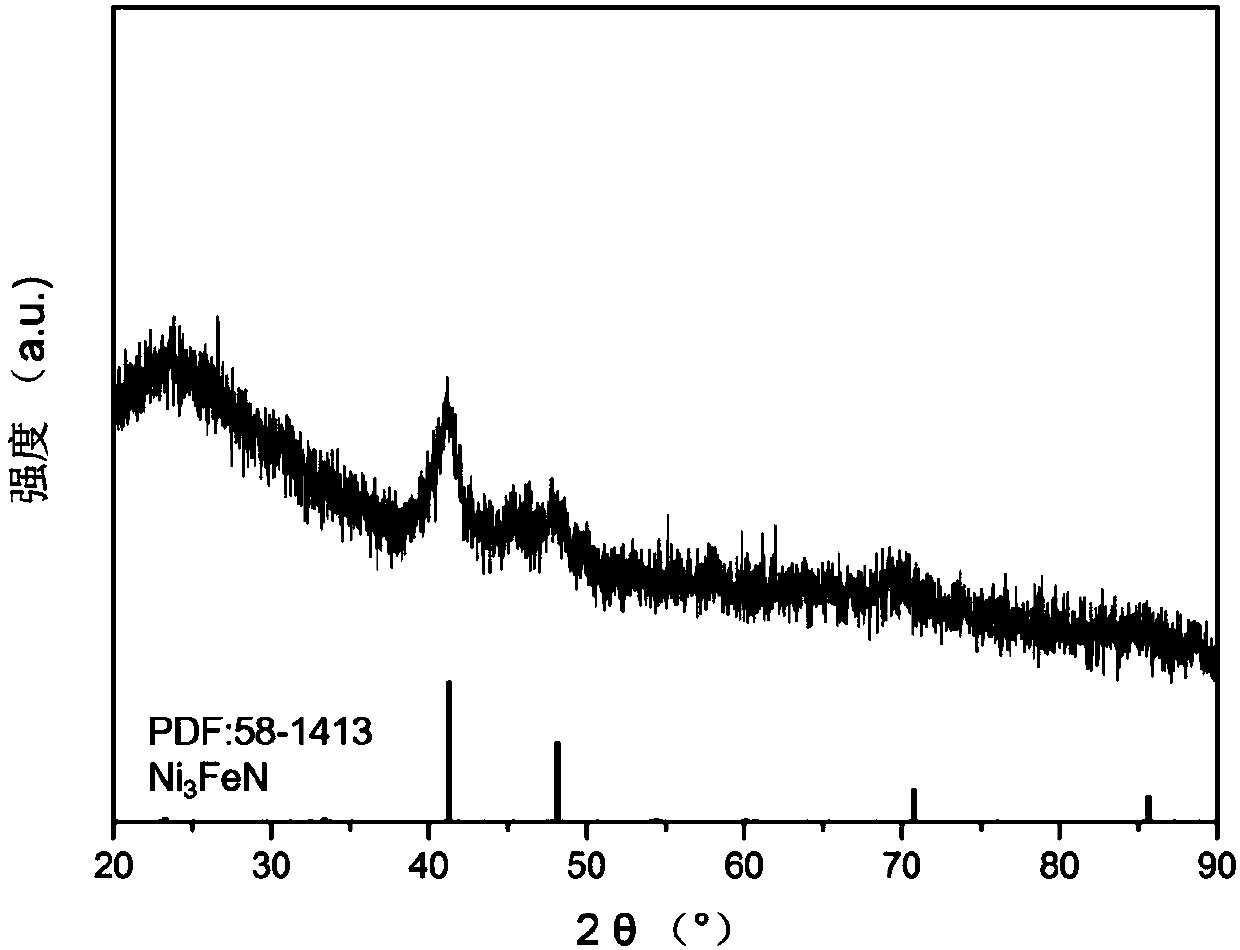
The invention discloses a mesoporous silica protected ultra-thin ferro-nickel nitride composite material and preparation thereof. The structure of the composite material is Ni3FeN@SiO2, and the composite material comprises an ultra-thin ferro-nickel nitride base layer and mesoporous silica coating the surface of the ultra-thin ferro-nickel nitride base layer. The preparation method comprises the following steps: obtaining a ferro-nickel hydrotalcite precursor through a reverse micro-emulsion method; under the existence of a surface active agent and a silicon source, using a sol-gel method to coat the silica material of a mesoporous structure on the surface of the ferro-nickel hydrotalcite precursor to obtain an original composite material; burning the original composite material in an ammonia gas atmosphere so as to obtain the final mesoporous silica protected ultra-thin ferro-nickel nitride composite material. The preparation method is mild in conditions and applicable generally, andagglomeration in the burning process is avoided; and the obtained mesoporous silica protected ultra-thin ferro-nickel nitride composite material has excellent activity in catalyzed ammonia borane hydrolysis hydrogen production reactions.
Owner:TECHNICAL INST OF PHYSICS & CHEMISTRY - CHINESE ACAD OF SCI
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com