Ternary transition-metal catalyst for ammonia borane hydrolysis and preparation method thereof
A transition metal and catalyst technology, applied in the field of ternary transition metal catalyst and its preparation, can solve the problems of high price, high production cost, difficult to realize industrialized production and the like
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
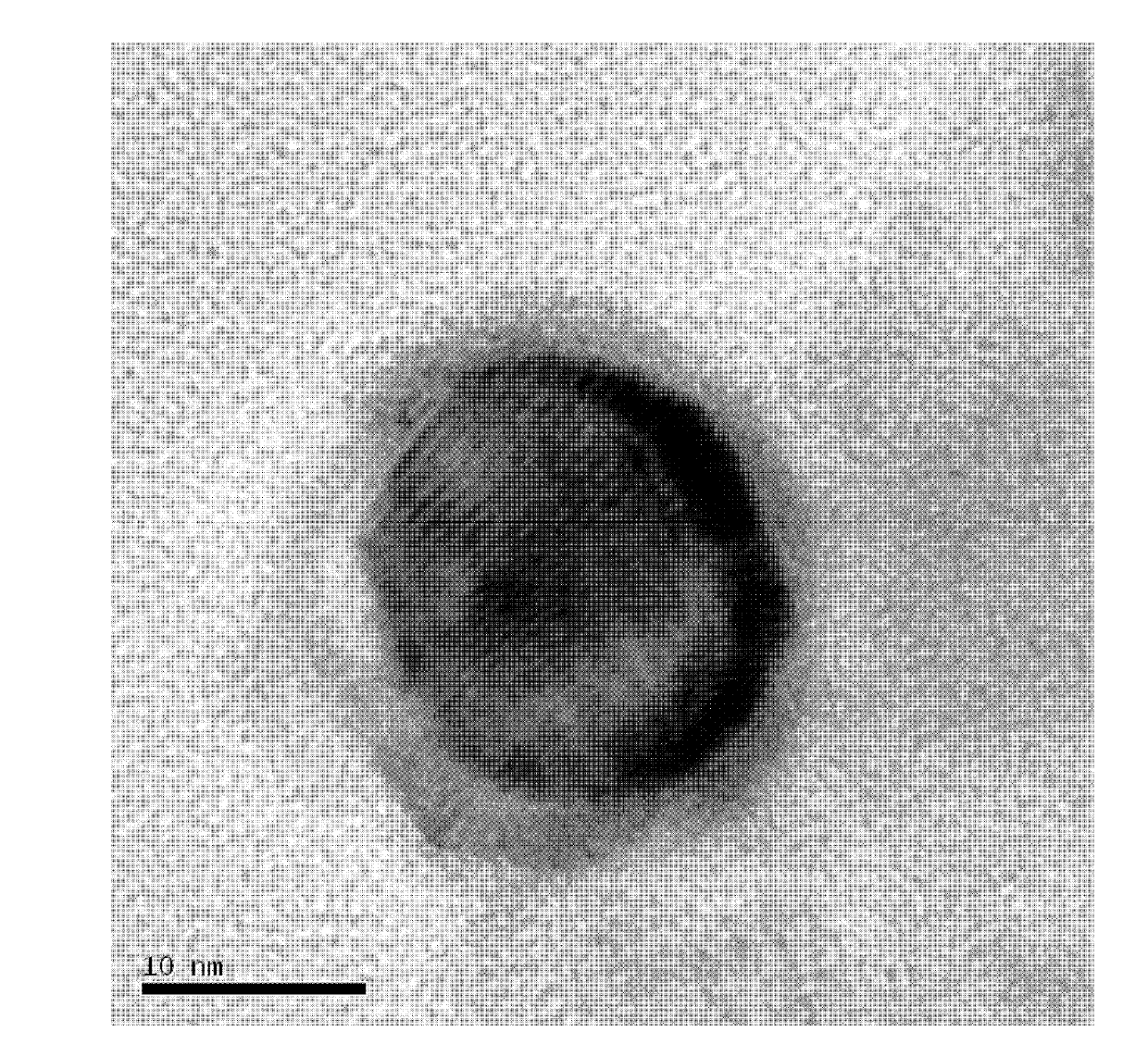
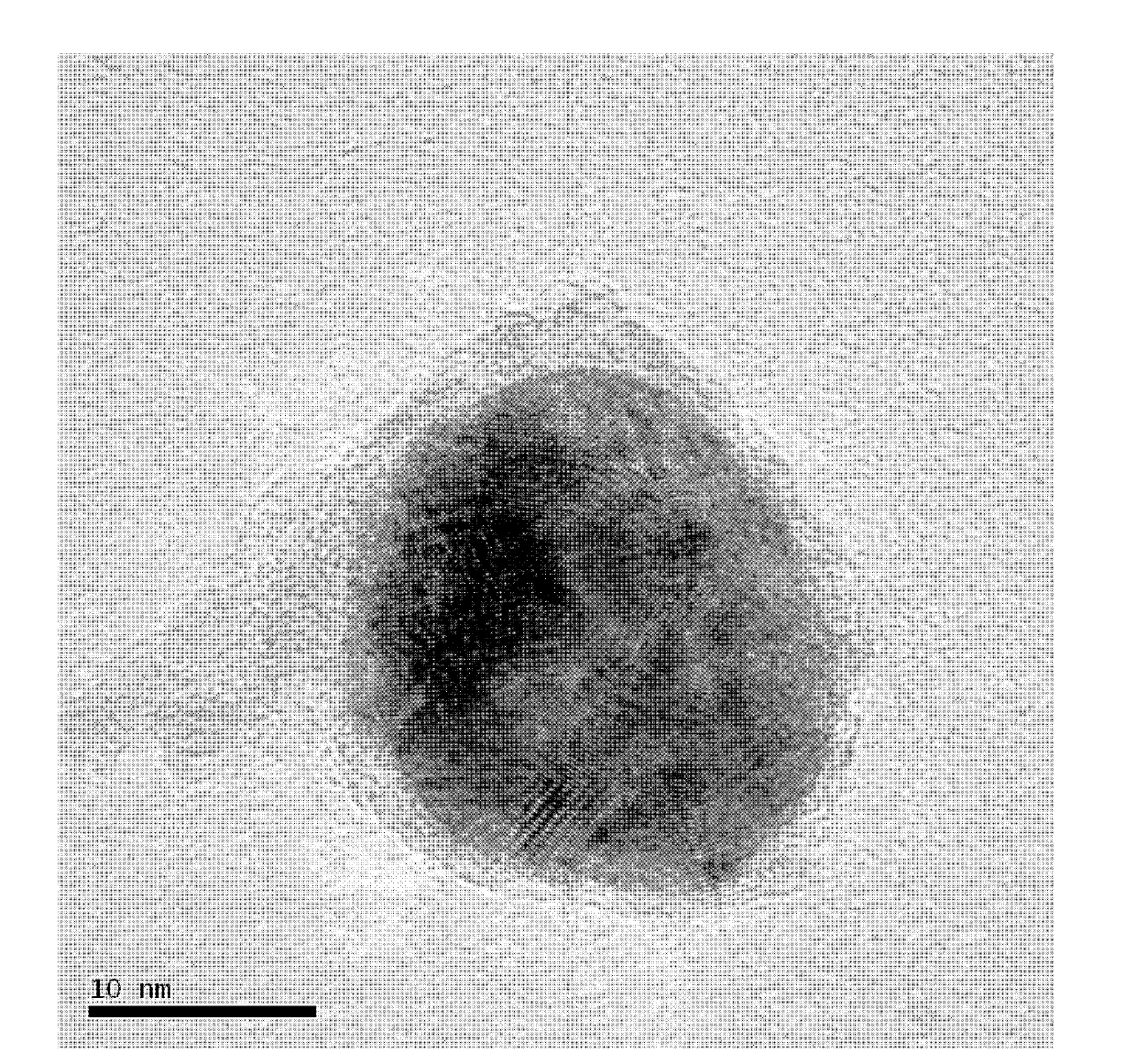
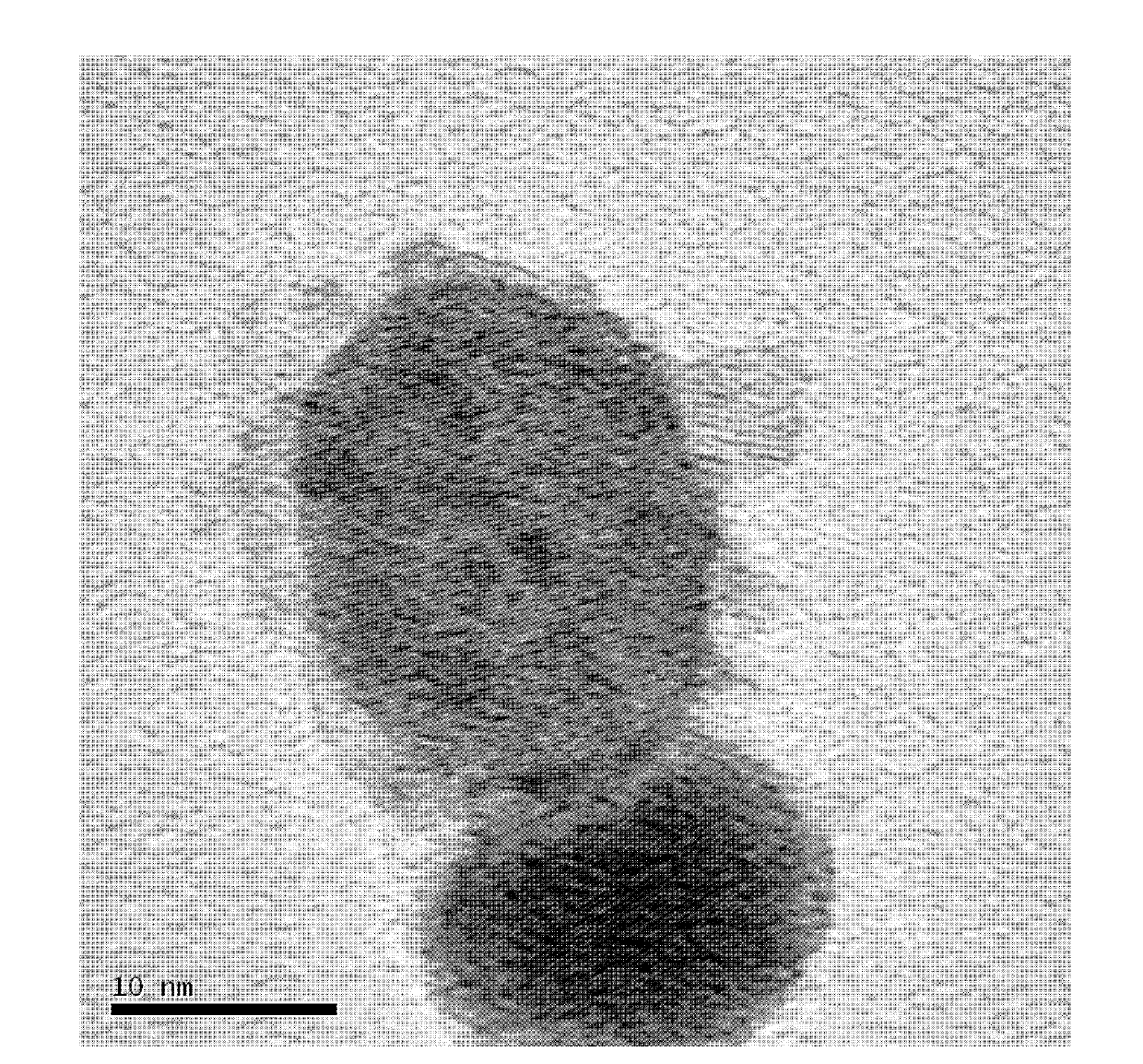
[0024] Weigh silver nitrate, cobalt acetate and nickel acetate according to the molar ratio Ag:Co:Ni=0.04:0:0.96 and mix them in a beaker, slowly add a solution of 1% PVP with a separating funnel, wherein the nitric acid The molar ratio of the mixture of silver, cobalt salt and nickel salt to polyvinylpyrrolidone PVP is 3:100, and it is fully dissolved by ultrasonication for 3 minutes to form a uniform solution; then add 0.2% ammonia borane in a mass percentage of the solution, Ultrasonic reaction for 30 minutes to complete the reaction; let the mixed liquid in the beaker stand still, pour off the supernatant, wash the solid at the bottom of the beaker with distilled water and ethanol three times, and dry it in an oven at 60°C to obtain a core-shell structure Ag 0.04 Ni 0..96 catalyst.
Embodiment 2
[0026] Weigh silver nitrate, cobalt chloride and nickel acetate according to the molar ratio Ag:Co:Ni=0.04:0.48:0.48 and mix them, place them in a beaker, add a PVP solution with a mass percentage of 1.5%, and a mass percentage of 0.25% ammonia Borane, wherein the mol ratio of the mixture of silver nitrate, cobalt salt and nickel salt to polyvinylpyrrolidone PVP is 3:150, and other preparation conditions are the same as embodiment 1, and obtain the Ag with core-shell structure 0.04 co 0.48 Ni 0..48 catalyst 。
Embodiment 3
[0028] Weigh silver nitrate, cobalt acetate and nickel sulfate according to the molar ratio Ag:Co:Ni=0.04:0.96:0 and mix them, put them in a beaker, add 2% PVP solution by mass percentage, and 0.3% ammonia boron alkane, wherein the molar ratio of the mixture of silver nitrate, cobalt salt and nickel salt to polyvinylpyrrolidone PVP is 3:200, and other preparation conditions are the same as in Example 1 to obtain Ag with core-shell structure. 0.04 co 0.96 catalyst.
[0029] The prepared above-mentioned catalyst is tested as follows for ammonia borane hydrolysis hydrogen release performance test and activation energy test:
[0030] Synthetic ternary transition metal catalyst Ag 0.04 Ni 0..96 、Ag 0.04 co 0.48 Ni 0..48 、Ag 0.04 co 0.96 Separately catalyze the hydrolysis of ammonia borane: take 10 mg of catalyst, 50 mg of ammonia borane, and 10 mg of sodium borohydride, mix them and place them at the bottom of a 25 ml round bottom flask, and place them in a water bath at 20...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com