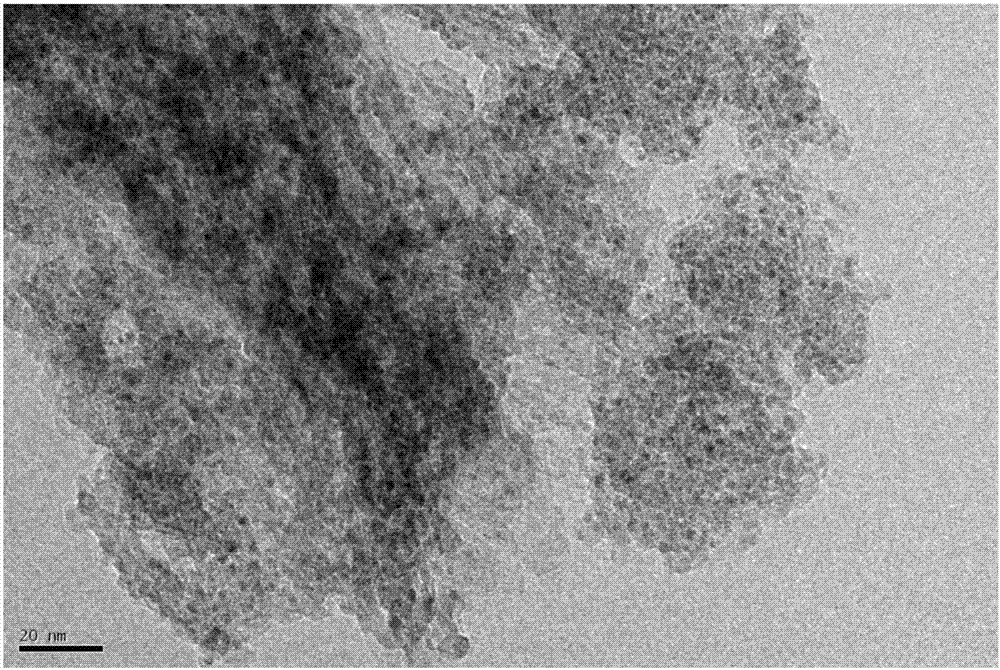
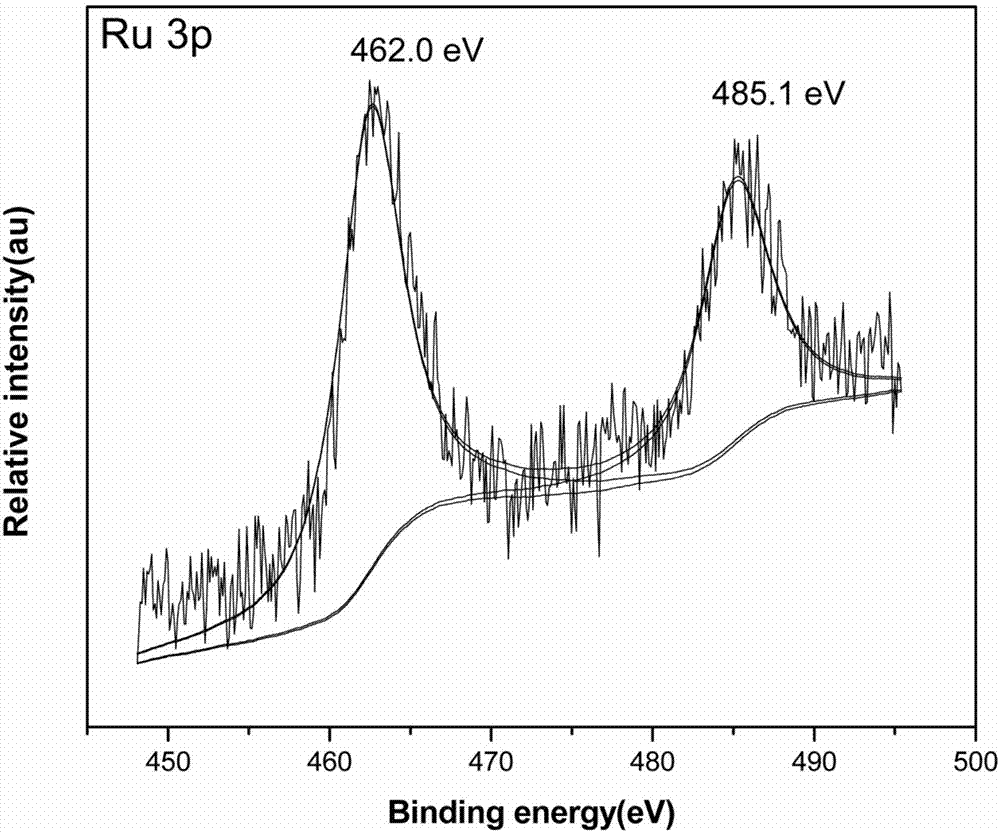
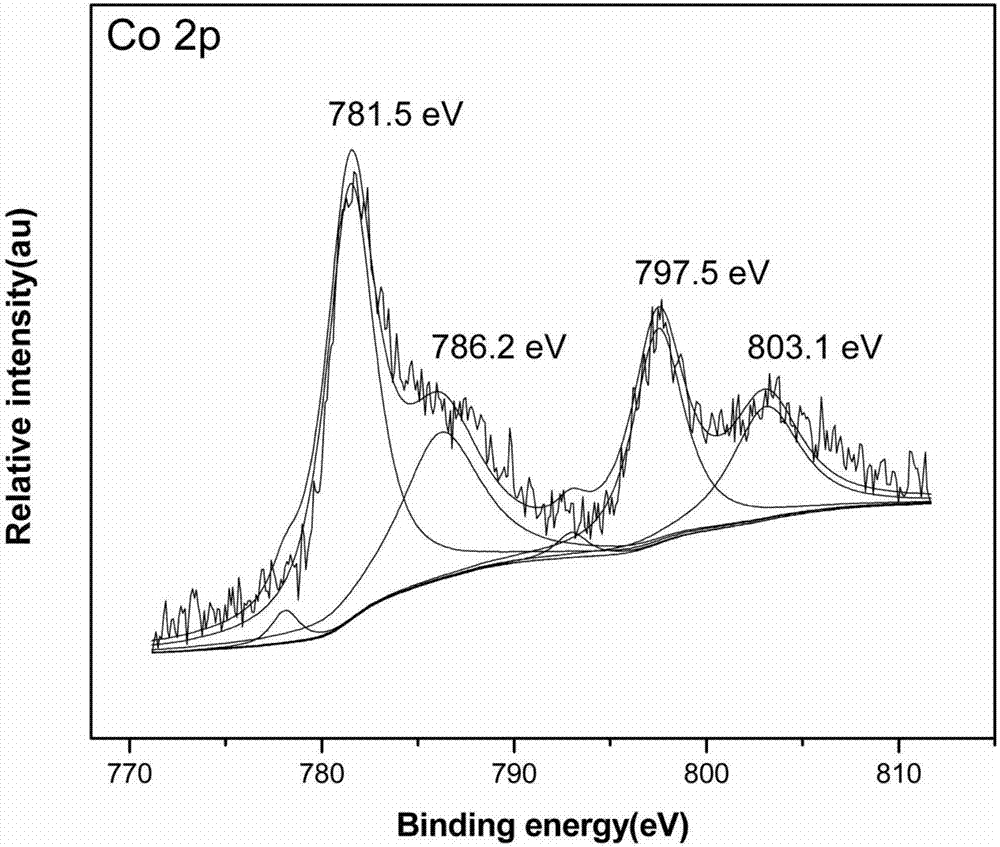
Ru-Co bimetallic nano supported catalyst for hydrolytic hydrogen release of ammonia borane and preparation method of Ru-Co bimetallic nano supported catalyst
A supported catalyst and bimetallic nanotechnology, applied in the field of nano-catalytic materials, can solve the problems of unsatisfactory catalytic effect and stability of the catalyst, and achieve the effects of low cost, easy availability of raw materials and cost reduction.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0026] Example 1: Preparation of carrier MIL-110 and bimetallic nano-supported catalyst RuCo@MIL-110
[0027] Al(NO 3 ) 3 9H 2 O, trimethyl trimesate (Me 3 btc), NaOH, and deionized water in a molar ratio of 1:0.5:2.3:309, then mixed and stirred evenly, added to a 50mL stainless steel reactor with a polytetrafluoroethylene liner, and reacted at 210°C for 3h to obtain a suspension liquid. Then cool naturally, filter the suspension with suction, wash and dry to obtain a white solid. The obtained white solid was added into a 50 mL reaction kettle containing N,N dimethylformamide (DMF) solution, and reacted at 150° C. for 5 h. After the reaction, the solid was refluxed in deionized water at 100° C. for 12 hours, washed, and vacuum-dried at 80° C. to obtain a white metal-organic framework MIL-110 powder.
[0028]Weigh 50 mg of the above MIL-110 white powder and add it to 30 mL of deionized water, sonicate for 20 min, then add 0.025 mmol of ruthenium salt and 0.025 mmol of cob...
Embodiment 2
[0032] Example 2: Preparation of single metal supported catalysts Ru@MIL-110 and Co@MIL-110
[0033] Weigh 50 mg of MIL-110 prepared in Example 1 and 2.5 mL of RuCl with a concentration of 0.01 mol / L 3 solution, and the two were added to 30mL deionized water, and ultrasonicated for 10min to obtain a uniformly dispersed suspension. Then weigh 50.0mg reducing agent NaBH 4 solid and dissolved it in 10 mL deionized water, the NaBH 4 The aqueous solution was added dropwise to the above suspension, and stirring was continued for 3 h after the dropwise addition was completed. The resulting product was filtered, washed, and vacuum-dried overnight to obtain the Ru@MIL-110 catalyst.
[0034] Weigh 50 mg of MIL-110 prepared in Example 1 and 2.5 mL of Co(NO 3 ) 2 solution, and the two were added to 30mL deionized water, and ultrasonicated for 10min to obtain a uniformly dispersed suspension. Then weigh 50.0mg reducing agent NaBH 4 solid and dissolved it in 10 mL deionized water, th...
Embodiment 3
[0035] Example three: Preparation of RuCo nanoparticles
[0036] Measure 2.5mL of RuCl with a concentration of 0.01mol / L 3 solution and 2.5 mL of Co(NO 3 ) 2 The solution was added to 30 mL of deionized water, and a uniformly dispersed mixed solution was obtained after ultrasonication for 10 min. Then weigh 50.0mg reducing agent NaBH 4 solid and dissolved it in 10 mL deionized water, the NaBH 4 The aqueous solution was added dropwise to the above mixed solution, and stirring was continued for 3 h after the dropwise addition was completed. The resulting product was filtered, washed, and vacuum-dried overnight to obtain a RuCo nanoparticle catalyst.
PUM
| Property | Measurement | Unit |
|---|---|---|
| The average particle size | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com