Novel ammonia borane composite material for hydrolysis hydrogen production
A composite material, ammonia borane technology, applied in the field of new ammonia borane composite materials, can solve the problems of low hydrogen storage density, inability to fully release hydrogen, and fail to meet the application requirements of hydrogen storage, achieve high hydrogen storage capacity, improve Activation, the effect of maintaining the amount of hydrogen released
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0018] With LiH+NH 3 BH 3 As the starting material, the molar ratio was 1:1, and LiH / NH was prepared by high-energy ball milling 3 BH 3 Complex.
[0019] Raw material: NH 3 BH 3 (purity 90%, 200 mesh), LiH (purity 95%, 200 mesh). LiH / NH with a molar ratio of 1:1 was placed in a 0.1 MPa argon atmosphere glove box. 3 BH 3 The composite and stainless steel balls were put into a ball mill jar, sealed and then milled on a high-energy ball mill with a ball-to-material ratio of 30:1 and a milling time of 10 minutes.
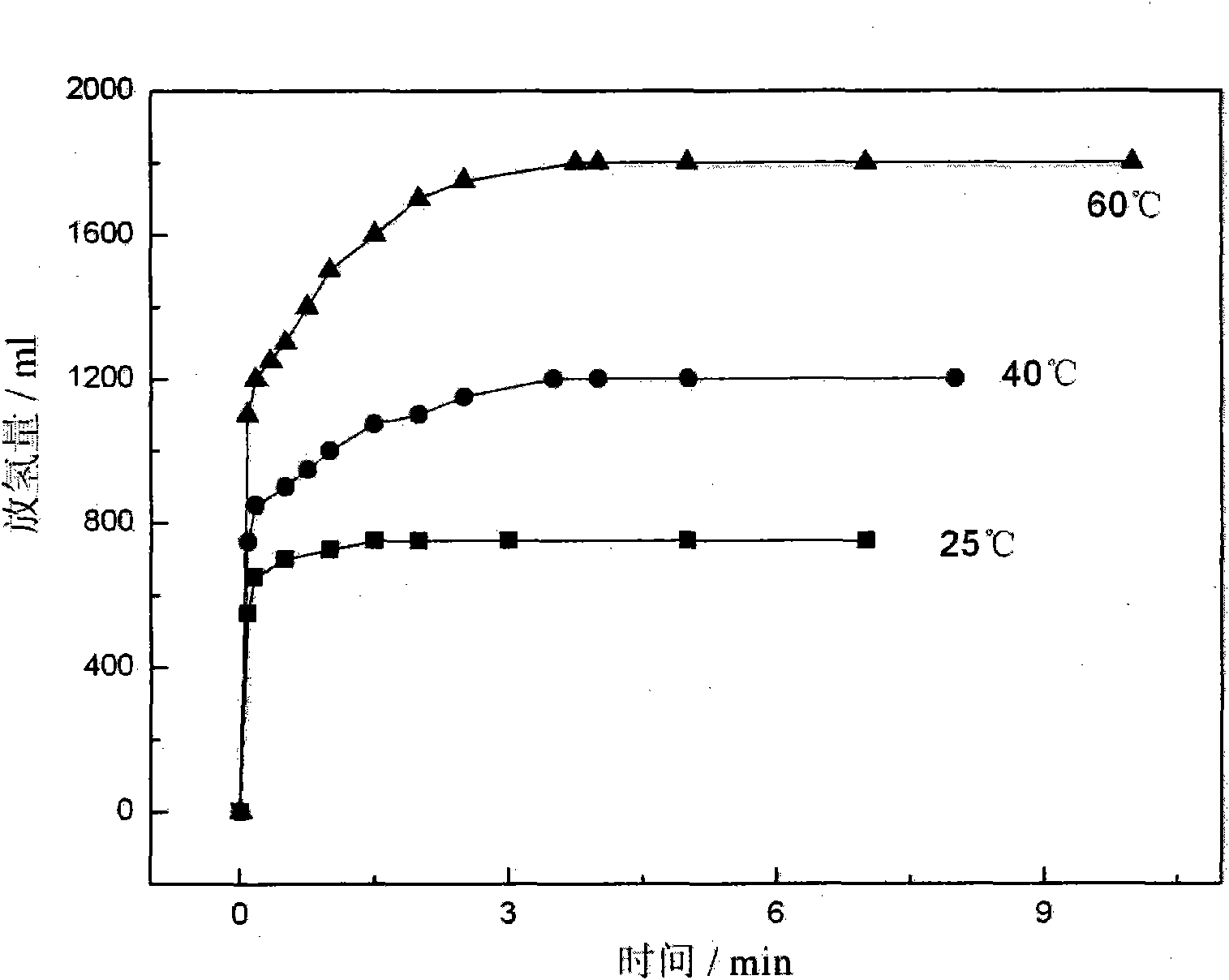
[0020] The hydrolysis hydrogen release performance of the material was tested by the drainage gas collection method. figure 1 gives LiH / NH 3 BH 3 Kinetic performance curves of hydrolysis and hydrogen release of the composite at different temperatures. The higher the temperature, the greater the amount of hydrogen released. At 60°C, the final hydrogen released is 1800ml / g, which is about 16.1wt% (mass without water).
Embodiment 2
[0022] as CaH 2 +NH 3 BH 3 As the starting material, the molar ratio was 1:2, and CaH was prepared by high-energy ball milling 2 / 2NH 3 BH 3 Complex.
[0023] Raw material: NH 3 BH 3 (purity 90%, 200 mesh), CaH 2 (purity 95%, 200 mesh). The molar ratio of raw materials is 1:2, the ball milling time is 10 minutes, and the other sample preparation conditions are the same as in Example 1.
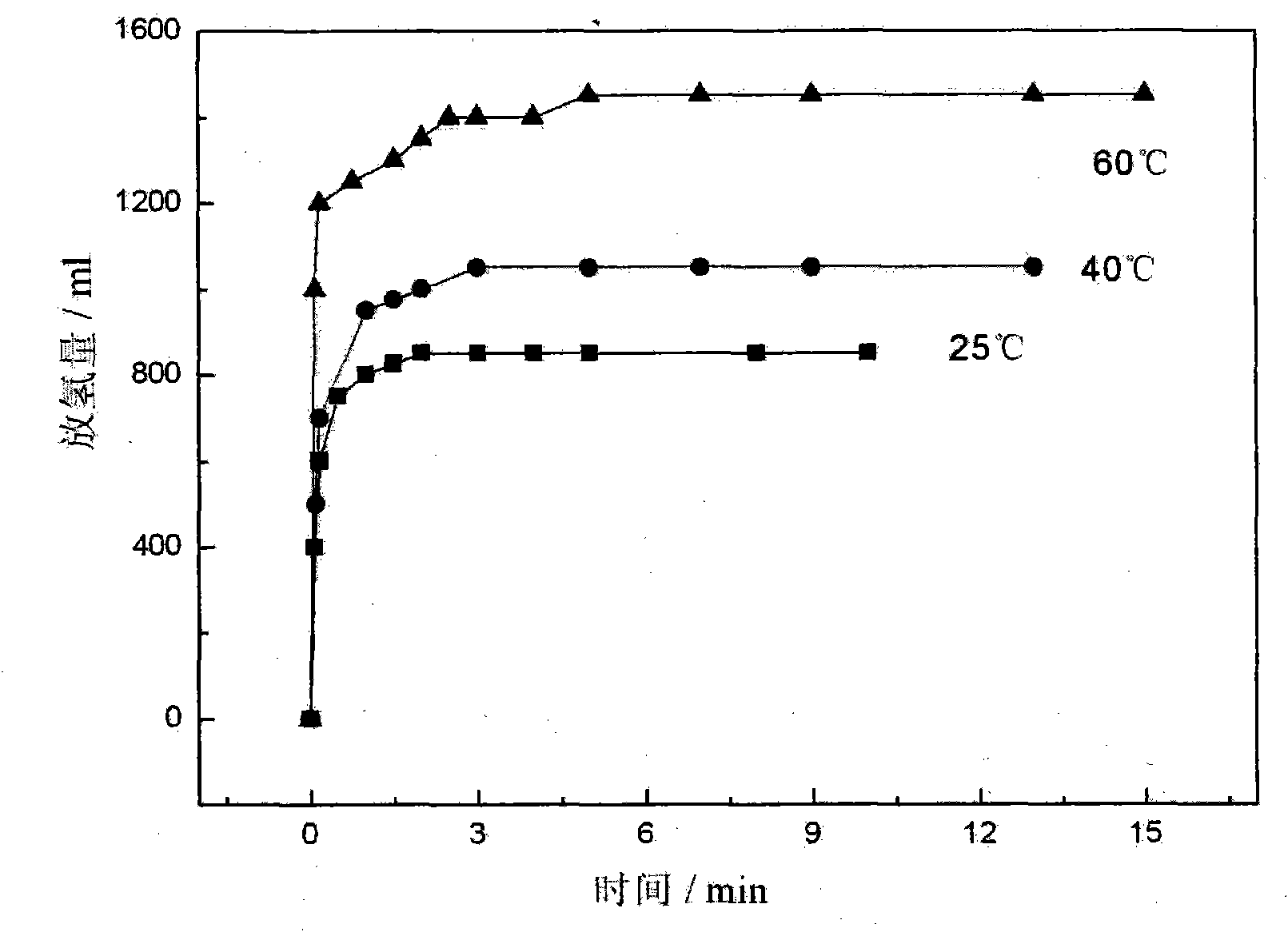
[0024] The hydrolysis hydrogen release performance of the material was tested by the drainage gas collection method. figure 2 for CaH 2 / 2NH 3 BH 3 Kinetic performance curves of hydrolysis and hydrogen release of the composite at different temperatures. 1450ml / g of hydrogen is released within 10 minutes at 60°C, which is about 12.9wt% (mass without water).
Embodiment 3
[0026] With NaCl+NH 3 BH 3 As the starting material, the molar ratio is 2:1, and the NaCl / NH 3 BH 3 Complex.
[0027] Raw material: NH 3 BH 3 (purity 90%, 200 mesh), NaCl (purity 99.9%, 200 mesh). The molar ratio of raw materials is 2:1, the ball milling time is 10 minutes, and the other sample preparation conditions are the same as in Example 1.
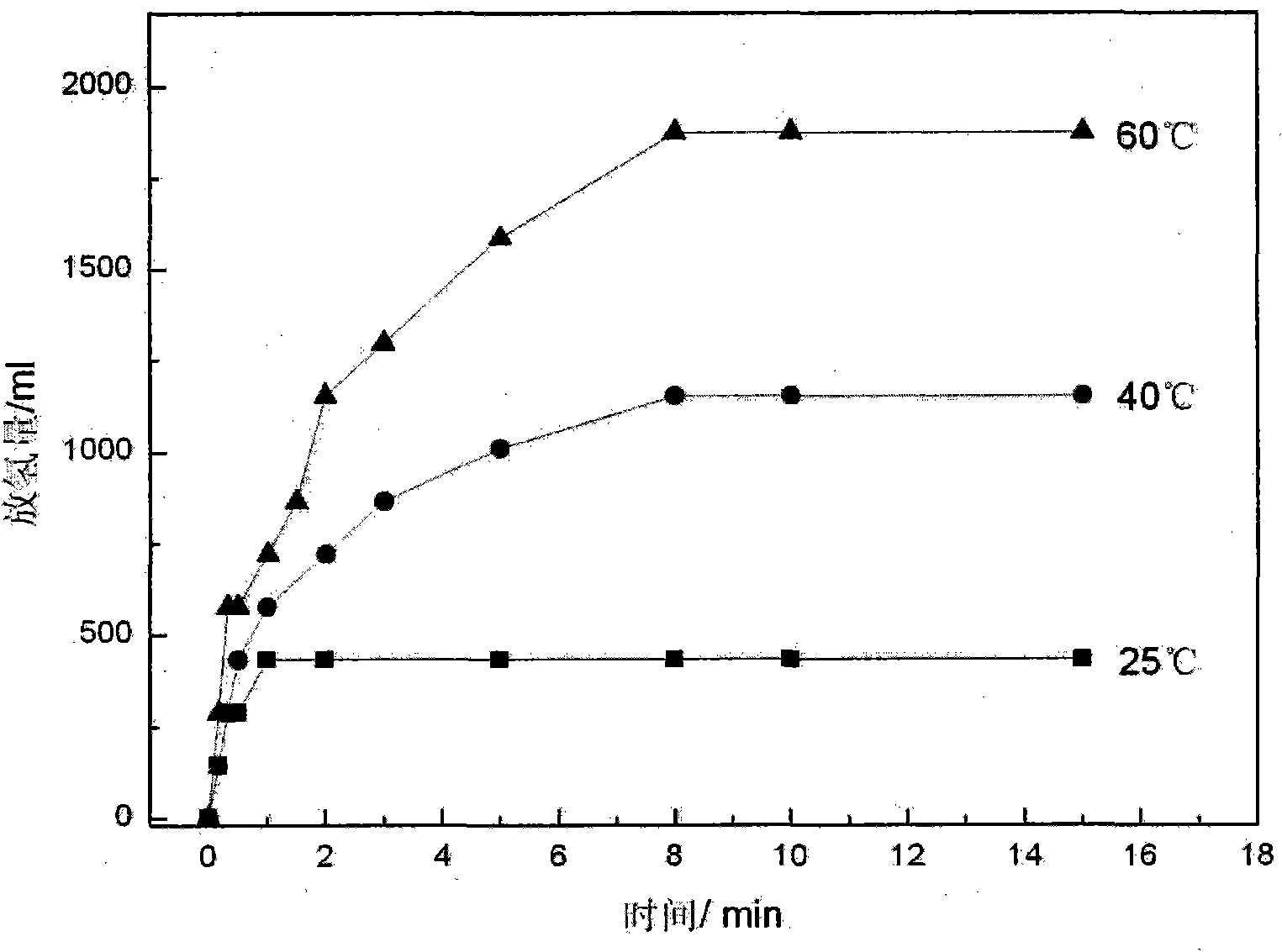
[0028] The hydrolysis hydrogen release performance of the material was tested by the drainage gas collection method. image 3 2NaCl / NH 3 BH 3 NH of the complex at different temperatures 3 BH 3 Hydrogen release performance curve. NH within 10 minutes at 60°C 3 BH 3 The generated hydrogen is about 1873.2ml / g, about 16.8wt% (mass excluding water and NaCl), and the hydrogen production rate is nearly 90%, which is significantly higher than that of pure AB under the same conditions (31.7%).
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com