Ammonia borane or hydrazine hydrate catalytic hydrolysis hydrogen release system containing nano-metal phosphide MxPy catalyst and application of catalytic hydrolysis hydrogen release system
A technology for hydrazine hydrate to catalyze water and nanometer metals. It is applied in the direction of physical/chemical process catalysts, phosphides, chemical/physical processes, etc. It can solve the problems of low hydrogen release efficiency and the need to improve the stability of non-precious metal catalysts. , to achieve the effect of safe use, low cost and cheap raw materials
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
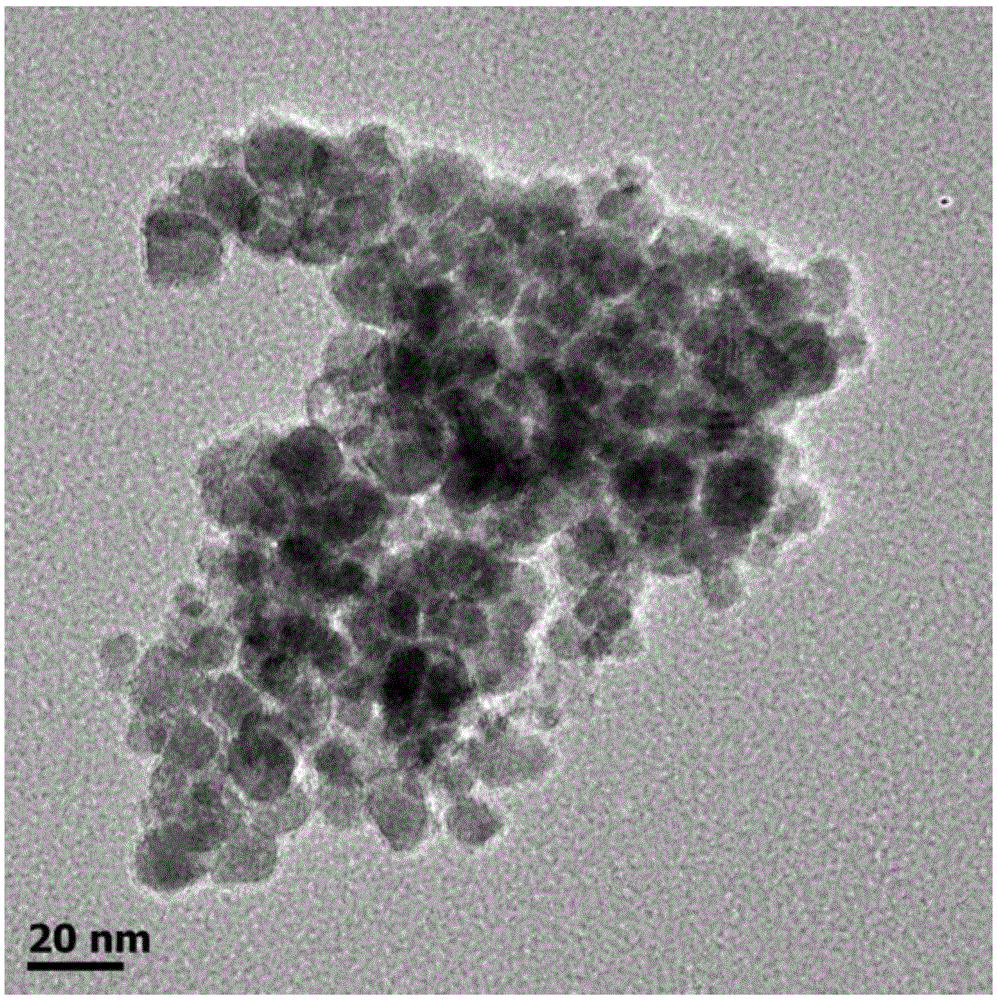
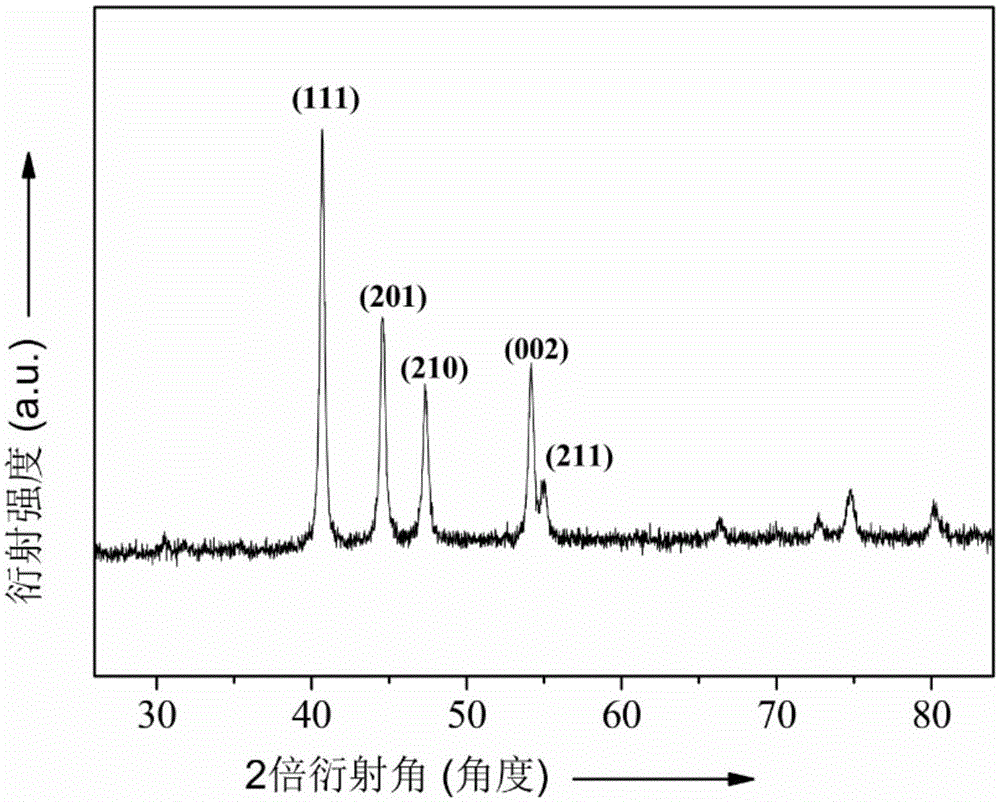
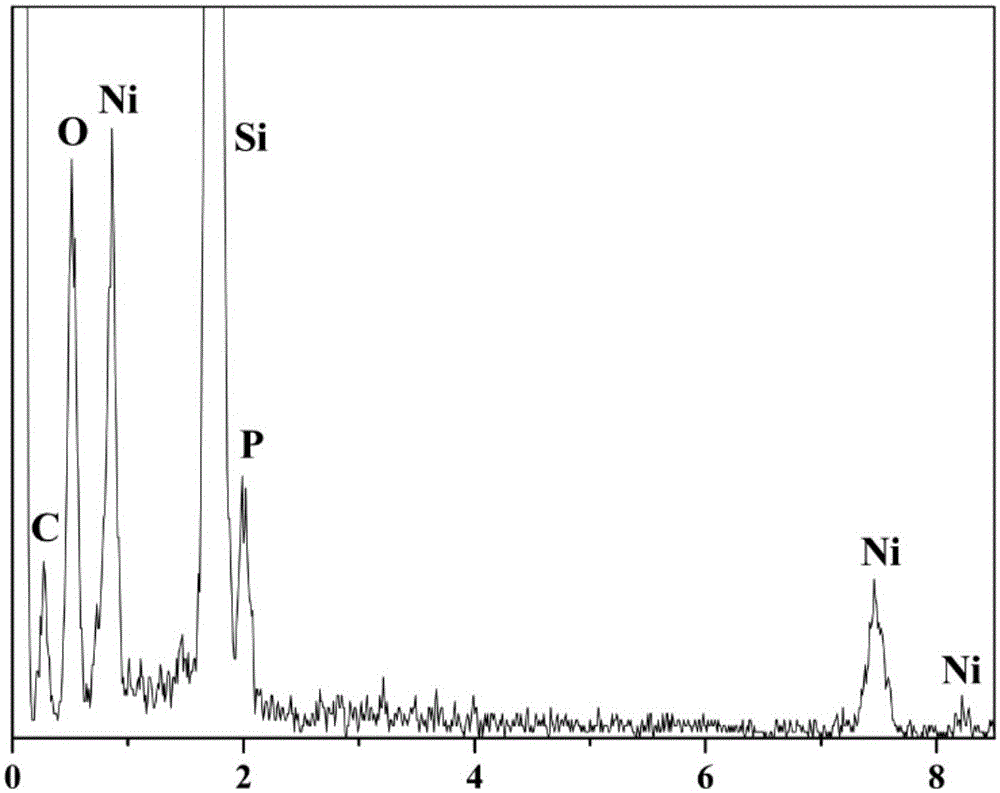
[0046] A kind of nanometer metal phosphide catalyst nickel phosphide (Ni 2 P), its preparation method is as follows:
[0047] 1) Preparation of catalyst precursor
[0048] In a round-bottomed flask, 1000.0 mg of nickel chloride hexahydrate, 250 mg of sodium citrate, 45 mL of distilled water, and 2.8 g of sodium hydroxide were added, and the mixture was stirred at room temperature for 40 min to obtain a green colloid. The green colloid in the mixture was collected by centrifugation, and after washing the green colloid with a large amount of distilled water, it was fully dried at 373K to remove water to obtain the precursor nickel hydroxide of the catalyst.
[0049] 2) Reduction of precursor by gas-solid reaction method
[0050] Thoroughly grind and mix 200mg nickel hydroxide and 1000mg sodium hypophosphite to obtain light green powder. Under argon flow, the green powder was placed in a porcelain boat of a tube furnace, heated slowly from room temperature to 543K, and maintai...
Embodiment 2
[0053] A kind of nanometer metal phosphide catalyst nickel phosphide (Ni 2 P), its preparation method is as follows:
[0054] 1) Preparation of catalyst precursor
[0055] In a round bottom flask, 200.0 mg of nickel sulfate, 150 mg of sodium citrate, 135 mL of distilled water, and 0.6 g of sodium hydroxide were added, and the mixture was stirred at room temperature for 10 min to obtain a green colloidal precipitate. The green colloidal precipitate in the mixture was collected by centrifugation, and after washing the green colloidal precipitate with a large amount of distilled water, it was fully dried at 353K to remove water to obtain the precursor nickel hydroxide of the catalyst.
[0056] 2) Reduction of precursor by gas-solid reaction method
[0057] 50mg of nickel hydroxide and 250mg of sodium hypophosphite were fully ground and mixed to obtain light green powder. Under argon flow, the green powder was placed in a porcelain boat of a tube furnace, slowly heated from roo...
Embodiment 3
[0059] A kind of nano metal phosphide catalyst iron phosphide (FeP), its preparation method is as follows:
[0060] 50mg of anhydrous ferric trichloride and 280mg of sodium hypophosphite were fully ground and mixed to obtain brown yellow powder. Under argon flow, the powder was placed in a porcelain boat of a tube furnace, heated slowly from room temperature to 623K, and maintained at the heating temperature for 1 h. After the reaction is completed, slowly cool down to room temperature, and wash the product with a large amount of distilled water and dilute hydrochloric acid to fully remove the inorganic salts inside, that is, to obtain a nanometer iron phosphide (FeP) catalyst.
PUM
| Property | Measurement | Unit |
|---|---|---|
| Particle size | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com