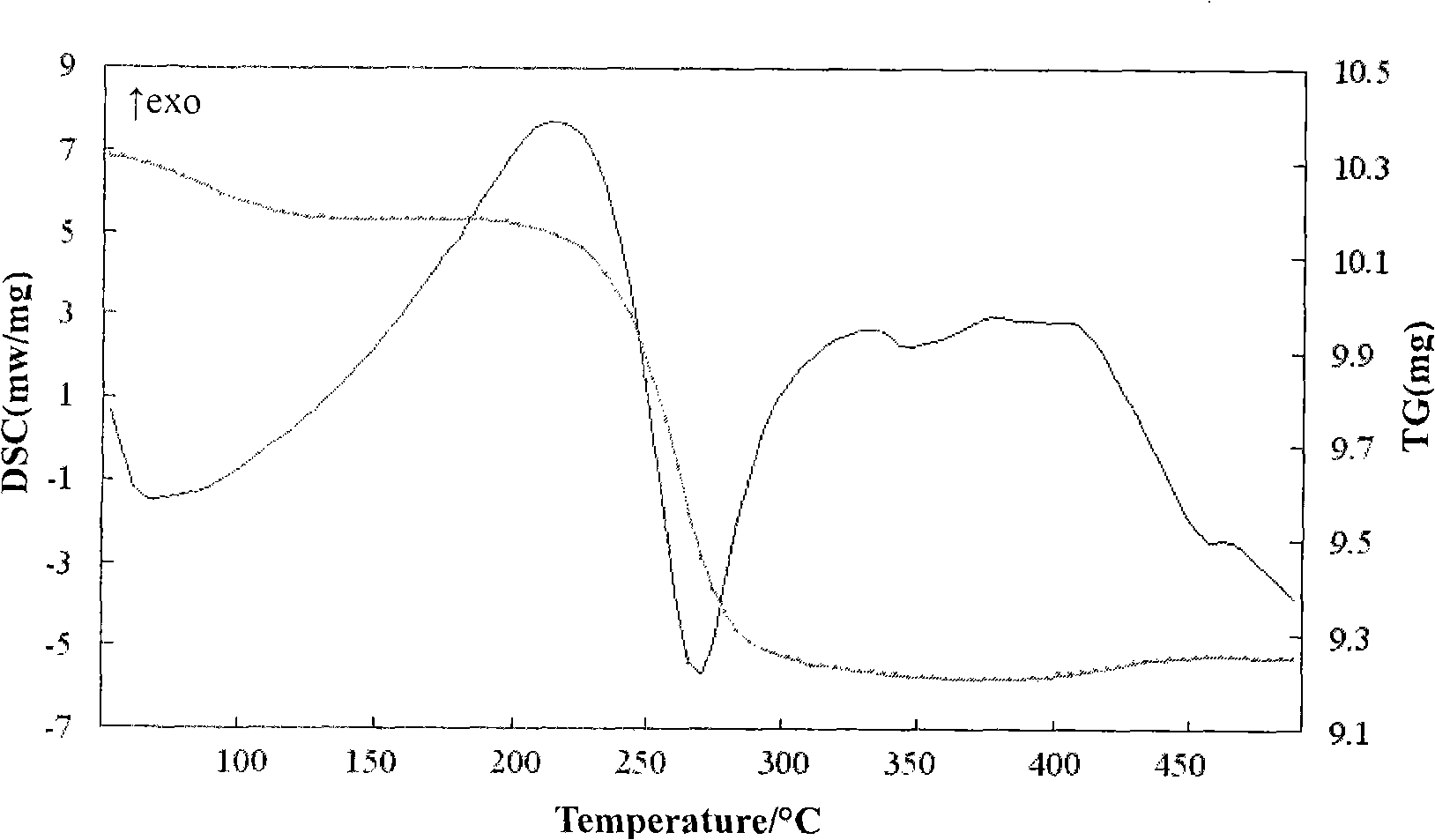
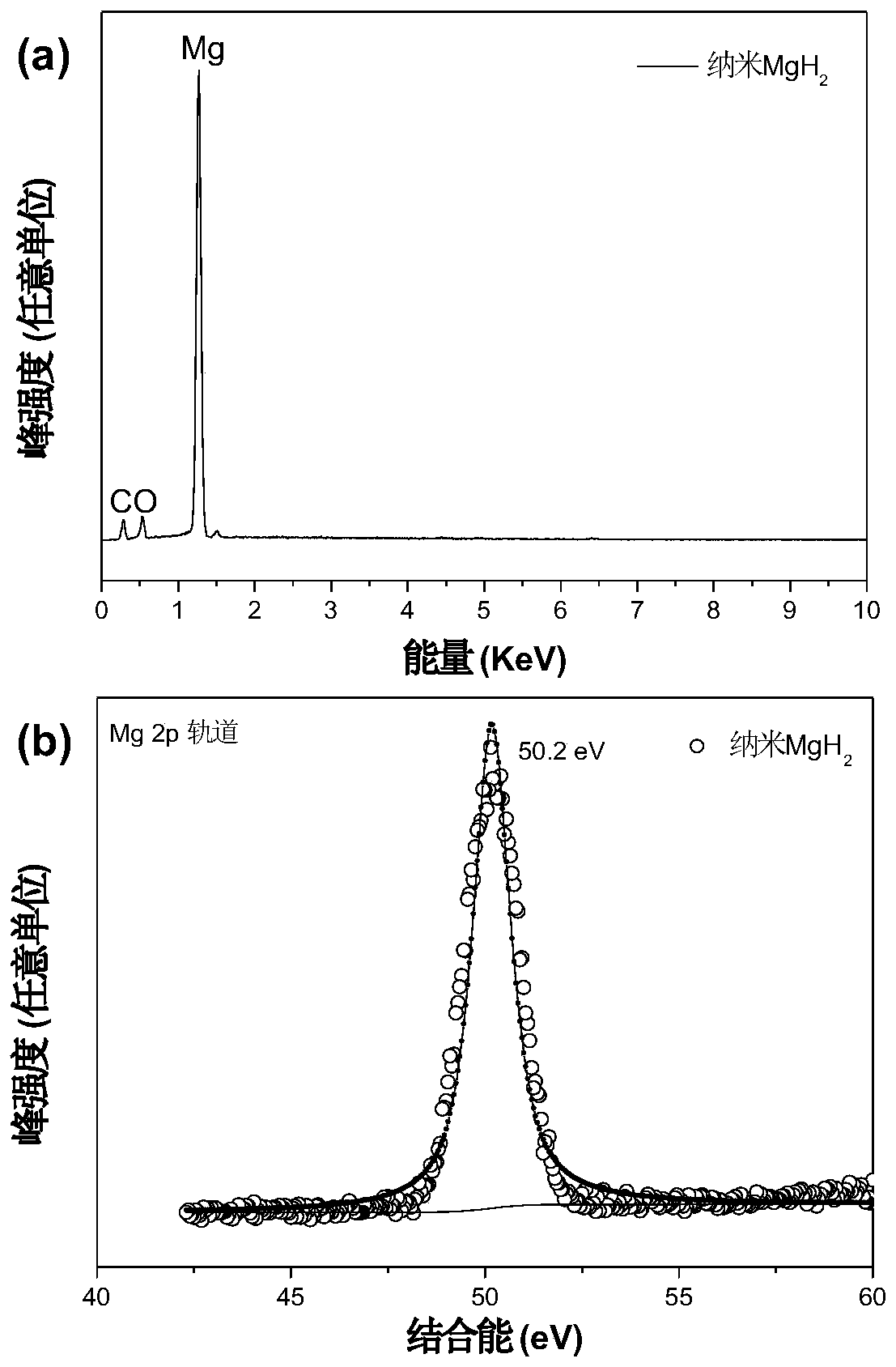
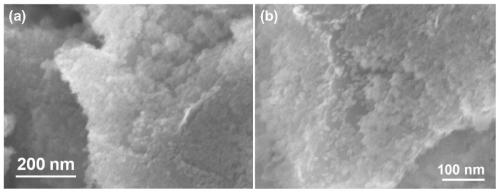
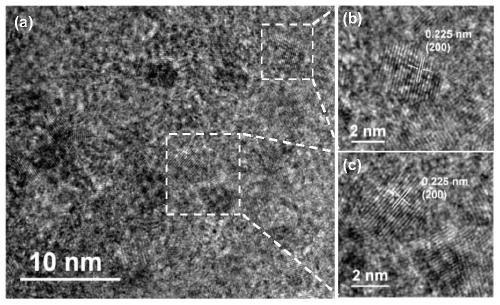
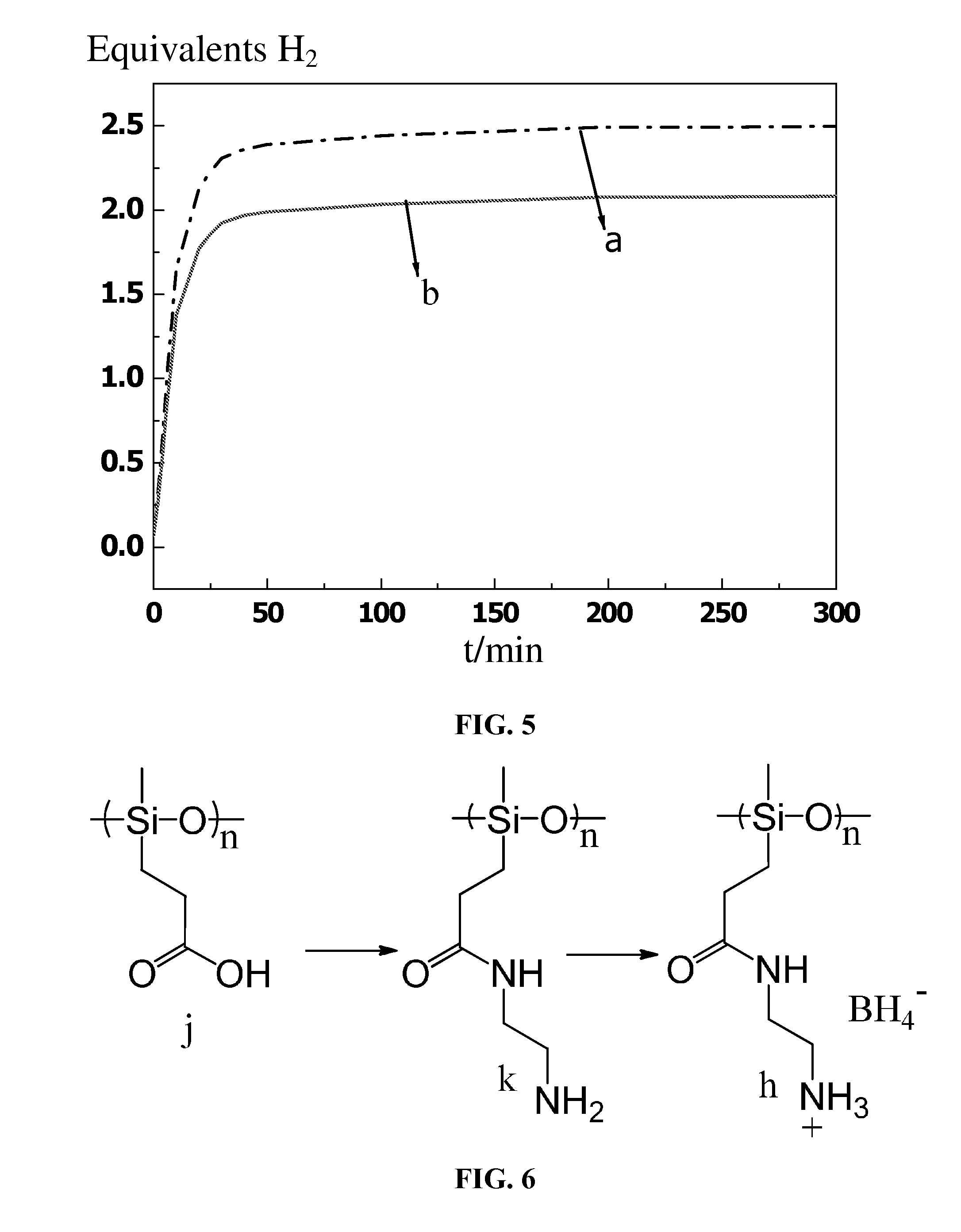
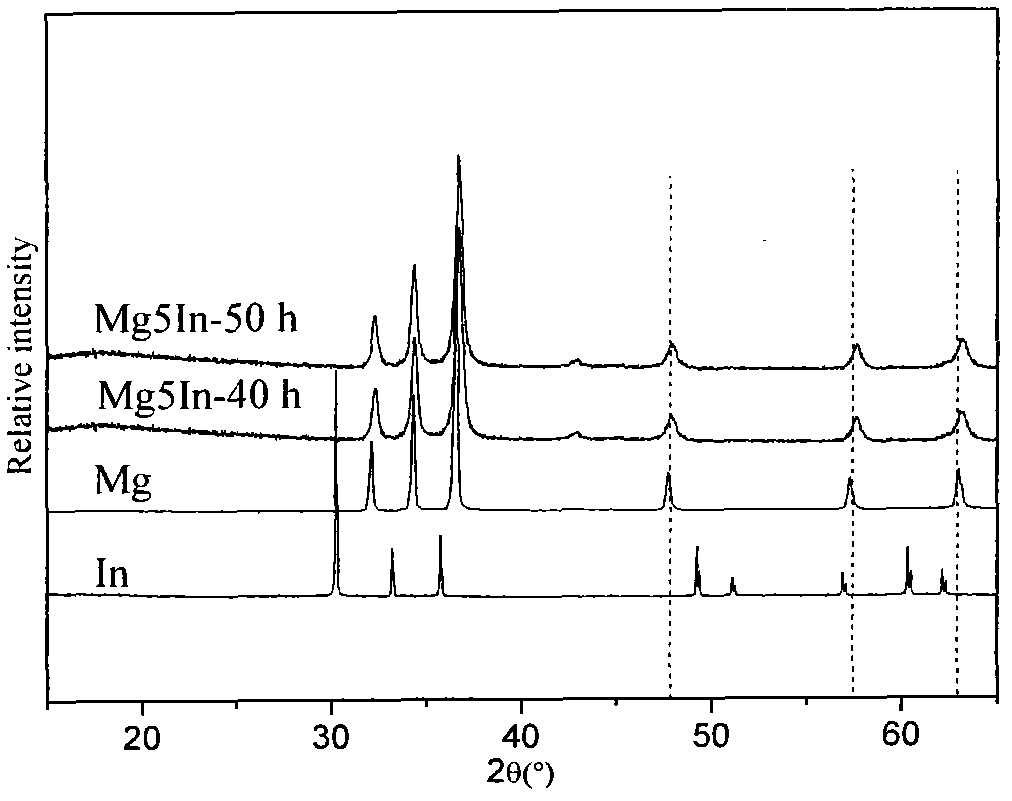
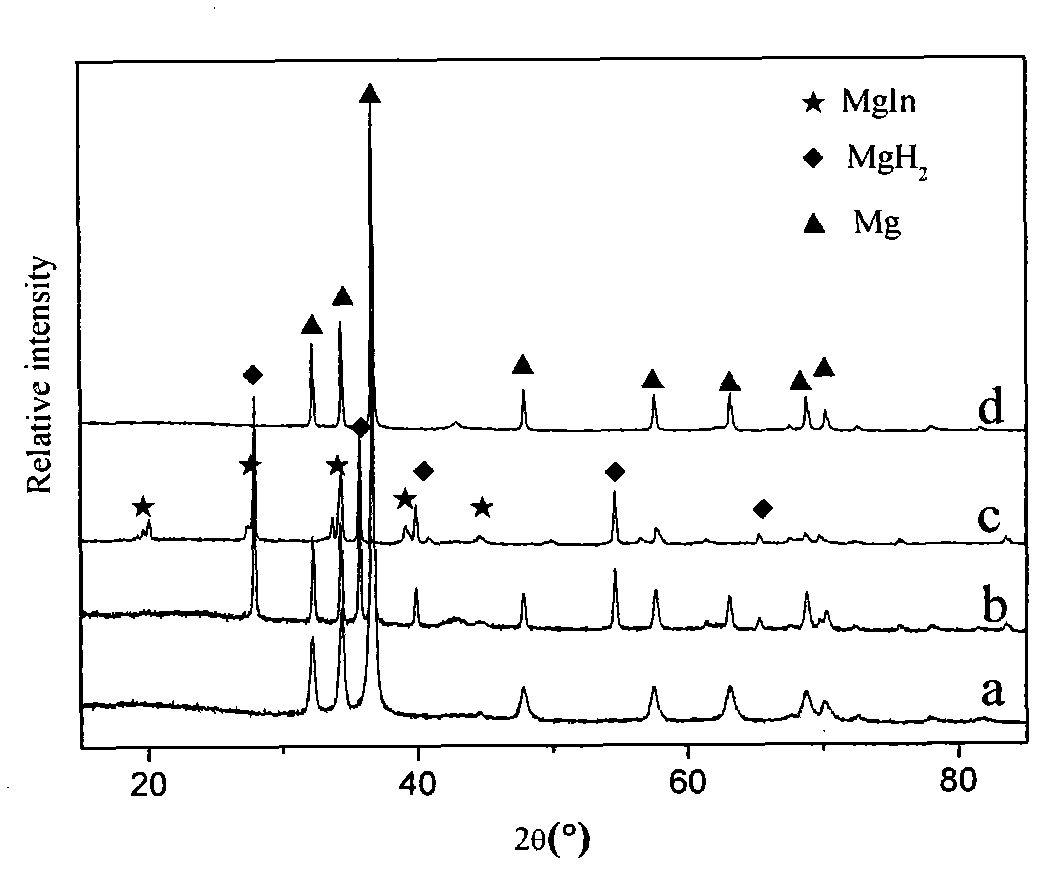
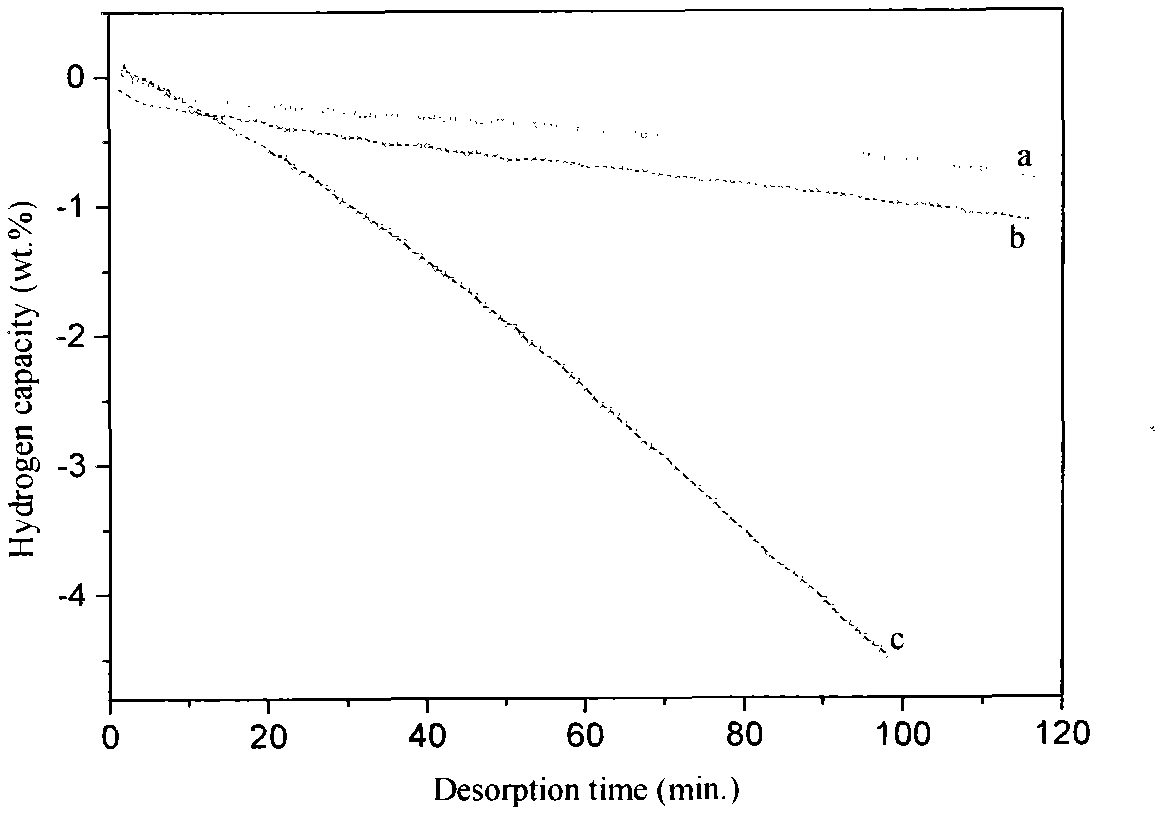
Patents
Literature
89results about How to "Low hydrogen release temperature" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
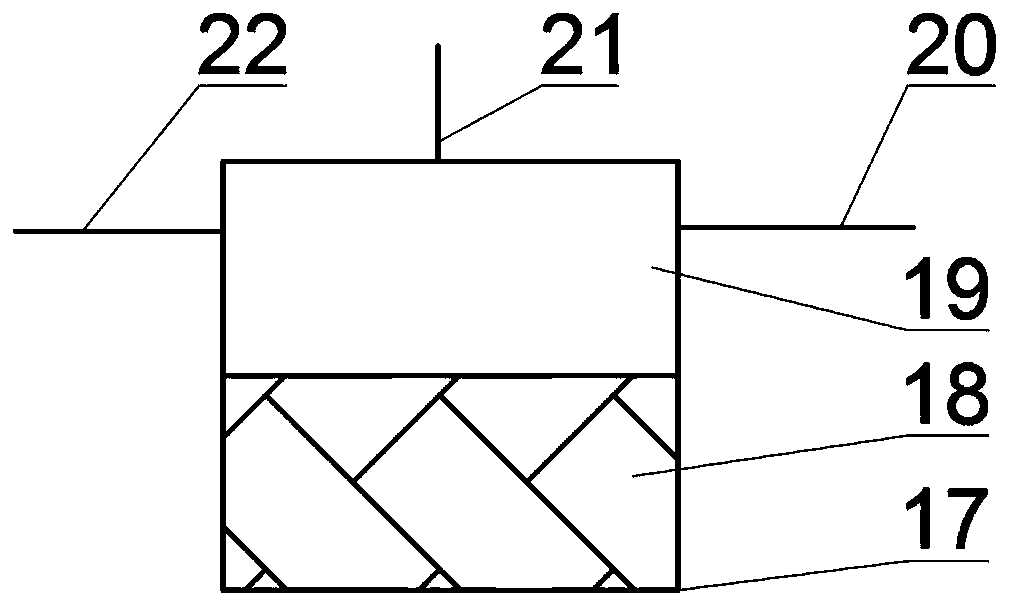
Hydrogen storage system for fuel cell vehicle
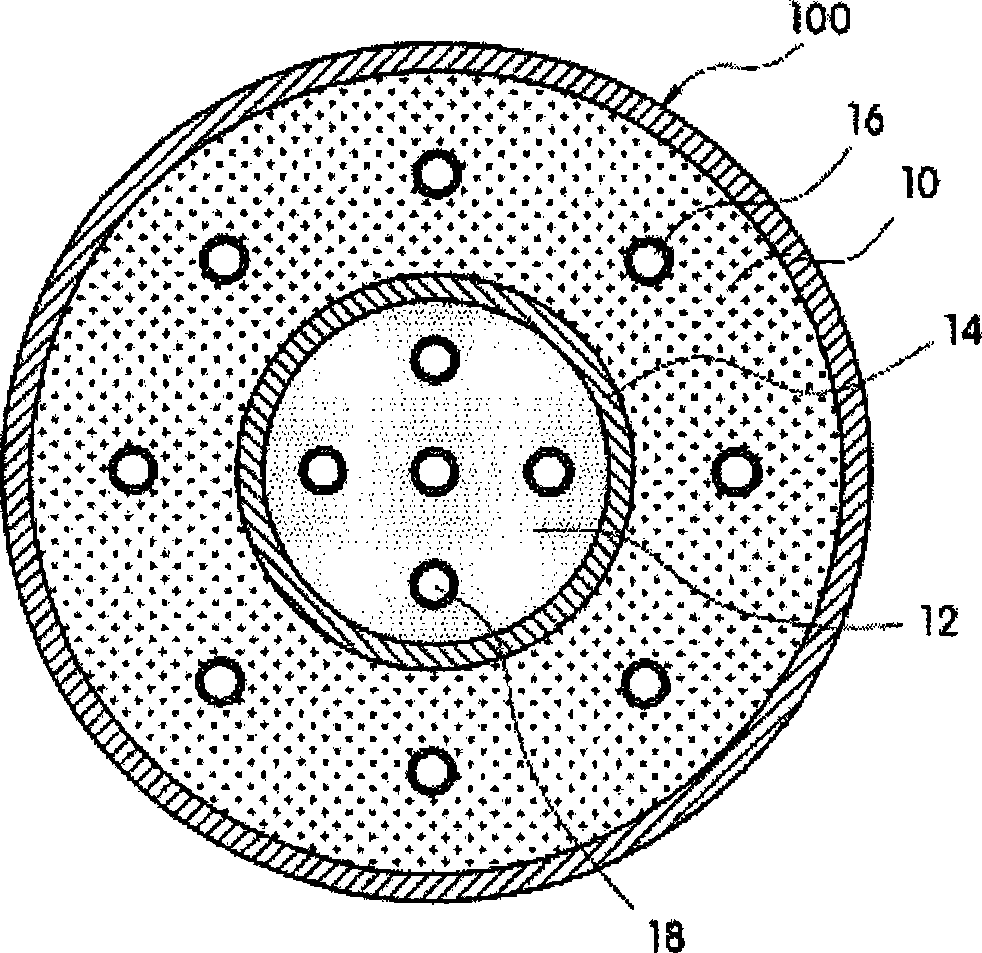
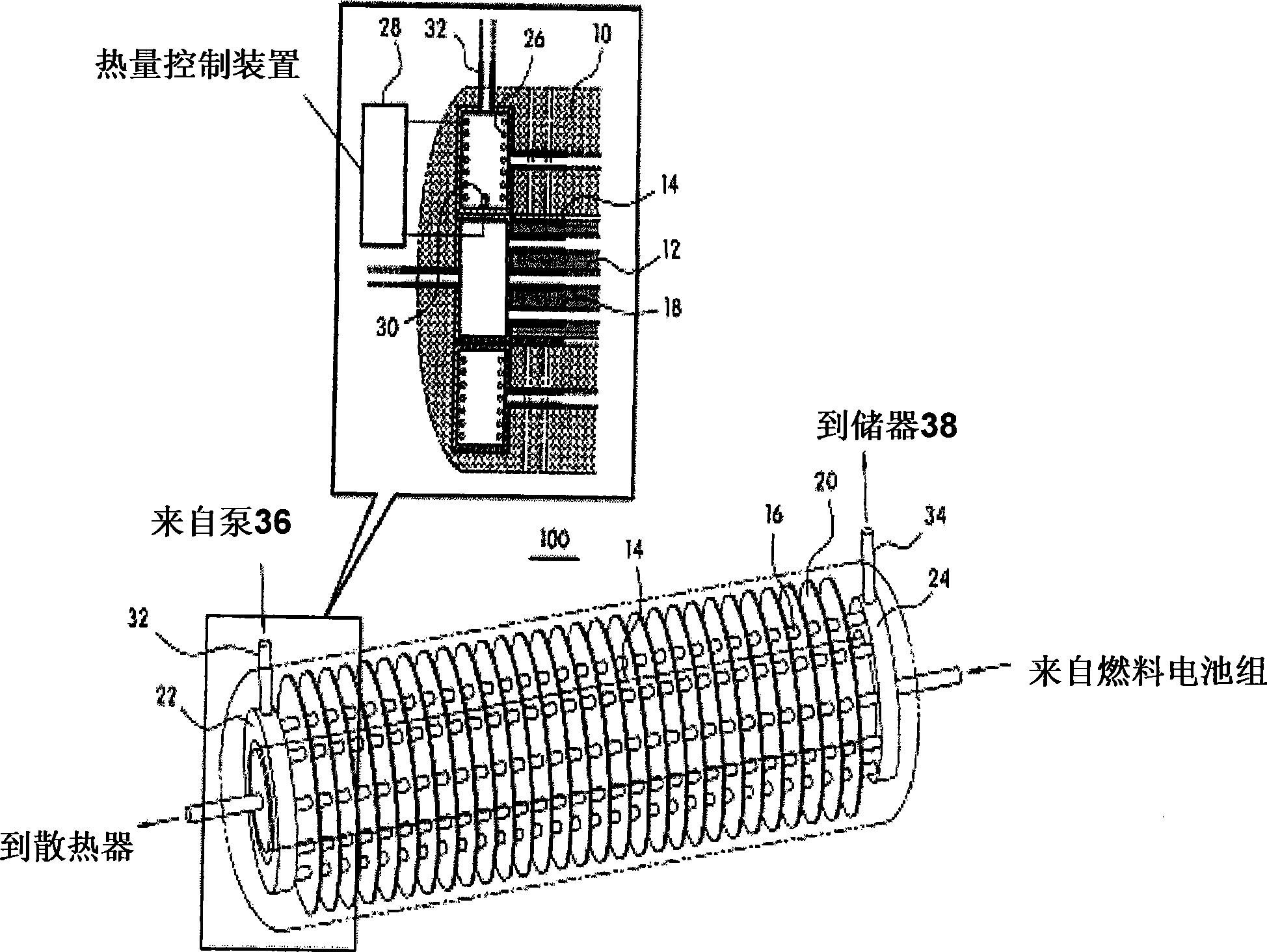
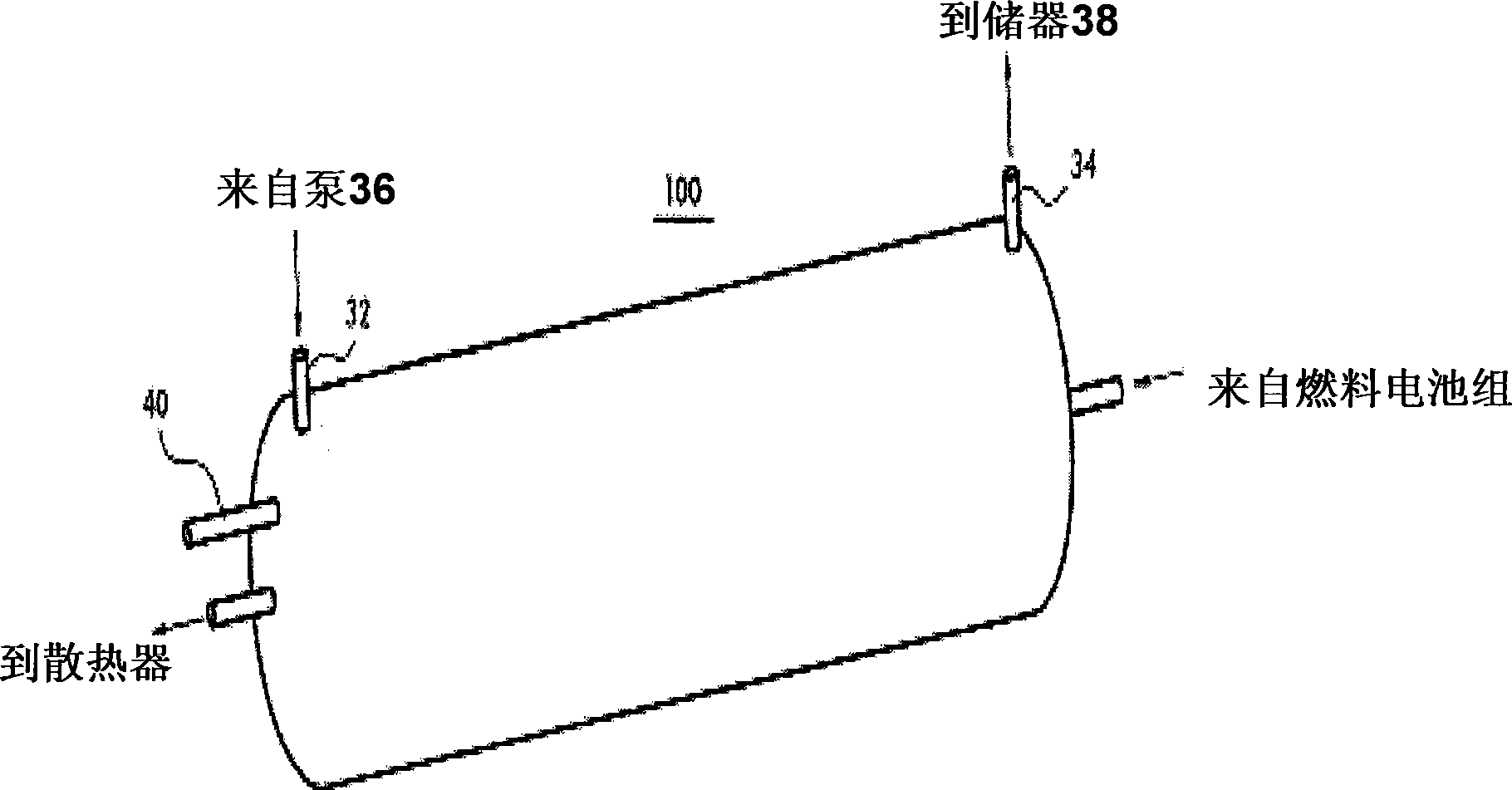
InactiveCN101459249AHigh hydrogen densityLow hydrogen release temperatureFuel cell auxillariesFixed capacity gas holdersInterior spaceFuel cells
The present invention provides a hydrogen storage system using a metal hydride (MH), which can increase volumetric storage density of hydrogen and total hydrogen storage capacity and improve system packaging. For this purpose, the present invention provides a hydrogen storage system for a fuel cell vehicle, the hydrogen storage system including: an outer space filled with a first storage alloy powder that is able to release hydrogen at a high temperature; an inner space filled with a second storage alloy powder that is able to release hydrogen only with heat generated from a fuel cell stack; a metal filter disposed between the outer and inner spaces so as to divide the outer and inner spaces; a second heat exchange tube provided between the fuel cell stack and a radiator to constitute a cooling loop and arranged along a longitudinal direction of the inner space; and an independent heat exchange loop independently connected to the outer space for the hydrogen release of the first storage alloy powder.
Owner:HYUNDAI MOTOR CO LTD +1
Lithium borohydride hydrogen storage material decorated by oxide and preparation method thereof
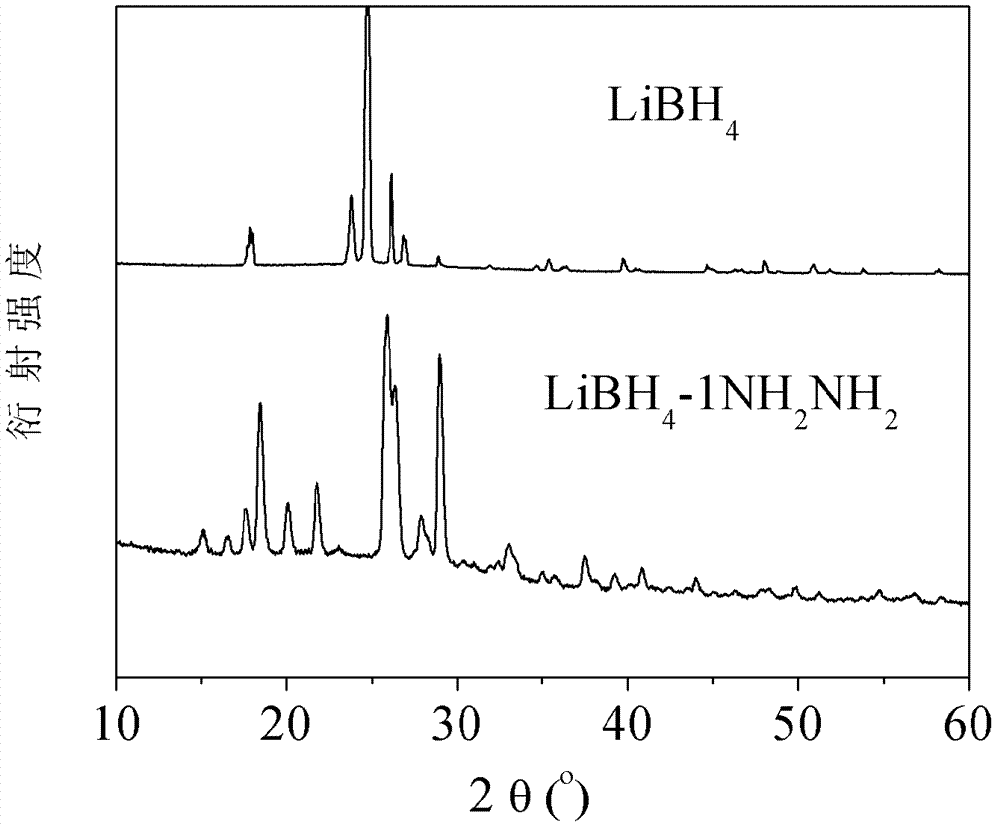
InactiveCN101054162ASimple methodLow hydrogen release temperatureOther chemical processesMonoborane/diborane hydridesBall millAtmosphere
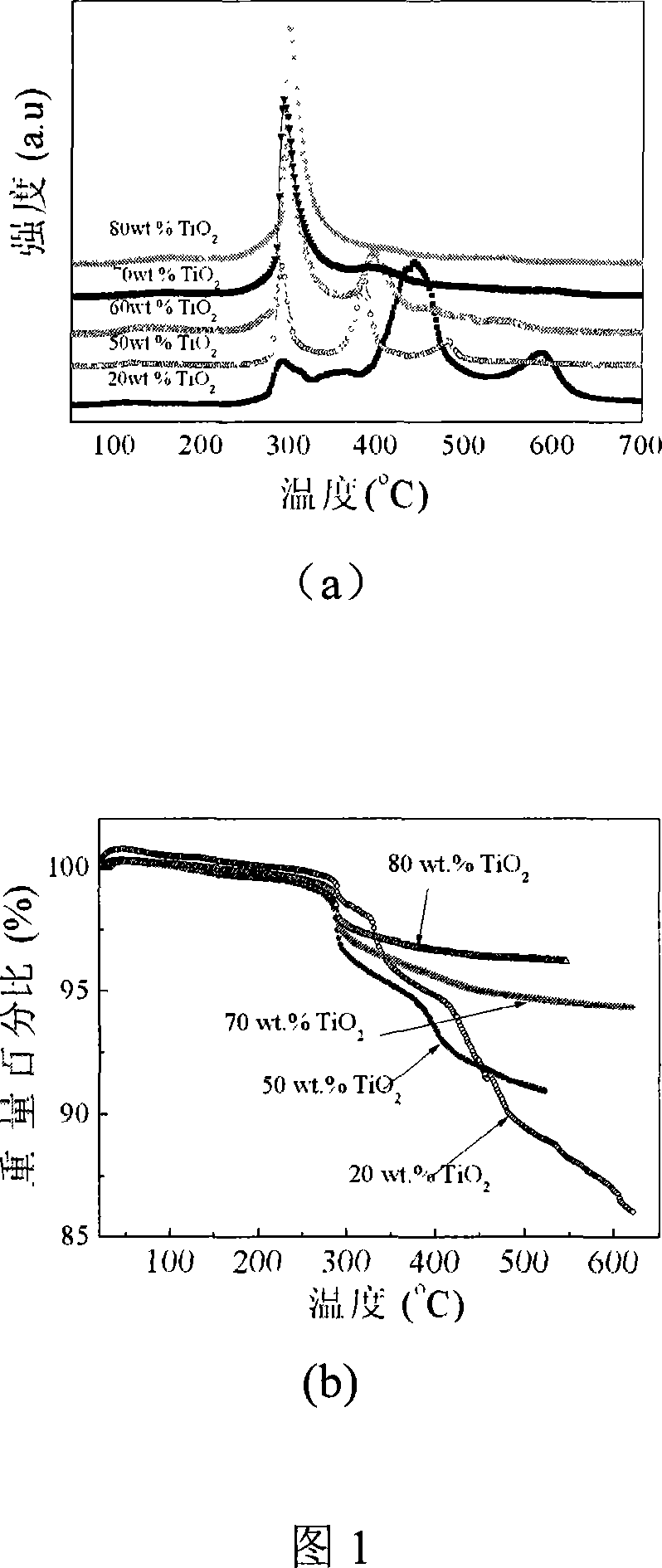
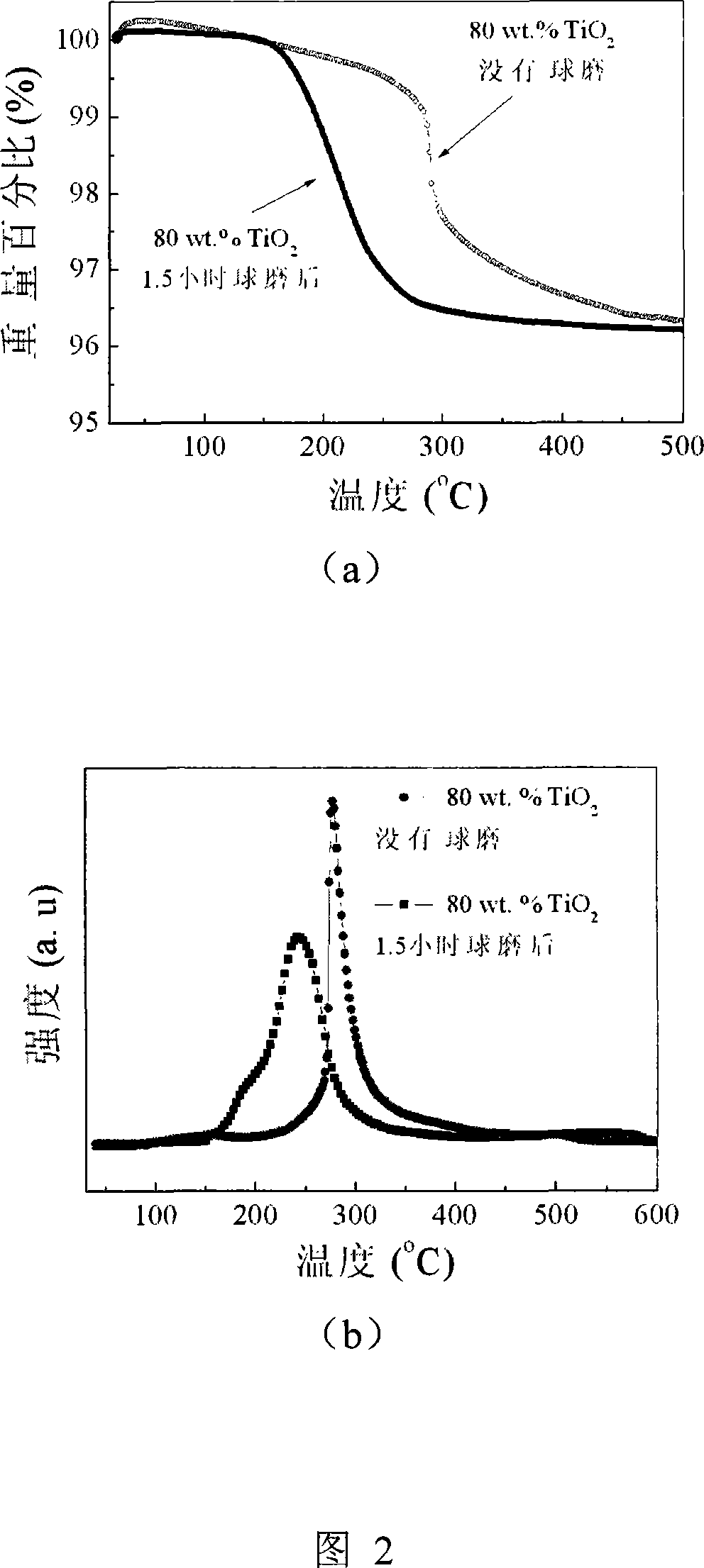
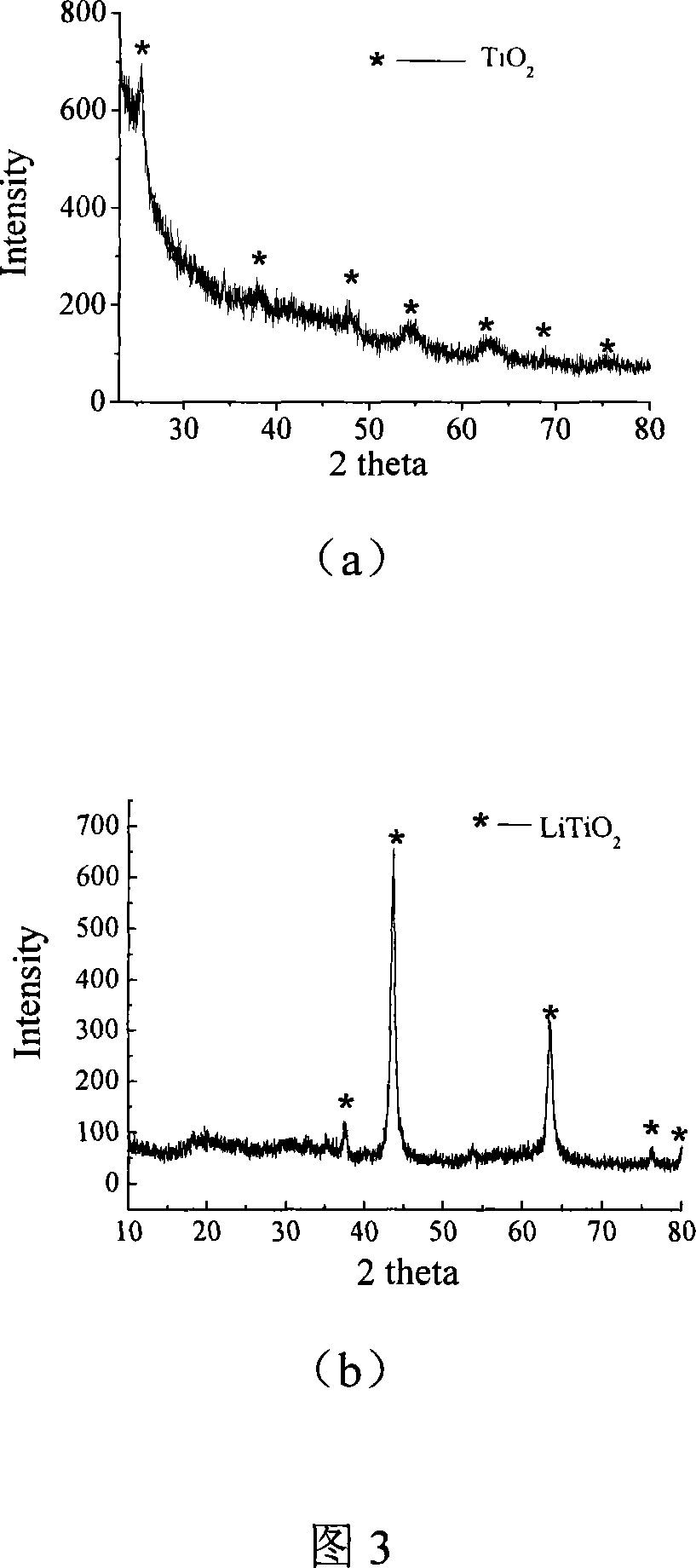
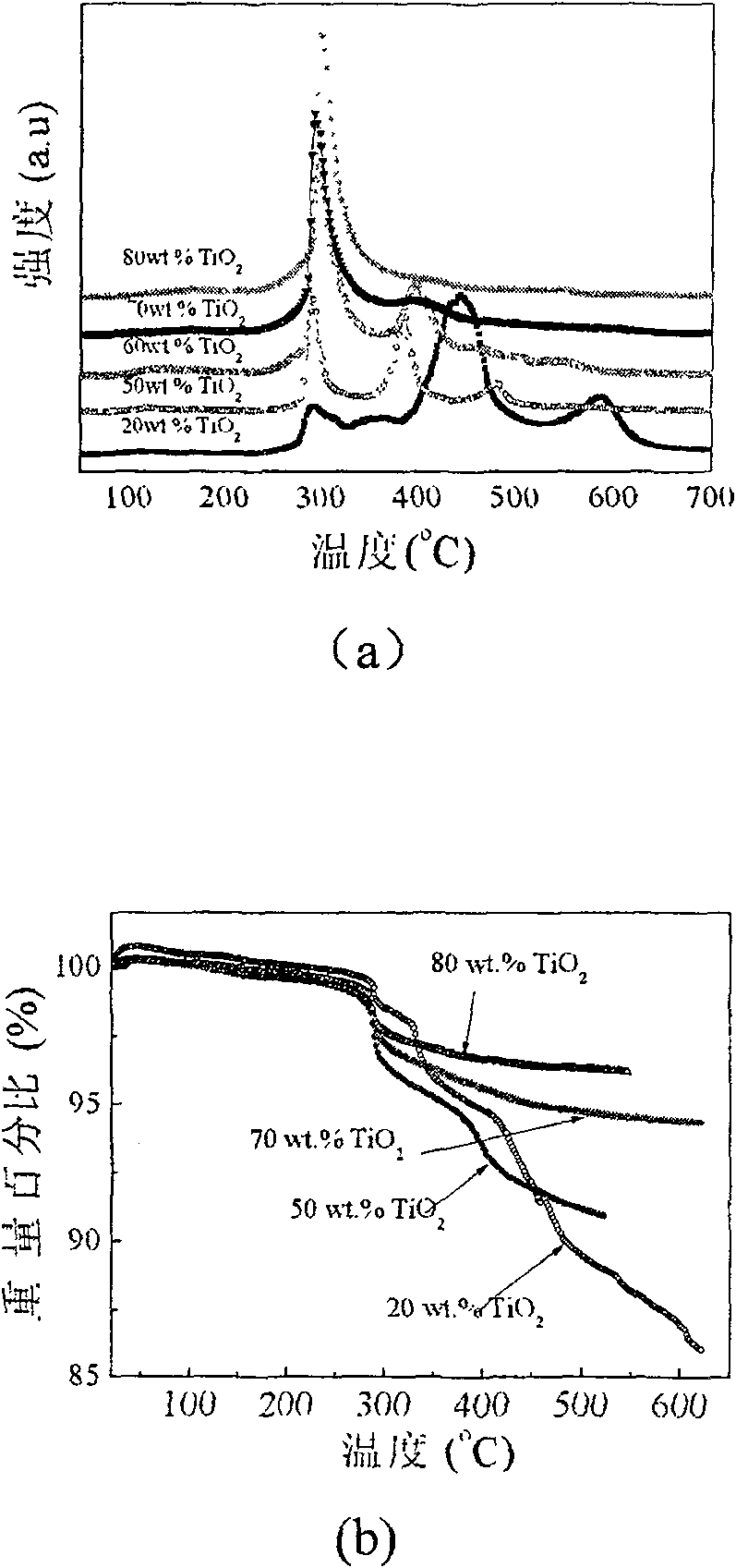
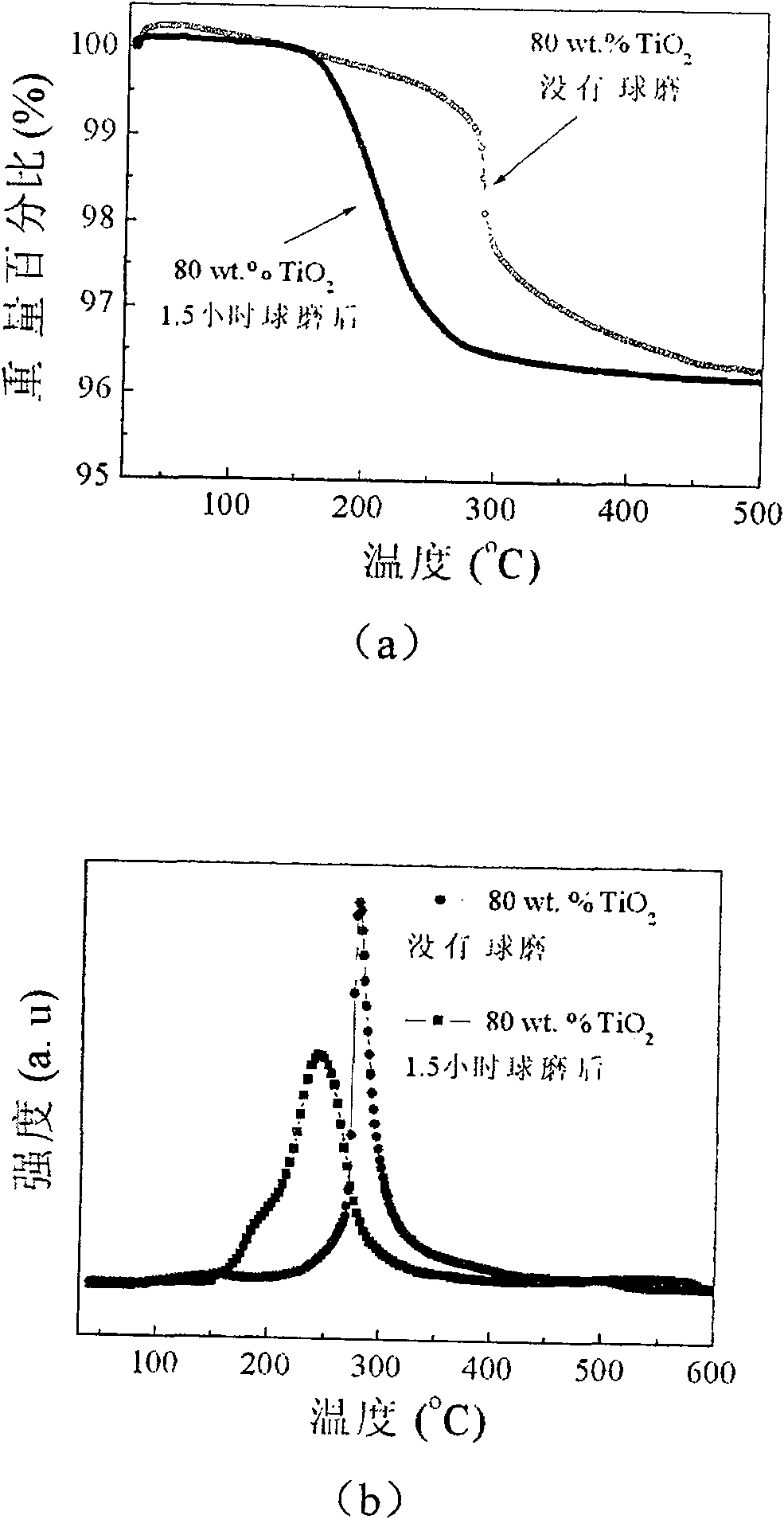
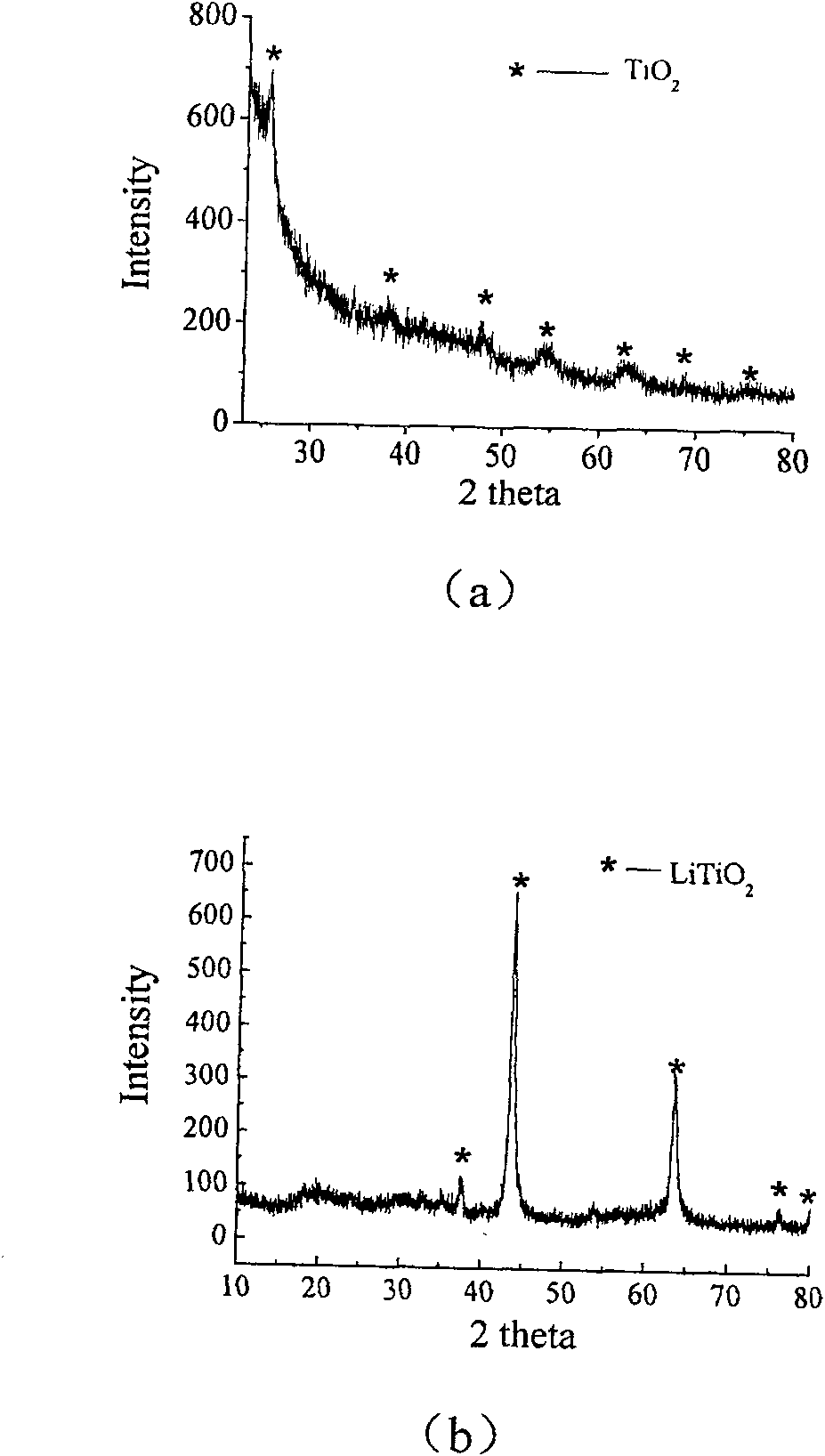
The invention relates to liborohydride hydrogen storage material and a preparation method thereof, which is characterized in that the formula of hydrogen storage material is (100-x)LiBH4+XMeO, wherein MeO is a adorning oxide, weight percent of X is 10-80%. After blending LiBH4 and the oxide according to the formula, the mixture is ball milled in protection of inert atmosphere to process surface treatment. The oxide is optional one of TiO2, Fe2O3, ZrO2, V2O5, SiO2, Al2O3, Al2O3-SiO2 or TiO2-SiO2. The intial hydrogen temperature of hydrogen storage material of the invention is lower than 100 DEG C, hydrogen amount is 3-6.5% at lower than 300 DEG C.
Owner:SHANGHAI INST OF MICROSYSTEM & INFORMATION TECH CHINESE ACAD OF SCI
Metal ammonia borane compound hydrogen storage material and preparation method thereof
InactiveCN101613083AImproved hydrogen release performanceStrong donating abilityHydrogen productionMetal hydridesHydrogen atmosphereImpurity
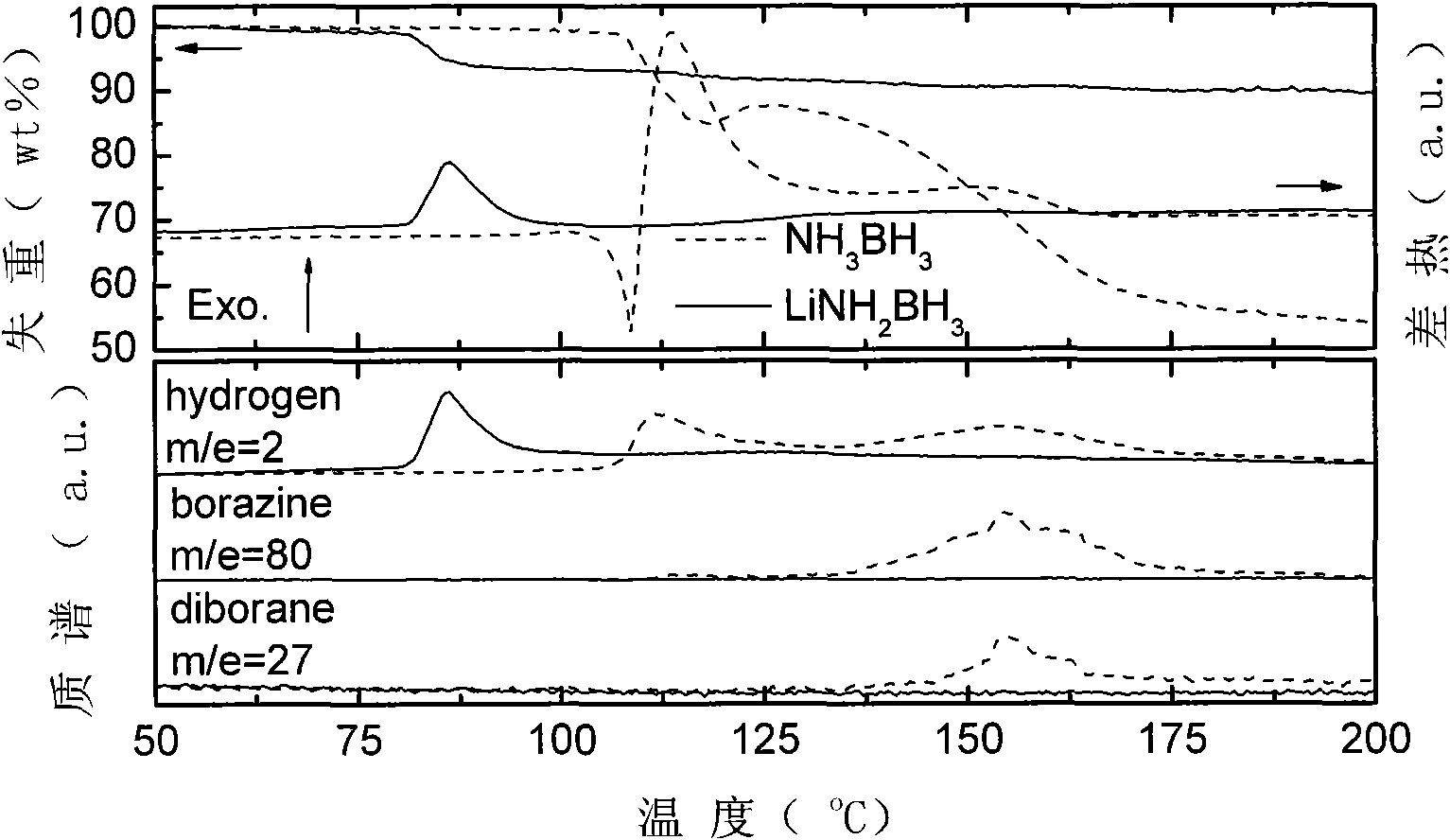
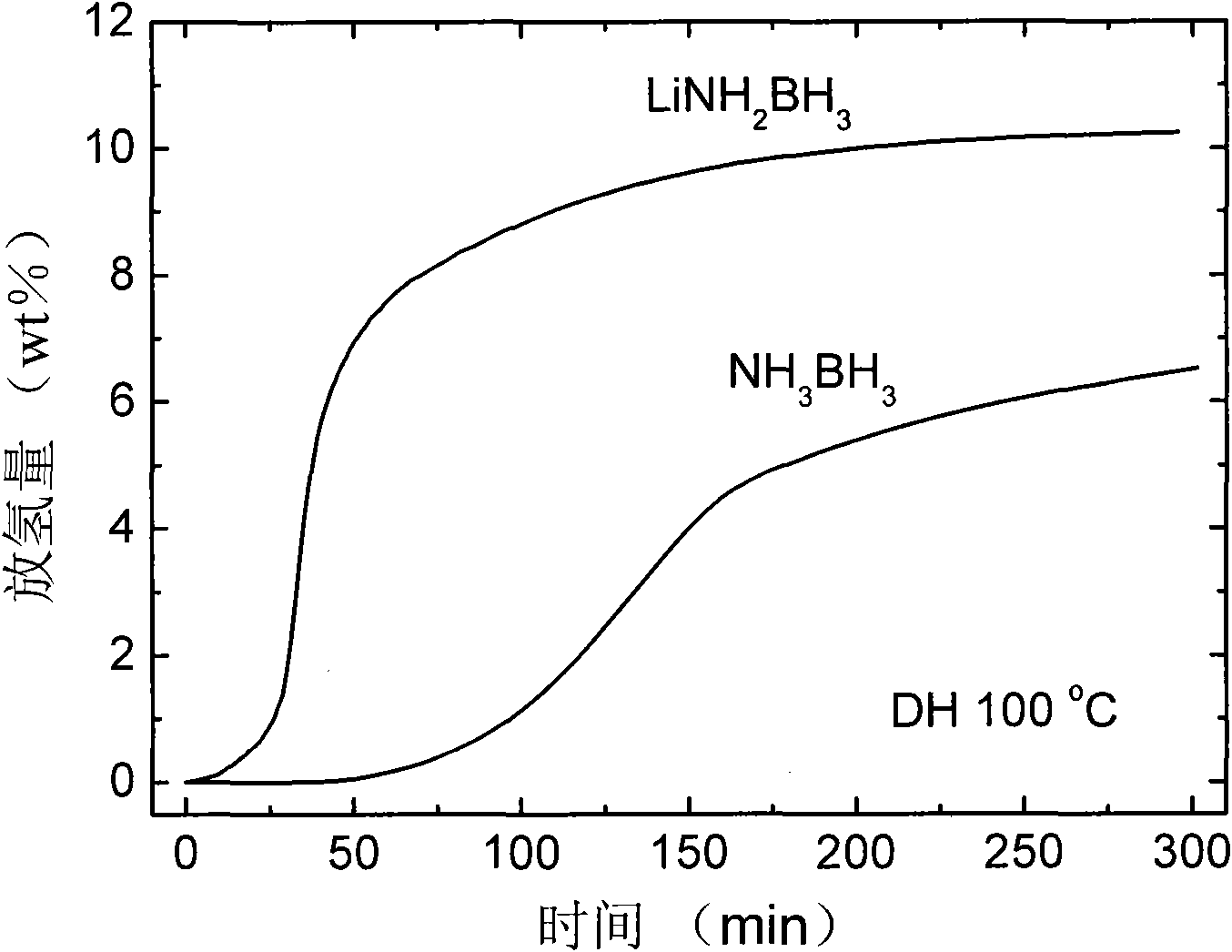
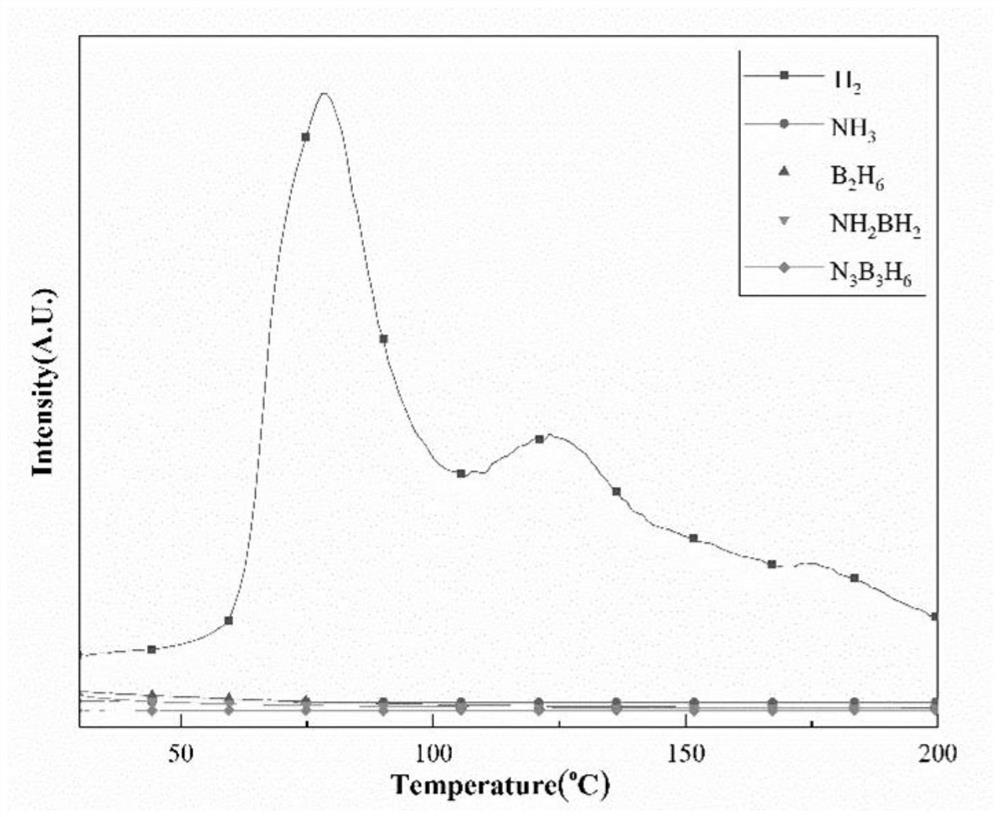
The invention relates to a material and technology for storing hydrogen, in particular to a novel metal ammonia borane compound hydrogen storage material and a preparation method thereof. The novel metal ammonia borane compound hydrogen storage material is prepared by taking the mixture of ammonia borane NH3BH3 and metal M or metal hydride NHy as an initial raw material and performing ball milling on the raw material in an inert protective atmosphere or a reactive hydrogen atmosphere. The molecular formula of the novel metal ammonia borane compound hydrogen storage material is MxNH(3-nx)BH3, wherein x is more than 0 and less than or equal to 1, and n is more than or equal to 1 and less than or equal to 3. The mole ratio of NH3BH3 to M or NHy in the phase composition of the raw material is 1-50:1. The preparation method provided by the invention has high efficiency and simple and easy operation. The novel metal ammonia borane compound hydrogen storage material provided by the invention has the advantages of high hydrogen storage capacity, low hydrogen production temperature, fast hydrogen production dynamics, no impurities, no gaseous pollutants and the like and has the application prospective of automobile-mounted hydrogen storage.
Owner:INST OF METAL RESEARCH - CHINESE ACAD OF SCI
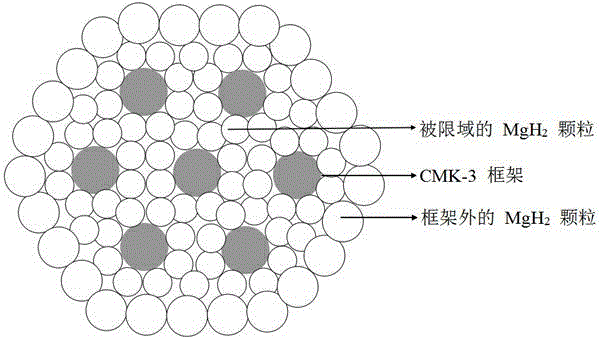
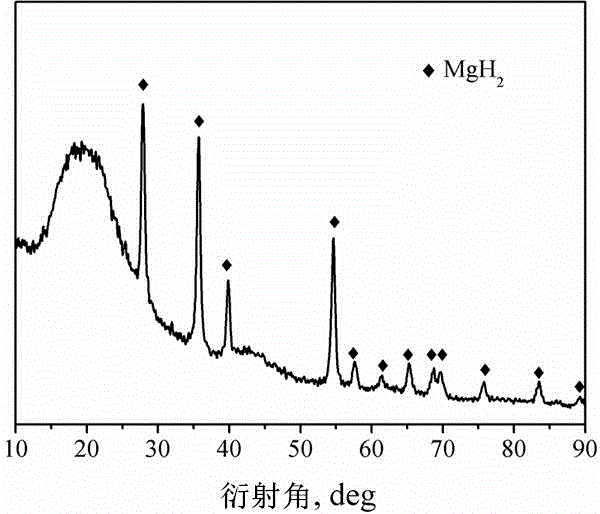
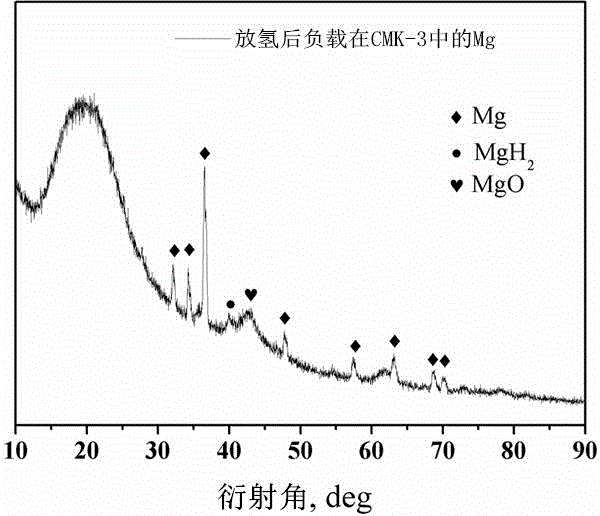
Method for preparing nanometer limited range magnesium-based hydrogen storage material
InactiveCN104649229AImprove mechanical propertiesLow costAlkali/alkaline-earth/beryllium/magnesium hydridesDispersityNew energy
The invention discloses a method for preparing a nanometer limited range magnesium-based hydrogen storage material. The nanometer limited range method belongs to the technical field of new energy materials. The method is characterized in that the hydrogen storage material is formed by loading magnesium hydride (MgH2) in nanopores of a mesoporous framework material. The method comprises the following steps: dipping dibutyl magnesium (MgBu2) and a mesoporous framework material, replacing MgBu2 with MgH2 loaded inside and outside the nanopores of the mesoporous framework material in a high-pressure reactor under high temperature and high pressure, washing MgH2 loaded outside the pores away by using pentane, drying, thereby obtaining the material. The nanometer limited range magnesium-based hydrogen storage material prepared by the method can release hydrogen at room temperature and has excellent properties in hydrogen absorption and desorption kinetics and hydrogen desorption thermodynamics. The method disclosed by the invention is easy to operate and high in synthesis speed and the prepared material is high in dispersity; therefore, the method has ideal application prospects.
Owner:SHANGHAI UNIV
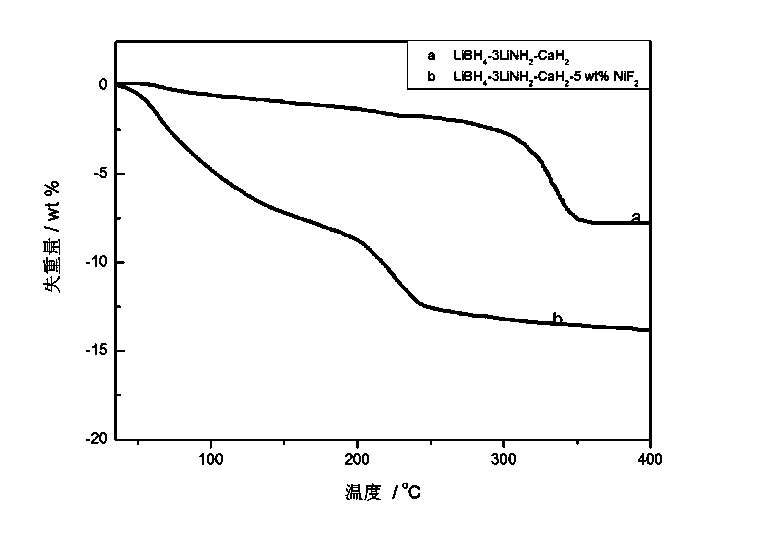
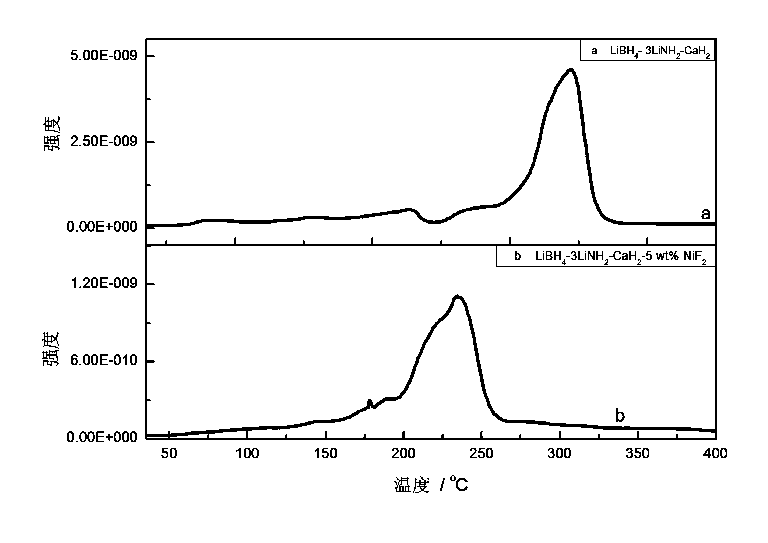
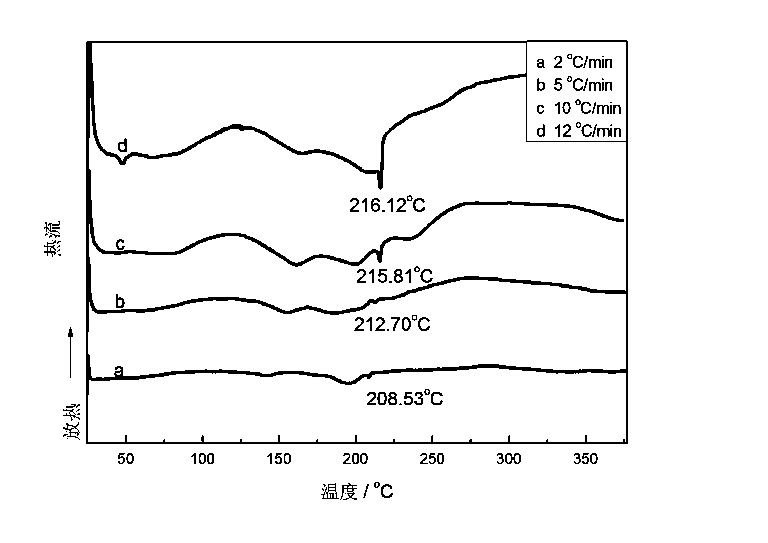
NiF2-dopped LiBH4-LiNH2-CaH2 composite hydrogen storage material and preparation method thereof
InactiveCN103539066AImprove hydrogen absorption and desorption performanceLow hydrogen release temperatureHydrogen productionHydrogen desorptionChemistry
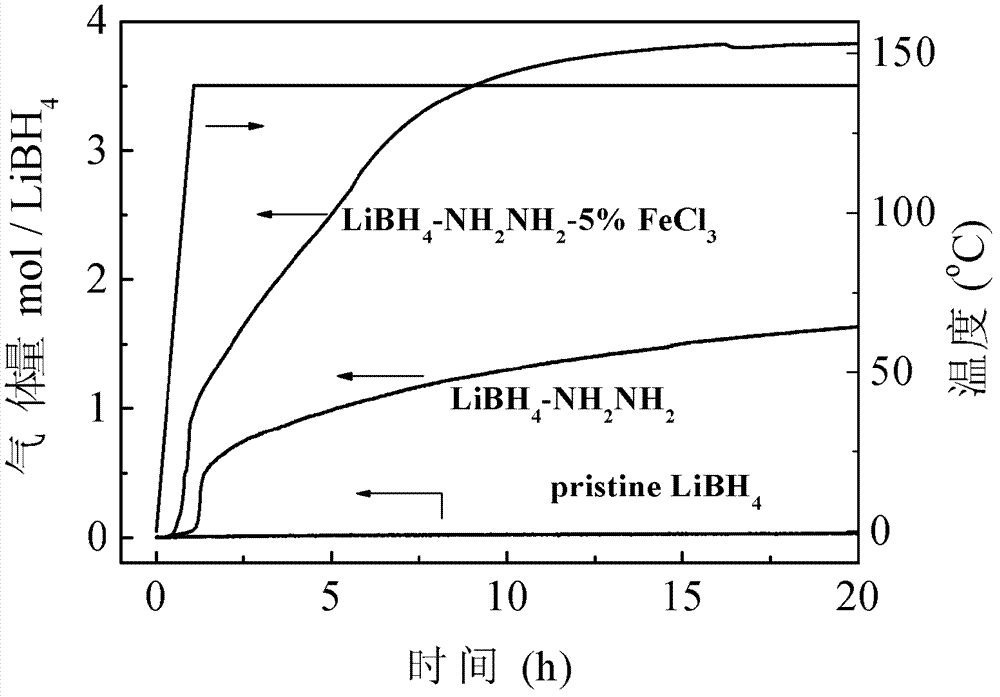
The invention relates to a preparation method of an NiF2-dopped LiBH4-LiNH2-CaH2 composite hydrogen storage material, in particular relates to a hydrogen storage material which improves the hydrogen desorption performance of an LiBH4-LiNH2-CaH2 system through doping NiF2 and a preparation method thereof, and belongs to the technical field of modification. The composite material is prepared by utilizing a mechanical milling method, when the doping amount of NiF2 is 5wt%, the system starts greatly desorbing hydrogen at 47 DEG C, the main hydrogen desorption peak temperature is 234 DEG C, and the hydrogen desorption amount reaches 3.75wt% at 175 DEG C within 5000s; the hydrogen desorption amount reaches 5.03wt% within 5h; the hydrogen desorption amount reaches 6wt% at 200 DEG C within 5000s; the hydrogen desorption amount reaches 6.55wt% at 270 DEG C within 1000s. The composite hydrogen storage material which is prepared by ball milling has good hydrogen storage and desorption performances, and the prepared NiF2-dopped LiBH4-LiNH2-CaH2 composite hydrogen storage material has good hydrogen desorption performance at low temperatures.
Owner:DALIAN INST OF CHEM PHYSICS CHINESE ACAD OF SCI
Method for improving hydrogen storage property of lithium borohydride
InactiveCN102502488AImprove hydrogen storage performanceImproved hydrogen releaseHydrogen productionAlkaline earth metalHydrogen fuel cell
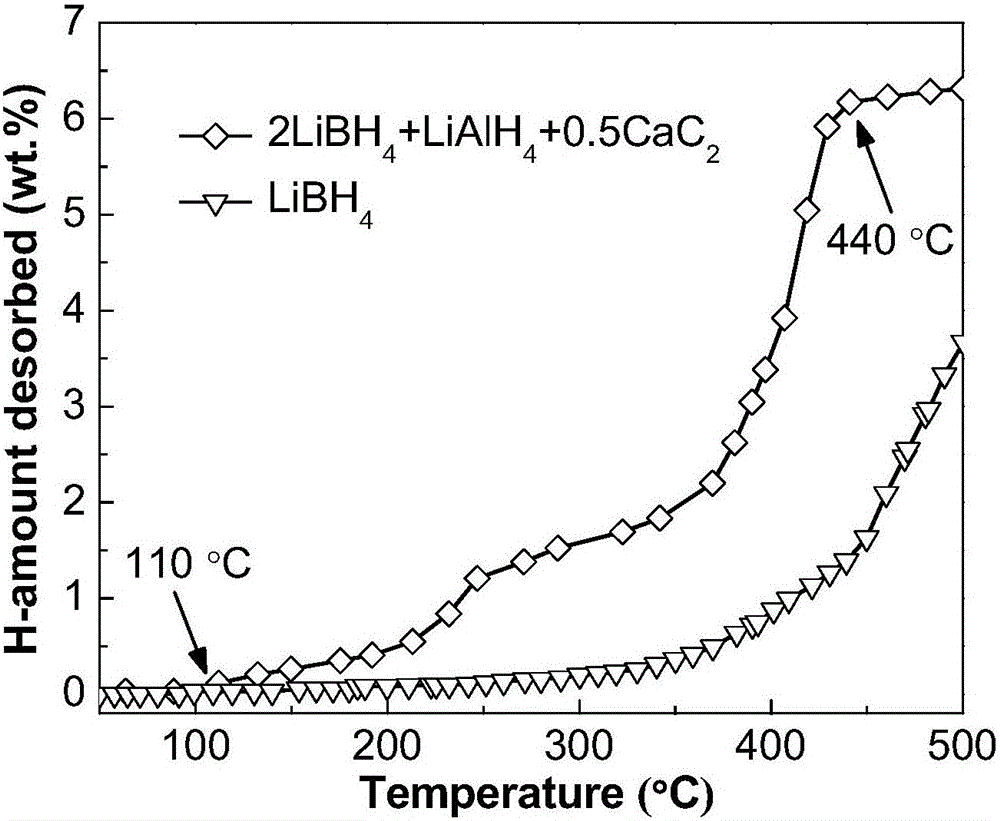
The invention provides a method for improving a hydrogen storage property of lithium borohydride, and belongs to the technical field of hydrogen storage materials. The method comprises the following steps of: mixing the lithium borohydride and alkaline-earth metal alanate according to the molar ratio of 2:1-10:1 under the protection of vacuum or inert gases, and heating mixed powder of the lithium borohydride and the alkaline-earth metal alanate to a certain temperature to ensure that the alkaline-earth metal alanate is decomposed into alkaline-earth metal hydride, aluminum or aluminum alloy in advance. By the method, double effects of in situ and concerted catalysis in the process of discharging and absorbing hydrogen of the lithium borohydride by using the alkaline-earth metal alanate are achieved, so that a hydrogen discharging temperature of the lithium borohydride is greatly reduced, and the hydrogen absorbing and discharging dynamic properties of the lithium borohydride are improved. The method is suitable for storing the hydrogen safely and efficiently and is particularly applied in fields of hydrogen fuel cells and the like.
Owner:ANHUI UNIVERSITY OF TECHNOLOGY
Multi-metal ammonia borane compound hydrogen storage material and preparation and composite hydrogen release method thereof
InactiveCN102530872AIncrease energy densityAdjustable ionic propertiesMonoborane/diborane hydridesHydrogen productionHydrogen atmosphereHydrogen storage system
The invention relates to the field of hydrogen storage material and hydrogen production, in particular to a multi-level ammonia borane compound hydrogen storage material and preparation and composite hydrogen release method thereof. The mixture of ammonia borane NH3BH3 and multi-metal hydride M1Mm2nHx is used as the starting material, and the multi-level ammonia borane compound hydrogen storage material is prepared by ball milling or auxiliary heat treatment in an inertia protection atmosphere or reactive hydrogen atmosphere, wherein the molecular formula of the multi-level ammonia borane compound hydrogen storage material is M1mM2n(NH2BH3)x, wherein 0<m<=4, 0<n<=4 and 1<=x<=10; the starting material comprises the phases of NH3BH3 and M1mM2nHx at a molar ratio of (1-10):1. The multi-level ammonia borane hydrogen storage material provided by the invention has obvious advantages of relatively high hydrogen storage capacity, low hydrogen release temperature, no impurity gas pollutant and the like. The composite hydrogen release technology provided by the invention effectively integrates the synthesis reaction and decomposition reaction of the multi-level ammonia borane so that the hydrogen storage system can realize high-capacity and fast hydrogen release in a proper temperature, and has application prospect in vehicular hydrogen storage.
Owner:INST OF METAL RESEARCH - CHINESE ACAD OF SCI
Preparation method of transition metal fluoride-doped composite hydrogen storage material, and application thereof in hydrogen storage material
ActiveCN107934913ALow hydrogen release temperatureShorten the induction periodReversible hydrogen uptakeSynthesis methodsReaction rate
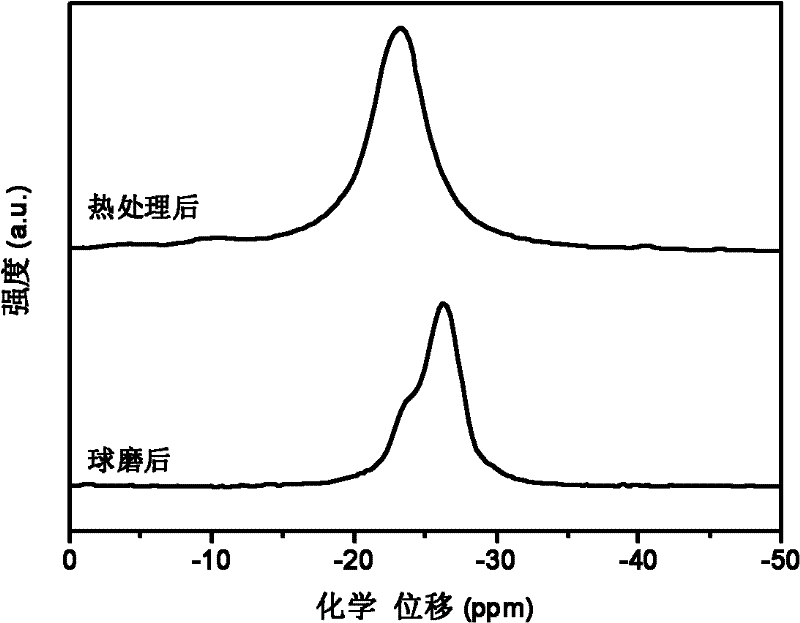
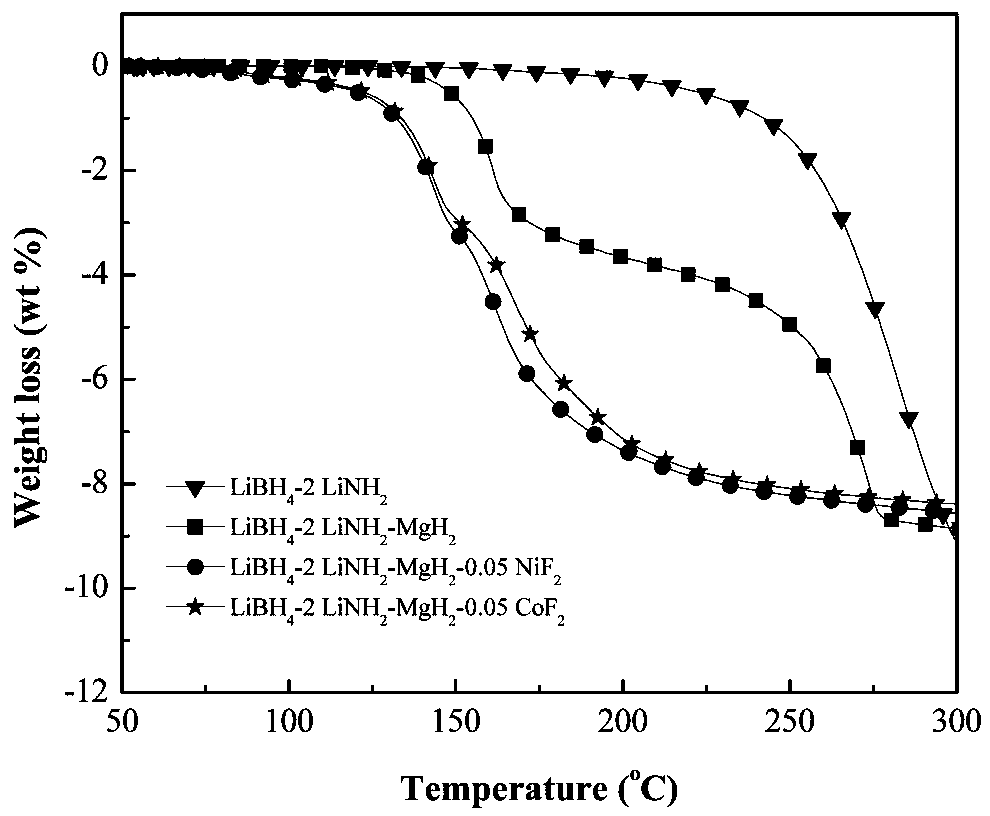
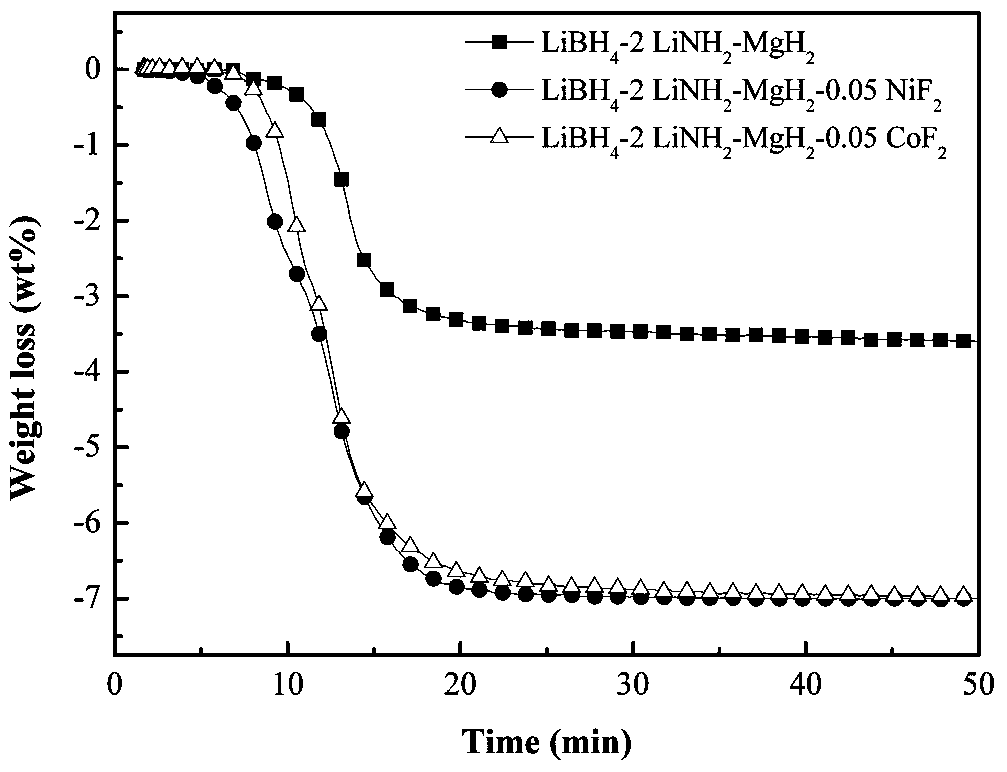
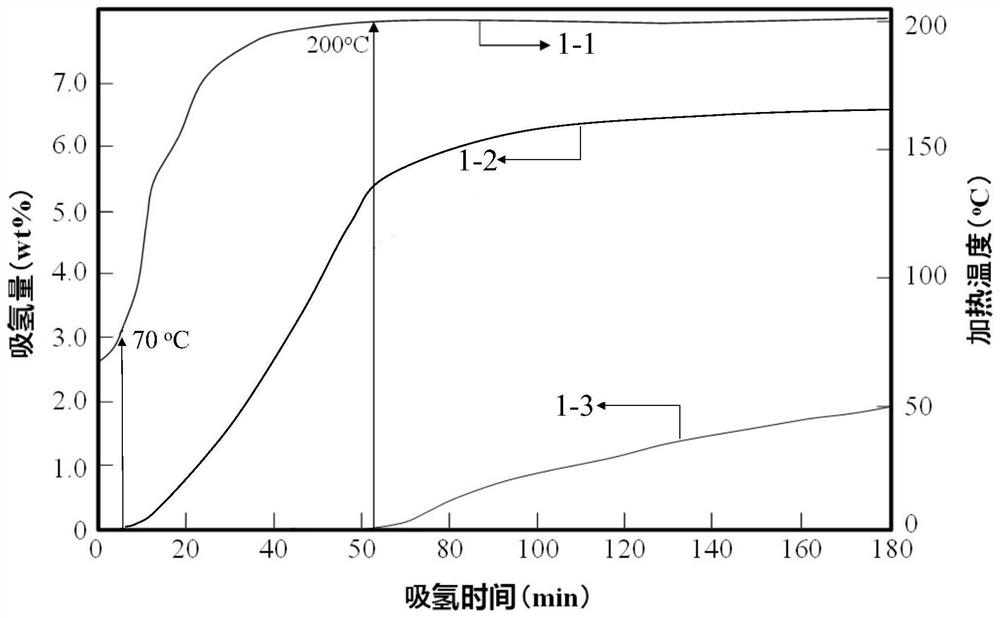
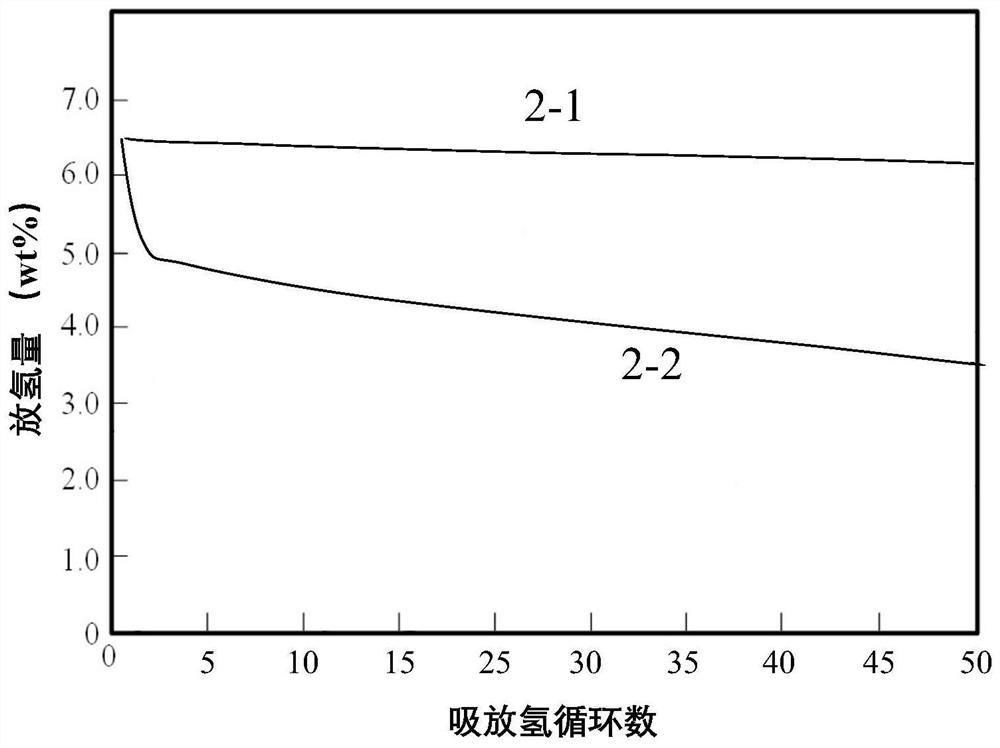
The invention discloses a transition metal fluoride-doped composite hydrogen storage material. The material is prepared through mixing and mechanically ball-milling LiBH4, LiNH2, MgH2 and transition metal fluoride. The initial hydrogen desorption temperature of the material is 90-100 DEG C, the second-step hydrogen desorption temperature is about 150 DEG C, hydrogen desorption is mainly completedat 180-200 DEG C, and the composite hydrogen storage desorbs 6.5-7.0 wt% of hydrogen when heated to 200 DEG C. A preparation method of the material comprises the following steps: 1, weighing raw materials; and 2, carrying out a ball-milling process to prepare the composite hydrogen storage material. The composite hydrogen storage material has the following advantages: 1, the hydrogen desorption temperature is low, and the amount of hydrogen desorption heat is large; 2, the hydrogen desorption quantity is large; 3, the induction period in a second-step hydrogen desorption process used as the speed control step in the hydrogen desorption process is greatly shortened, the second-step hydrogen desorption temperature is reduced, the hydrogen desorption processes in two steps are coordinated, the hydrogen desorption reaction rate is fast, and the dehydrogenation kinetics performance is good; and 4, the cost of raw materials is low, and the synthesis method has a simple process. The preparation method has a certain application prospect in the field of hydrogen storage materials.
Owner:GUILIN UNIV OF ELECTRONIC TECH
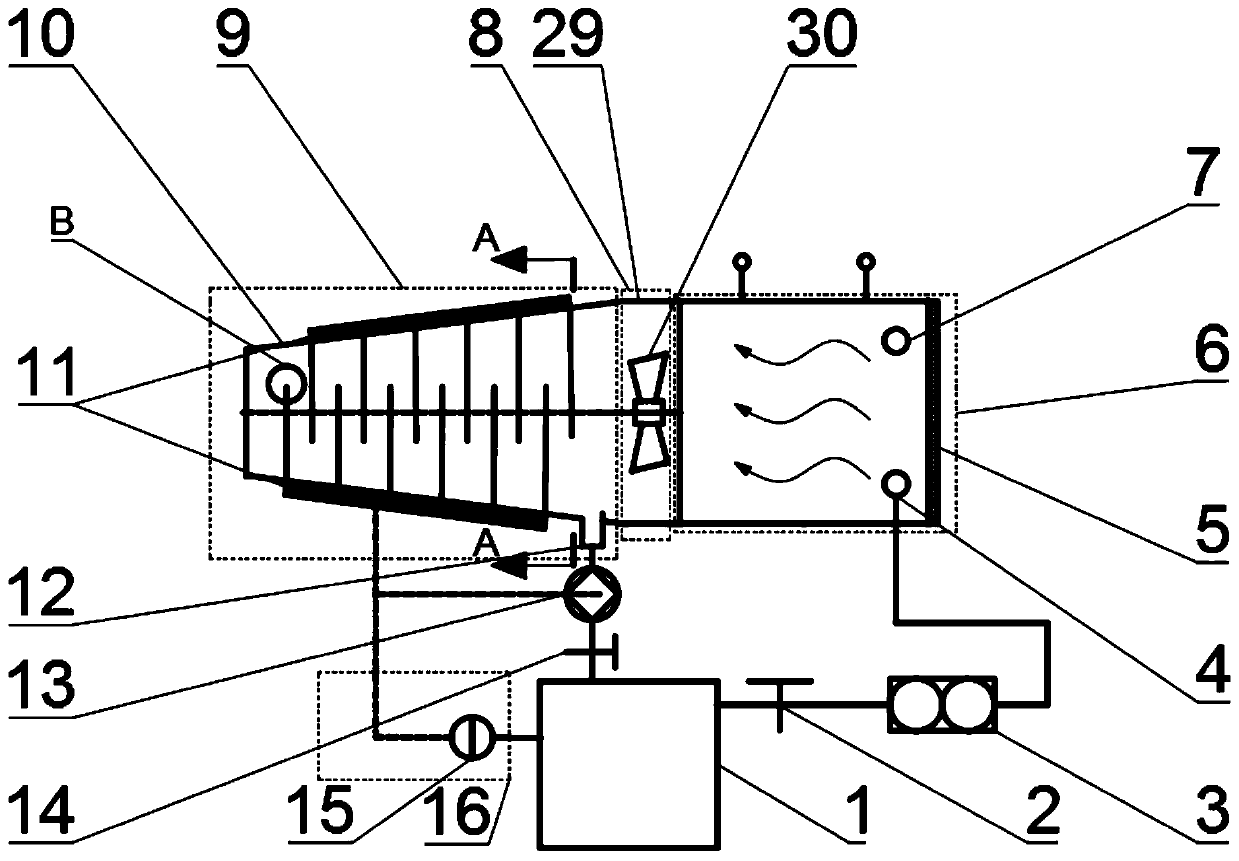
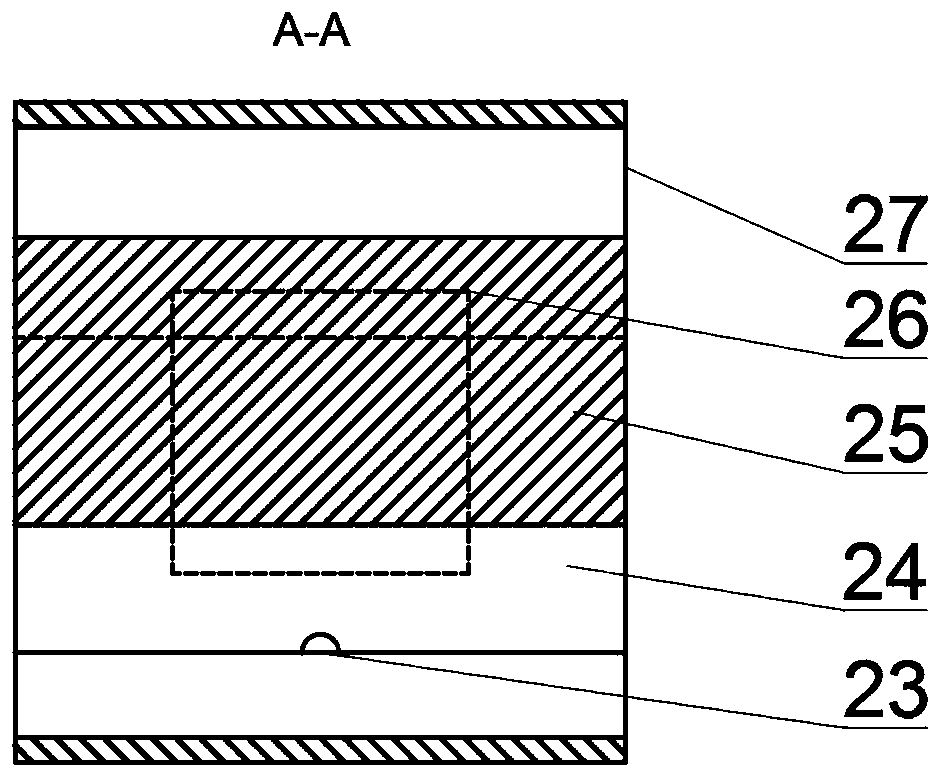
Light solid-state hydrogen storage power system for water reuse of fuel cell exhaust gas
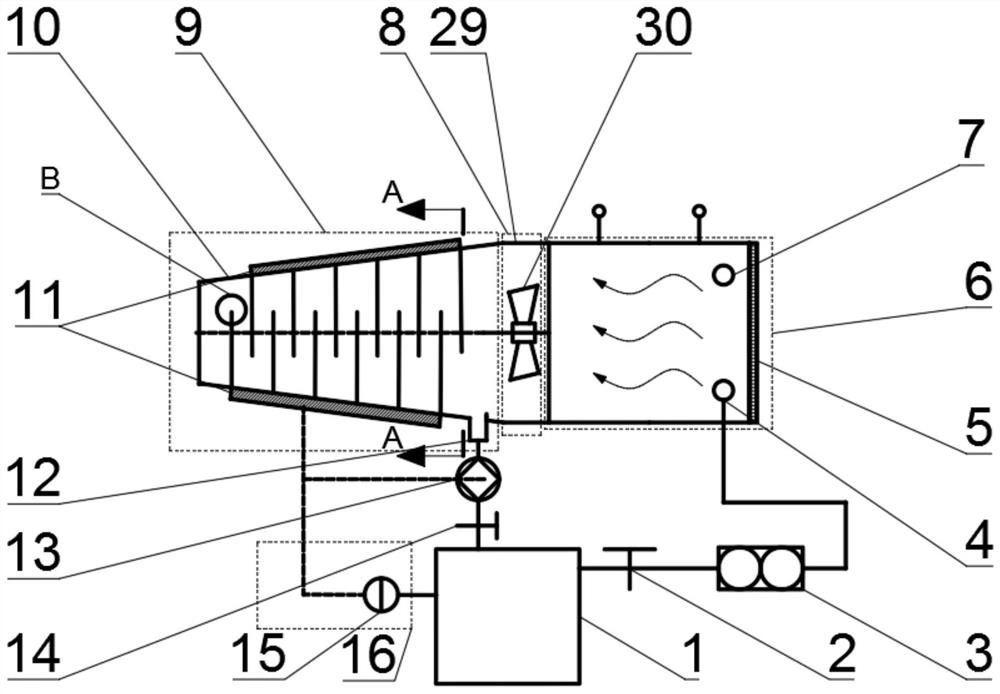
The invention discloses a light solid-state hydrogen storage power system for water reuse of fuel cell exhaust gas. The system is characterized in that hydrogen released from a hydrogen source systementers a hydrogen fuel cell, the exhaust gas generated by the hydrogen fuel cell and a part of the air enter a water vapor cooling device, and the cooling water condensed by the water vapor cooling device is injected into the hydrogen source system through a water outlet pipe and a water inlet pipe. The system is advantaged in that light element hydride (a light element hydride bed) is utilized, the water produced after hydrogen electrochemical reaction is recovered and returned to the hydrogen source system for hydrolysis reaction with the light element hydride to produce hydrogen, the weightof the reactant water is eliminated to increase the mass hydrogen storage amount of the system, under the same hydrogen release effect, the system mass is reduced.
Owner:XI AN JIAOTONG UNIV
Magnesium-based complex phase hydrogen storage material and preparation thereof
InactiveCN101279717ALarge hydrogen storage capacityLow hydrogen release temperatureMultiple metal hydridesHydrogen fuelMagnesium
The invention provides high-volume mg-based multiphase hydrogen storage material and a preparation method thereof. A fusion casting and ball milling method is adopted, which comprises the specific steps: (1) under vacuum or argon protection, Mg-Li-Al alloy ingot is melted and cast in an induction furnace; (2) the ingot is milled into fines with a milling machine and is thinning ground in a ball milling machine for 2-20 hours; (3) reaction milling is carried out under hydrogen atmosphere; the hydrogen pressure is 0.1-1 MPa; hydrogen is filled in a ball milling tank to the initial value of the hydrogen pressure at short intervals during the ball milling process; and the way of compulsory cooling is adopted to control the working temperature in the ball milling tank less than 50-60 DEG C; then the mg-based multiphase hydrogen storage material is prepared after argon packaging. The preparation process of the method and modification of the material are accomplished simultaneously. The initial hydrogen desorption peak temperature of the prepared hydrogen storage material is only 62 DEG C and the hydrogen storage amount is as high as 10.6wt percent. The hydrogen storage material is black nano-powder and has large hydrogen storage amount, low temperature and rapid speed for hydrogen desorption. The material can be used for hydrogen fuel vehicles, rechargeable batteries and fuel cells, etc. The method has moderate preparation condition, simple device and convenient operation.
Owner:CHONGQING UNIV
In-situ preparation method of nanometer magnesium hydride
ActiveCN110116990AFacilitate chemical reactionsHigh hydrogen storage capacityMaterial nanotechnologyAlkali/alkaline-earth/beryllium/magnesium hydridesCavitationChemical reaction
The invention discloses an in-situ preparation method of nanometer magnesium hydride. The method comprises the steps that under protection of an inert atmosphere, magnesium chloride and lithium hydride are added into an organic solvent, and through stirring, organic turbid liquid of a mixture is obtained; the organic turbid liquid is subjected to ultrasonic treatment, a chemical reaction of the mixture is promoted, and after the reaction is finished, filtration is conducted; a solid reaction product is washed, centrifuged and dried, remaining organic matter is removed, and the nanometer magnesium hydride is obtained. According to the preparation method, the energy provided by the cavitation effect, generated in a liquid medium, of ultrasonic waves is used for promoting the chemical reaction between the magnesium chloride and the lithium hydride, due to the fact that compared with traditional energy supply through heating and mechanical force, the cavitation effect can provide a large amount of energy instantaneously in a quite small range, the prepared product particles cannot easily become large, and the breaking effect of the ultrasonic waves is utilized for inhibiting nanometerparticle aggregation; the side effects caused by addition of various carrier materials for inhibiting growth of the particles are avoided, therefore the nanoscale magnesium hydride is obtained, and the effective hydrogen storage capacity of the product is increased.
Owner:ZHEJIANG UNIV
Metal amine hydrogen storage material and preparation method thereof
The invention discloses a novel metal amine hydrogen storage material and a preparation method thereof. The material is an amine material, and comprises a compound or mixture prepared through mixing organic amine, partially boron-substituted organic amine or boron-amine or other amine compounds with lithium hydride, magnesium hydride, titanium hydride or other metal hydrides. The preparation method comprises the following steps: uniformly mixing the amine compound with the metal hydride according to a certain quantity ratio, preheating the obtained mixture to carry out a certain degree reaction, and adding a certain amount of a transition metal compound as a catalyst to obtain the hydrogen storage material. The hydrogen storage material prepared in the invention has the advantages of simple preparation method, simple and easily available raw materials, low price, large hydrogen storage capacity, and adjustable and easily-adjusted structure and performances, is very close to or has already reaches hydrogen storage material standards of American DOE, is a very promising hydrogen storage material, is suitable for large-scale production and application in the future, and has wide market prospect.
Owner:赵前永
High-volume light-weight graphene catalysis rare earth aluminum magnesium based hydrogen storage material and preparation method thereof
ActiveCN108220728ALow hydrogen release temperatureRapid hydrogen charge and discharge capabilityCell electrodesRare-earth elementHysteresis
The invention relates to a high-volume light-weight graphene catalysis rare earth aluminum magnesium based hydrogen storage material and a preparation method thereof. The hydrogen storage material isprepared from rare earth aluminum magnesium based hydrogen storage alloy and graphene catalysts GR, wherein the rare earth aluminum magnesium based hydrogen storage alloy has a formula chemical formula of ReaMg100-a-b-cAlbNic, wherein the Re is one kind of materials of rare earth elements of lanthanum, cerium, praseodymium and neodymium; the a, the b and the c are the atom percentage of the corresponding element; the a is greater than or equal to 5 but smaller than or equal to 20; the b is greater than or equal to 5 but smaller than or equal to 40; the c is greater than or equal to 0 but smaller than or equal to 10; the sum of the b and the c is greater than or equal to 10 but smaller than or equal to 40; the proportion of the mass percentage of the graphene catalysts GR in the final hydrogen storage material is greater than or equal to 1 percent but smaller than or equal to 10 percent. The Mg and AL which has rich reserves in the nature and low price are used as major composition elements; meanwhile, different kinds and contents of rare earth elements are added in the alloy side A; different contents of Ni elements are added at the side B; graphene is added for ball milling. The hydrogen storage material prepared by the method has the characteristics of high hydrogen adsorption and release speed, high hydrogen storage capacity, small platform hysteresis and low hydrogen release temperature.
Owner:CENT IRON & STEEL RES INST
High-capacity organic-inorganic composite hydrogen storage material and preparation method thereof
ActiveCN105062033AInhibit mutual agglomerationReduce absorption efficiencyHydrogen productionEnd-groupSide chain
The invention discloses a high-capacity organic-inorganic composite hydrogen storage material. The organic-inorganic composite hydrogen storage material comprises an inorganic porous material and organic materials uniformly dispersed in pores of the inorganic porous material, wherein the inorganic porous material adopts porous silicon dioxide or aluminum oxide, the aperture is 0.5-20 nm, and the specific surface area is 300-500 m<2> / g; the organic materials comprise polymers serving as main chains and borane ammonia derivatives, and the borane ammonia derivatives are prepared as follows: side chains and / or end groups of the polymers are subjected to amination by polyamine compounds and grafted to the side chains and / or ends of the polymers through reaction with a borohydride. The invention further discloses a preparation method of the high-capacity organic-inorganic composite hydrogen storage material. The high-capacity organic-inorganic composite hydrogen storage material prepared with the method has the following advantages: mutual agglomeration of the polymers can be inhibited effectively, the hydrogen storage and release efficiency is high, little environmental pollution is produced, the material can be regenerated and recycled, and the cost is saved.
Owner:WUHAN KAIDI ENG TECH RES INST CO LTD
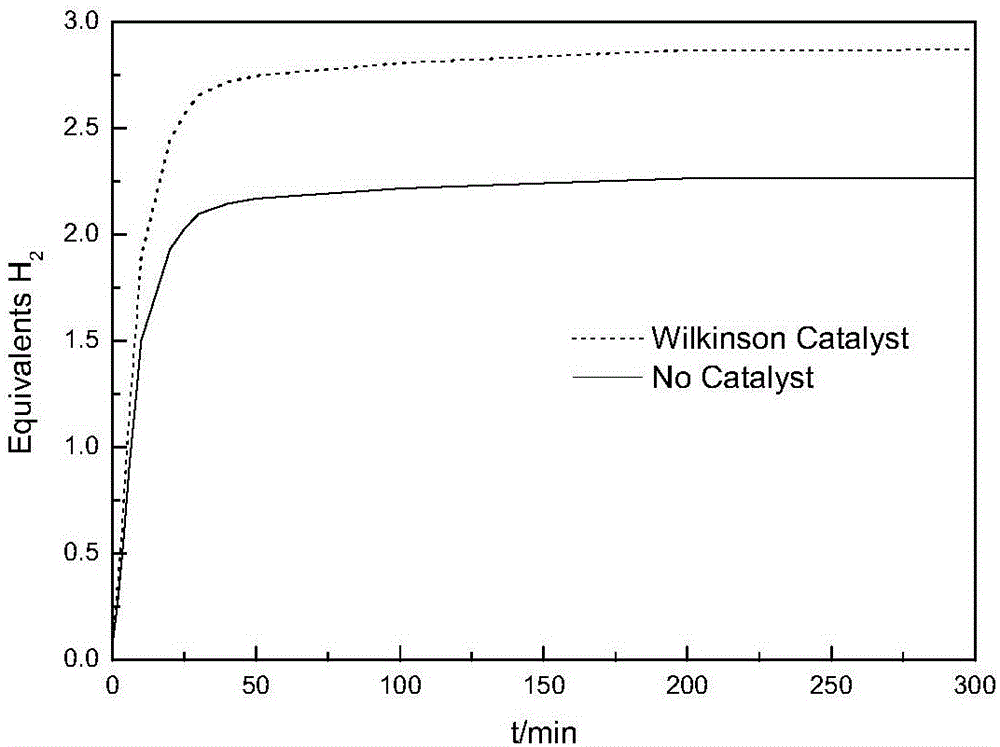
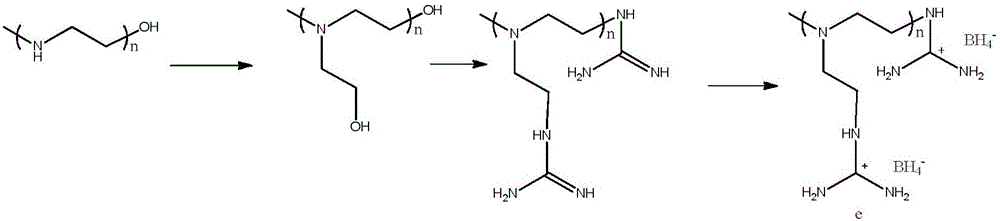
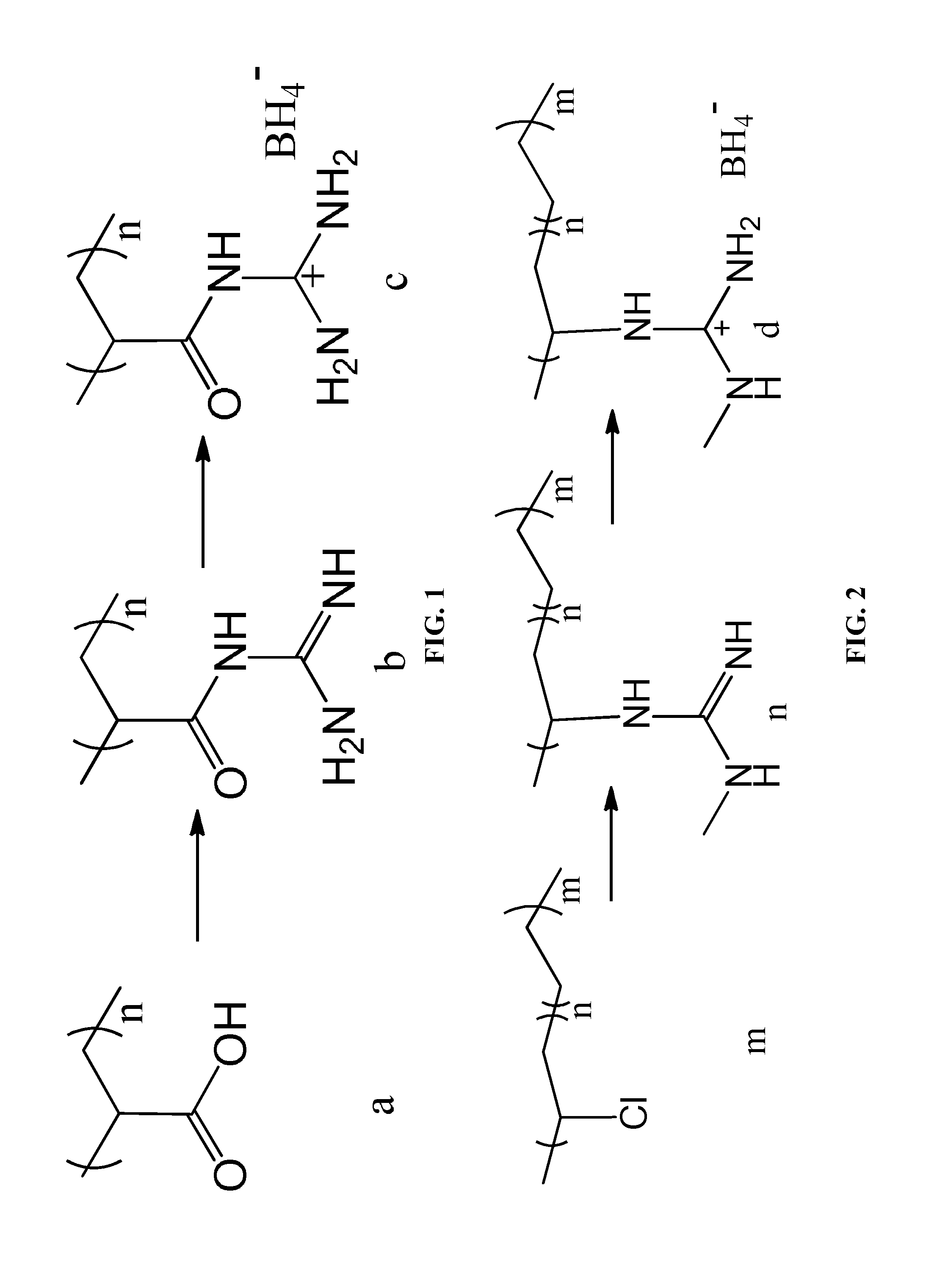
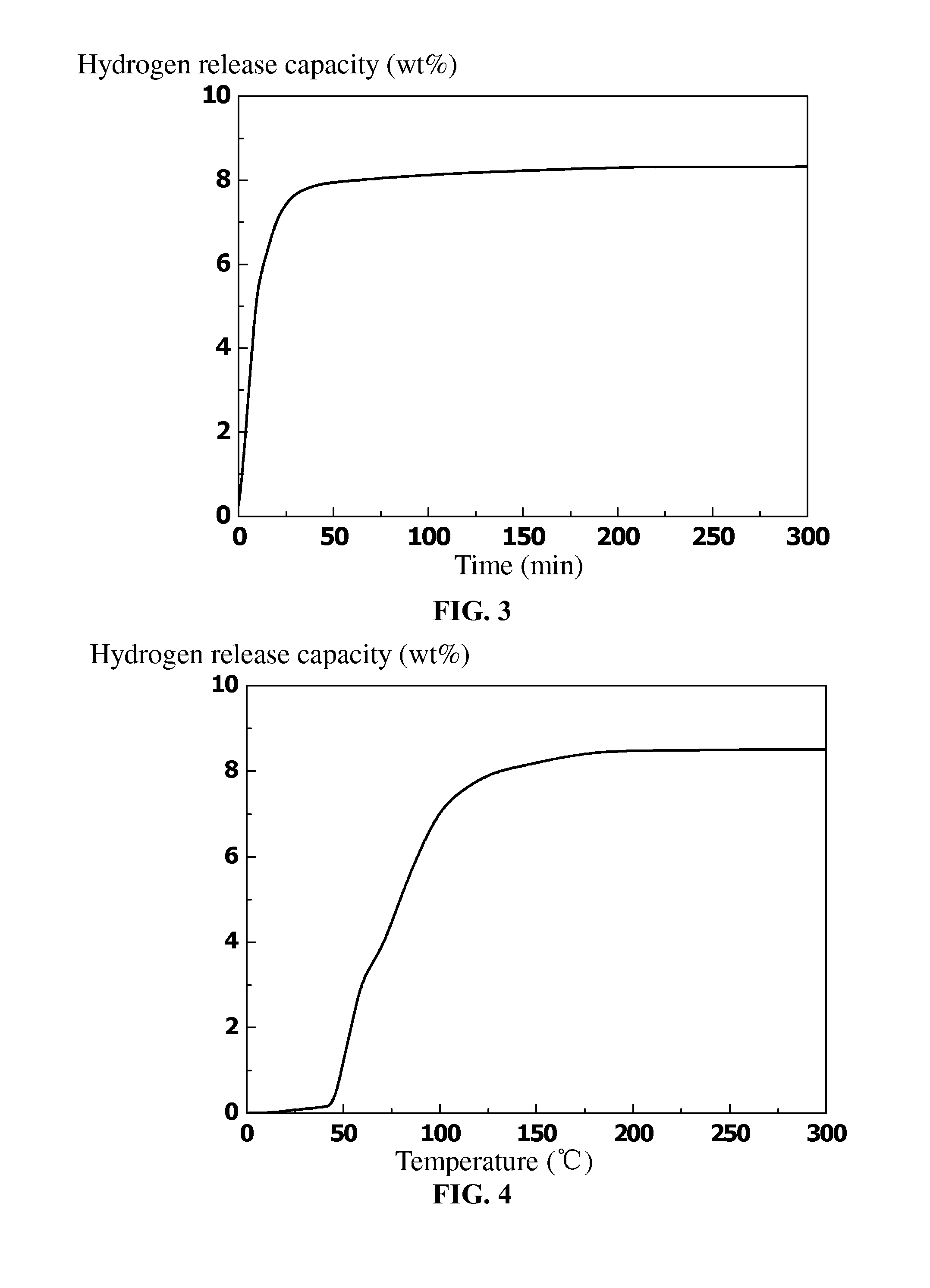
Polymer material having high capacity for hydrogen storage and preparation method thereof
InactiveUS20150191573A1Preventing uncontrollabilityGood film formingHydrogenPolyamine CompoundPolymer science
A high capacity polymer hydrogen storage material, including a linear high molecular polymer as a main chain. At least one side chain or a terminal group of the linear high molecular polymer is first aminated using a polyamine compound and then reacts with a borohydride to yield an ammonia borane derivative grafted to the side chain or the terminal group of the linear high molecular polymer.
Owner:WUHAN KAIDI ENG TECH RES INST CO LTD
Magnesium indium solid solution and preparation method thereof
ActiveCN101967590AImprove the kinetic performance of hydrogen absorption and desorptionAchieve destabilizationIndiumSolid solution
The invention discloses magnesium indium solid solution and a preparation method thereof. The preparation method comprises the following steps of: preparing a sample A in a molar ratio of magnesium powder to indium powder of (100-x):x, wherein x is more than 0 and less than 12; mixing the sample A on a ball mill to obtain mixed powder B, and then pressing and forming to obtain a green body C; introducing argon in a vacuum furnace for protecting and sintering the green body C, crushing the sintered green body C, and then performing ball-milling on the crushed green body C in an argon atmosphere to obtain the magnesium indium solid solution; and adding an appropriate amount of indium to prepare the magnesium indium solid solution. Therefore, a hydrogen adsorption and desorption reaction path of the magnesium is changed so as to achieve the effects of reducing hydrogen adsorption and desorption enthalpy change and reducing the stability of thermodynamics of magnesium hydride (MgH2). A magnesium indium alloy also has high reversibility, and the problem of irreversibility or low reversibility is solved.
Owner:SOUTH CHINA UNIV OF TECH
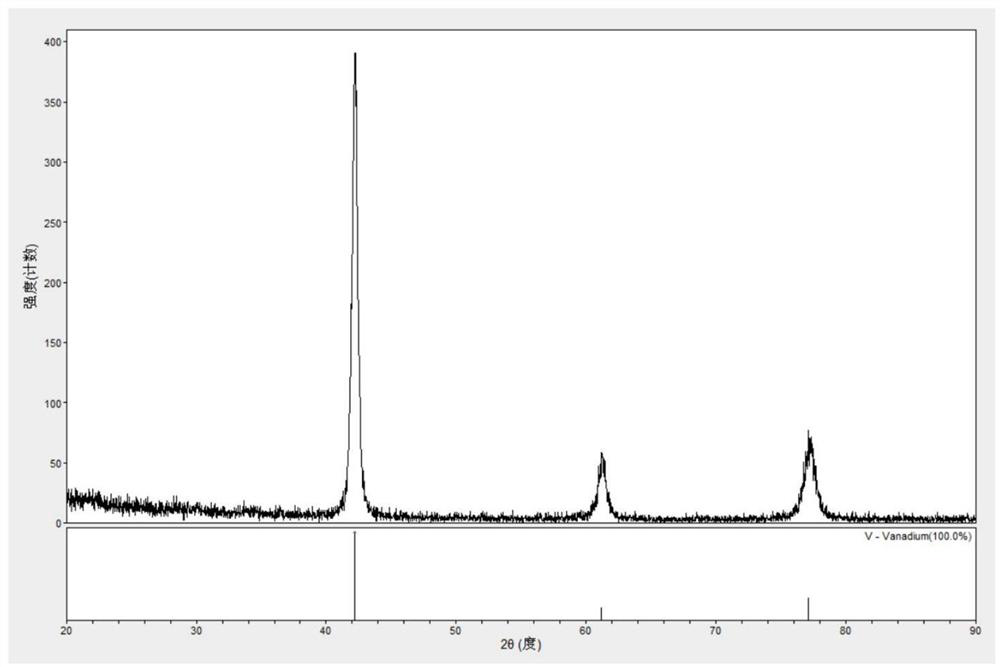
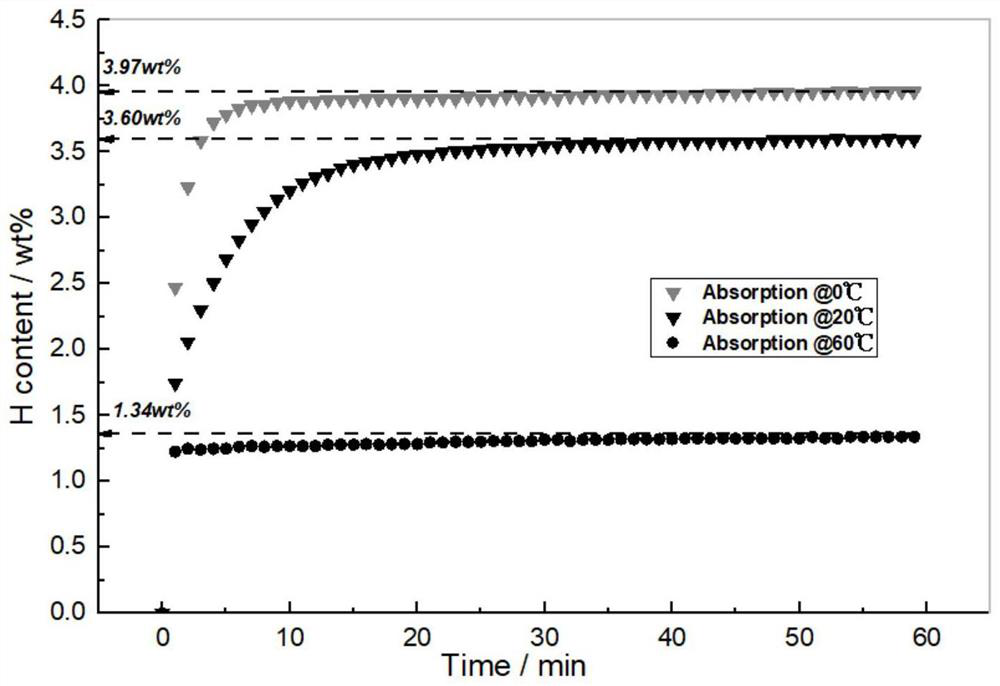
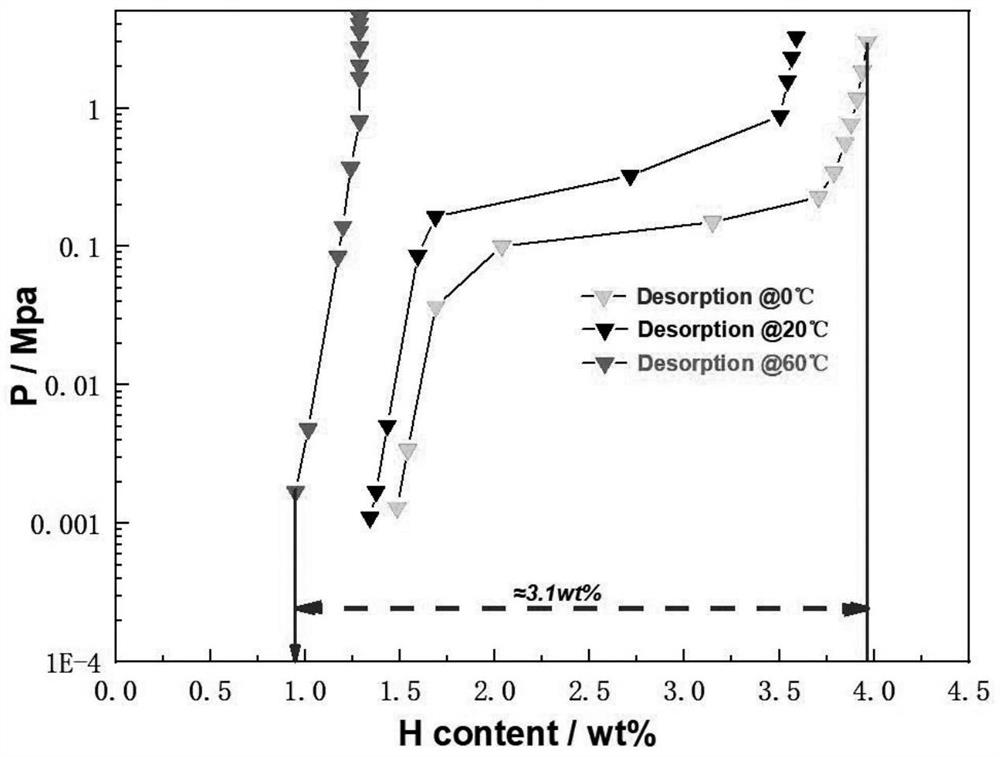
High-uniformity vanadium-titanium-based hydrogen storage alloy and preparation method thereof
The invention discloses a high-uniformity vanadium-titanium-based hydrogen storage alloy and a preparation method thereof. The preparation method comprises the following steps: S1, weighing raw materials according to a component proportion; s2, the high-melting-point raw materials are added into a water-cooled copper crucible of a vacuum induction suspension smelting furnace, and the low-melting-point raw materials are added into a feeder of the vacuum induction suspension smelting furnace; s3, after the high-melting-point raw materials are completely melted, adding the low-melting-point raw materials; s4, after smelting is finished, furnace cooling is carried out to obtain an alloy ingot, and the alloy ingot is turned over and then smelted again; and S5, after the S4 is finished, casting is conducted through a casting mold, and the vanadium-titanium-based hydrogen storage alloy ingot is obtained. By adopting and improving a vacuum induction suspension smelting technology, the problems of serious alloy burning loss, crucible corrosion and the like can be avoided, an alloy purification function is achieved in the preparation process, macrosegregation of components is effectively inhibited, the hydrogen absorption pressure of the prepared hydrogen storage alloy is 3-5 MPa, the reversible hydrogen storage capacity can reach 3.1 wt% when hydrogen is absorbed at the temperature of 0 DEG C and desorbed at the temperature of 60 DEG C, and the hydrogen storage alloy is suitable for being used as a hydrogen storage material. The technical advantages are obvious.
Owner:HOPE CLEAN ENERGY (GRP) CO LTD
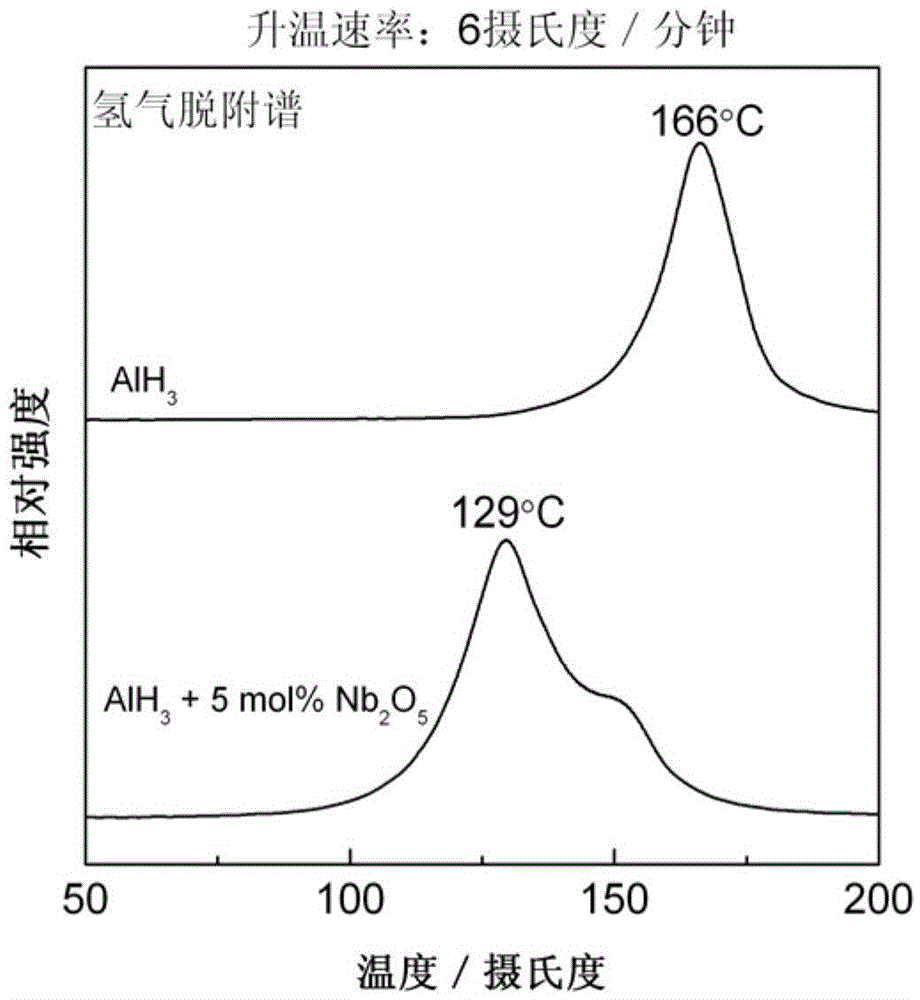
Aluminum hydride hydrogen storage material and preparation method thereof
InactiveCN105645351ALow hydrogen release temperatureThe initial hydrogen release temperature decreasesHydrogenHydrogen desorptionALUMINUM HYDRIDE
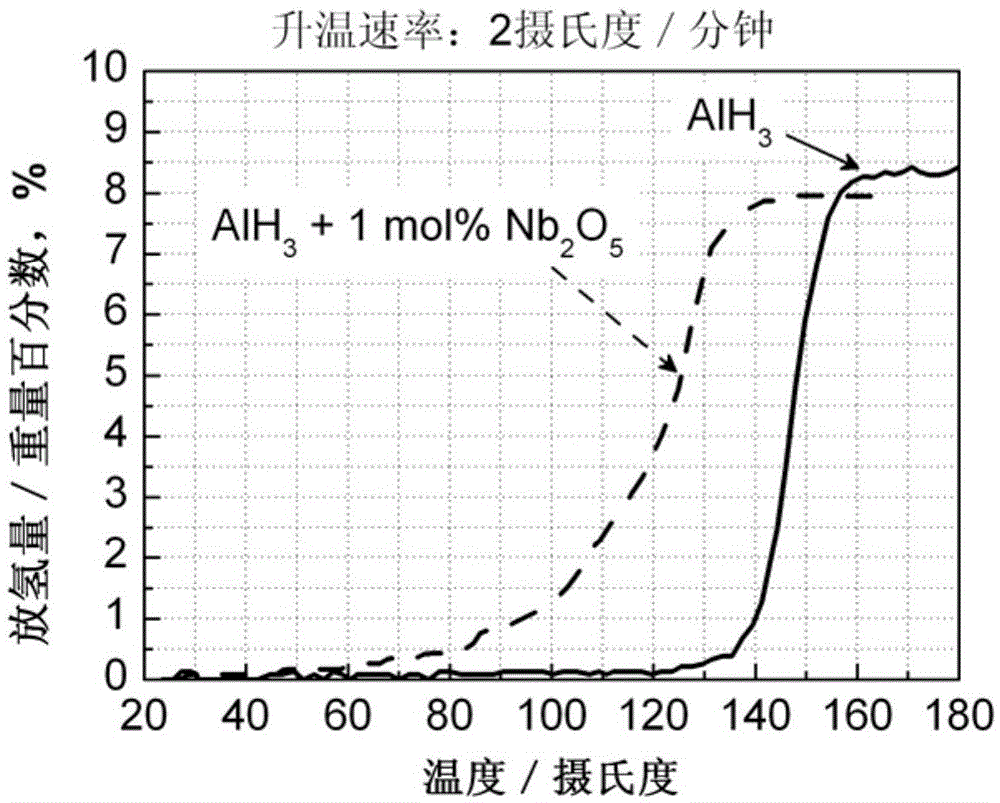
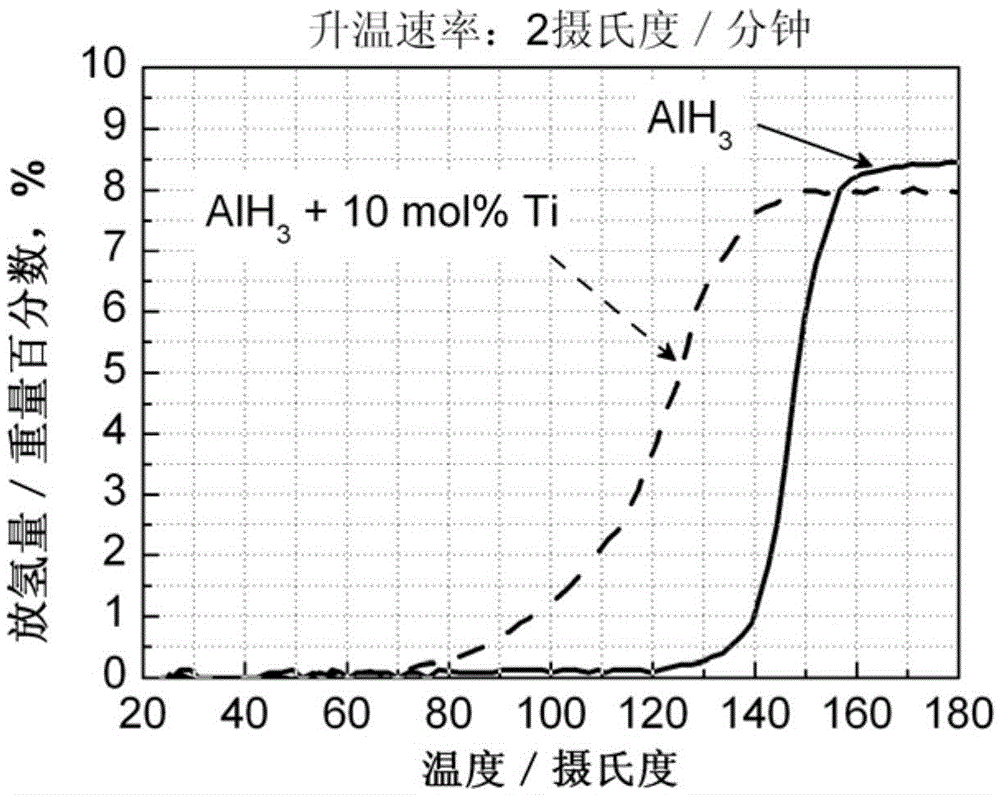
The invention discloses an aluminum hydride hydrogen storage material and a preparation method thereof. The hydrogen storage material is made from aluminum and a hydrogenation catalyst. The catalyst has a general formula of M or MxOy; M represents one or more transition metals selected from Sc, Ti, V, Cr, Mn, Fe, Cu and Nb; x equals to 1 or 2; y equals to 1,2,3,4 or 5; and the molar ratio of the hydride hydrogen and the catalyst is 100:0.1-10. The invention also discloses a preparation method of the aluminum hydride hydrogen storage material. The method is as below: sealing aluminum hydride and the catalyst in a ball milling tank for mechanical milling to obtain the aluminum hydride hydrogen storage material. The aluminum hydride hydrogen storage material modified by the method can reduce hydrogen desorption temperature of the aluminum hydride hydrogen storage material, can release hydrogen at the temperature of 60 DEG C, has high hydrogen desorption amount up to 8.0 wt%, which is higher than that of the aluminum hydride hydrogen storage material modified by the prior art.
Owner:ZHEJIANG UNIV
High-capacity reversible hydrogen storage composite material of LiBH4 doped fluoride, and preparation method thereof
InactiveCN105036074ALow hydrogen release temperatureHigh hydrogen discharge capacityHydrogen productionFuel cellsGeneration rate
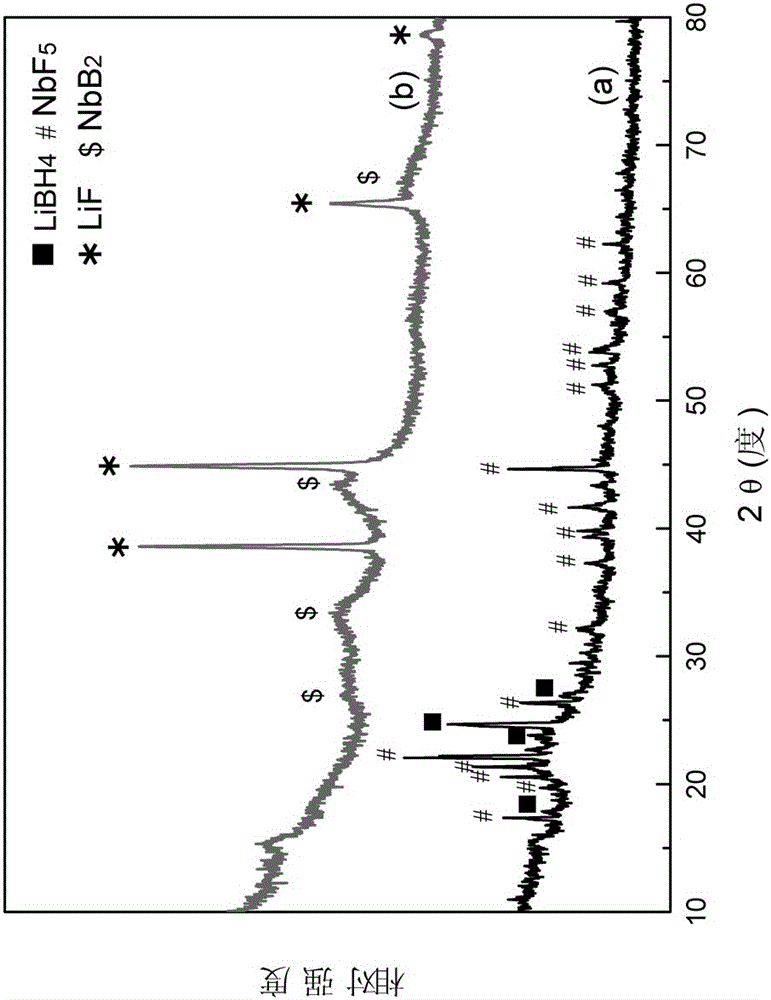
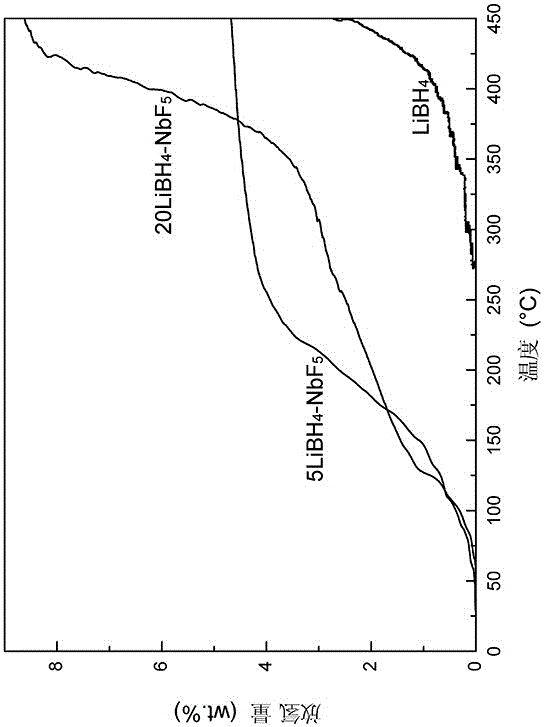
The invention discloses a high-capacity reversible hydrogen storage composite material of LiBH4 doped fluoride, and a preparation method thereof. The purpose of the invention is to solve the problems of high hydrogen desorption temperature, slow hydrogen desorption dynamics, strict afresh hydrogen absorption conditions, low generation rate and poor circularity of LiBH4 as a hydrogen storage material. A determination result shows that the hydrogen storage composite material can desorb hydrogen at about 60DEG C, and the hydrogen desorption amount at about 250DEG C can be greater than 4wt%; and the hydrogen desorption amount after multi-time cycle is still higher than 4.4wt%, so the hydrogen storage composite material has good cycle hydrogen desorption ability. The composite material can effectively solve the problems of high hydrogen desorption temperature and poor cycle hydrogen desorption performance of LiBH4, and effectively improves the hydrogen storage performance of LiBH4. The composite material can provide hydrogen for fuel cells and hydrogen-powered cells as a hydrogen source, can be widely used in the fields of electric automobiles, electronic products and military equipment, and can also be used to make mobile and portable power supplies. The preparation method has the advantages of simple process, high efficiency, reliability, and facilitation of industrial batch production.
Owner:MATERIAL INST OF CHINA ACADEMY OF ENG PHYSICS
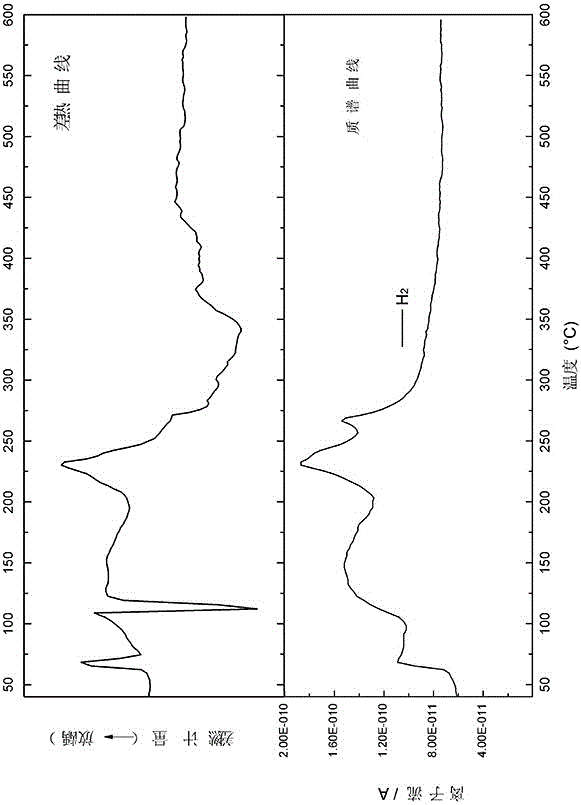
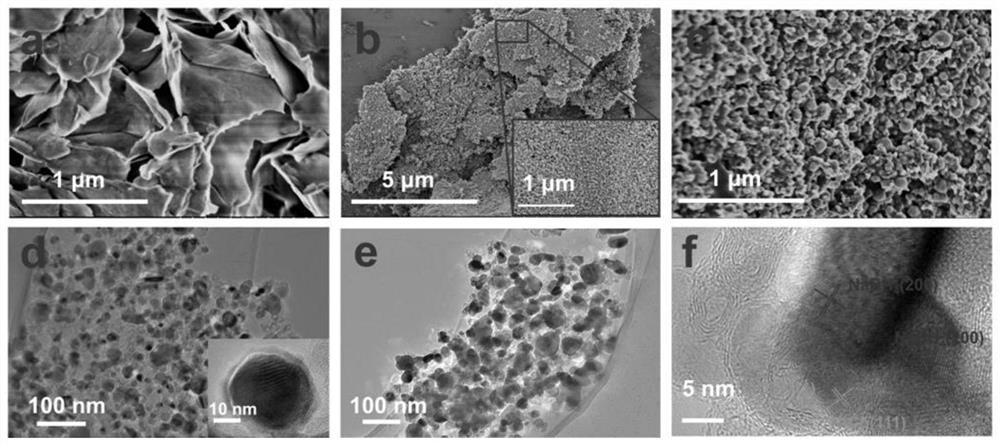
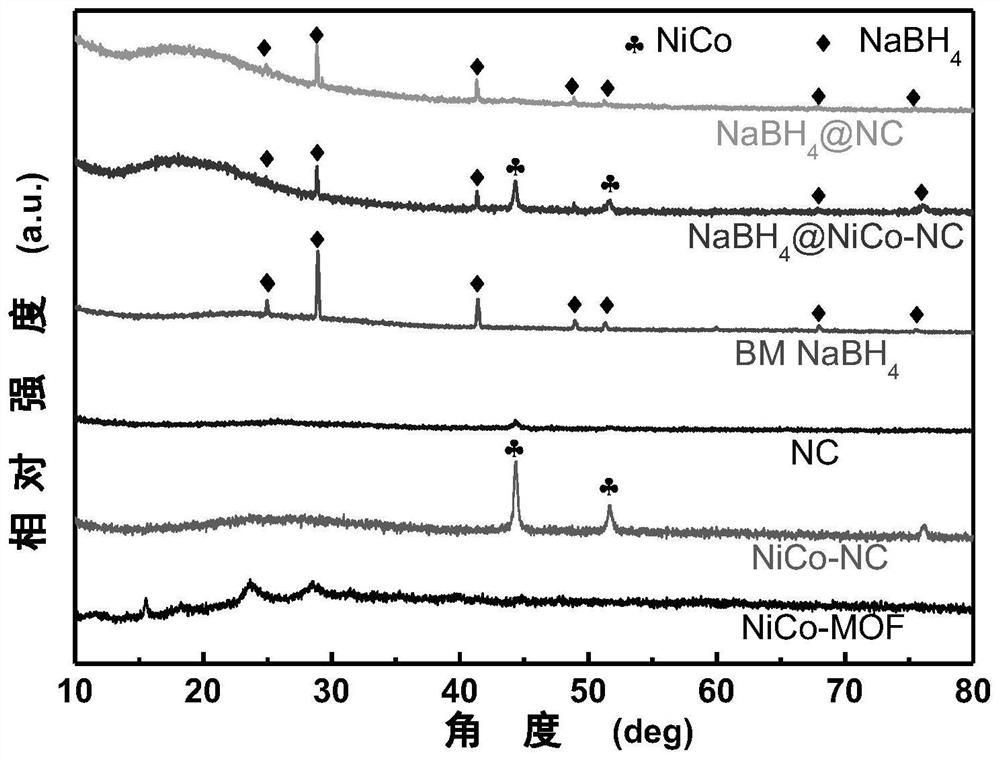
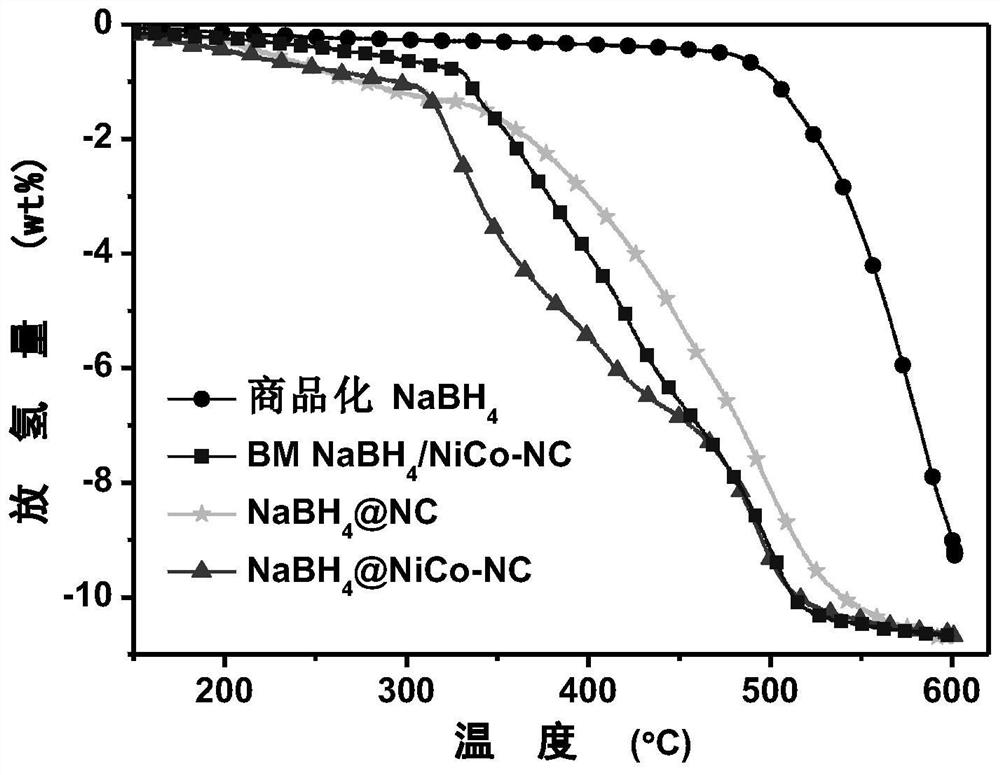
Composite hydrogen storage material NaBH4@NiCo-NC and preparation method thereof
ActiveCN113336188AImprove hydrogen storage performanceAchieve cyclic hydrogen storage capacityHydrogenPorous carbonHydrogen desorption
The invention belongs to the technical field of hydrogen storage materials, and particularly relates to a composite hydrogen storage material NaBH4@NiCo-NC and a preparation method thereof. The method disclosed by the invention comprises the following steps: preparing a NiCo-MOFs nanosheet; preparing a sheet-shaped carrier NiCo-NC porous carbon material; and preparing the NaBH4@NiCo-NC. Wherein the synthesis of the flaky NiCo-NC template material is controlled by controlling the temperature rise process; the loading capacity of the nano NaBH4 is 20 to 60 percent, and the mass fraction of the NiCo-NC is 80 to 40 percent. NaBH4 serving as a hydrogen storage material is poor in kinetic performance and cyclic reversibility, while by means of the method, NaBH4 in the composite material is completely reversible at the temperature of 400 DEG C, and the hydrogen desorption kinetic performance is obviously improved. Therefore, the prepared material has excellent hydrogen storage performance. The method is simple in process, easy to operate, convenient to synthesize and easy to implement.
Owner:FUDAN UNIV
Preparation method of ammonia borane-metal catalyst composite hydrogen storage material
InactiveCN103011074AImprove catalytic performanceRich varietyMolecular sieve catalystsCatalyst activation/preparationOrganic solventMetal catalyst
The invention discloses a preparation method of an ammonia borane-metal catalyst composite hydrogen storage material. The preparation method comprises that through magnetron sputtering, metal atoms having catalytic effects are uniformly deposited on a mesoporous material base so that catalyst powder is obtained; the catalyst powder and ammonia borane are mixed uniformly in an anhydrous organic solvent; and the organic solvent is volatilized so that the ammonia borane-metal catalyst composite hydrogen storage material is obtained. The catalyst in the ammonia borane-metal catalyst composite hydrogen storage material has good catalytic effects on a thermolysis hydrogen desorption reaction of ammonia borane so that a hydrogen desorption temperature of ammonia borane is reduced and foreign gas escape can be inhibited effectively and hydrogen desorption dynamic features can be improved. The preparation method adopts simple equipment, and has a fast synthesis speed and a low cost. The ammonia borane-metal catalyst composite hydrogen storage material obtained by the preparation method has good product dispersibility, a wide metal selection range and obvious catalysis performances, can be massively produced easily and has a good application prospect.
Owner:PEKING UNIV
Magnesium, aluminum, boron and nickel-based hydrogen storage material and preparing method thereof
The invention discloses a magnesium, aluminum, boron and nickel-based hydrogen storage material. The chemical component of the magnesium, aluminum, boron and nickel-based hydrogen storage material is xAl-yB-zNi-(1-x-y-z)Mg, wherein the mass fraction, by weight, of x is larger than or equal to 1% and smaller than or equal to 5%, the mass fraction, by weight, of y is larger than or equal to 0.5% and smaller than or equal to 1%, and the mass fraction, by weight, of z is larger than or equal to 5% and smaller than or equal to 20%. A preparing method for the hydrogen storage material comprises the steps that magnesium powder, nickel powder, boron powder and aluminum powder are evenly mixed according to the composition and pressed into a cylinder with the diameter of 10 mm and the height of 8 mm; and then a cubic press is used for heat preservation and pressure maintaining for 30-60 min at 4-6 GPa and 1200-1800 DEG C; and liquid nitrogen is immediately used for cooling an alloy, and the magnesium, aluminum, boron and nickel-based hydrogen storage material is obtained. The preparing method is simple and short in production period, and the prepared hydrogen storage alloy has the good performance of being short in activation period, high in hydrogen absorbing and desorbing rate and the like.
Owner:YANSHAN UNIV
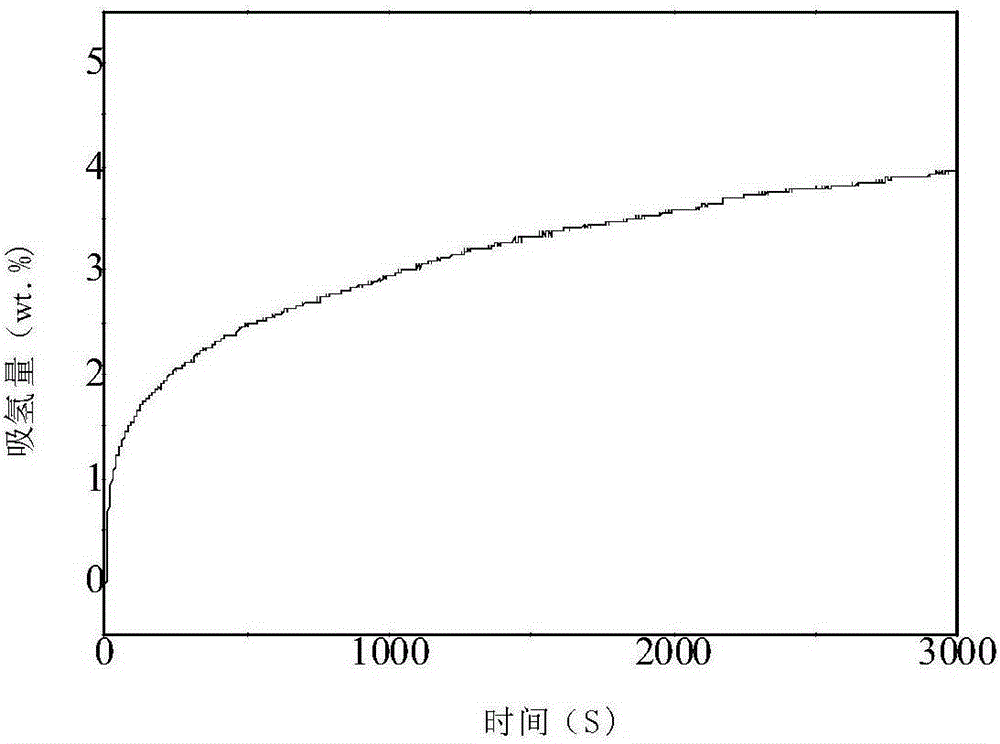
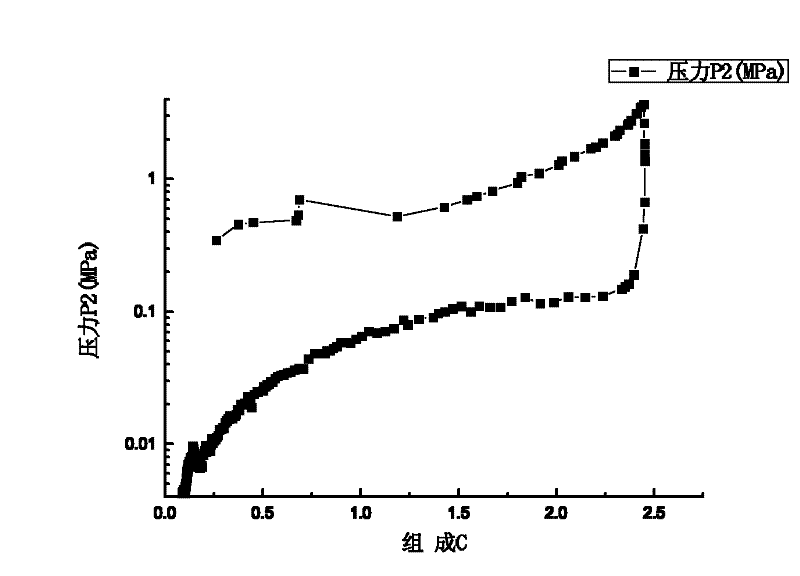
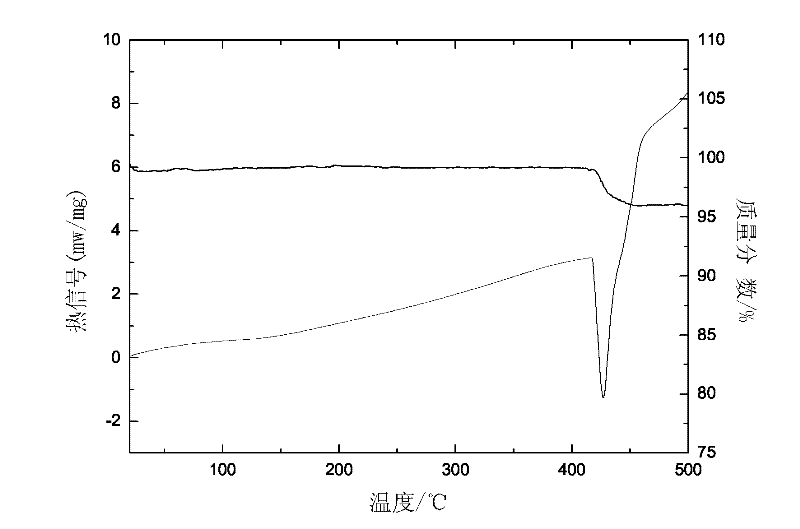
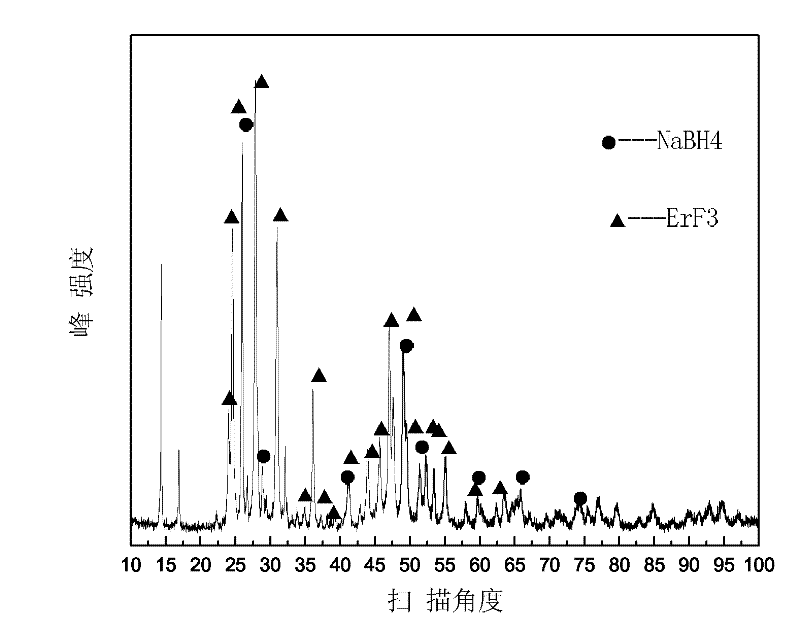
ErF3-containing rare earth composite reversible hydrogen storage material and preparation method thereof
InactiveCN102198932AImprove dynamic performanceLow hydrogen release temperatureHydrogen productionRare earthBall mill
The invention relates to an ErF3-containing rare earth composite reversible hydrogen storage material and a preparation method thereof. The reversible hydrogen storage material comprises the following components in percentage by mass: 10 to 65.25 percent of ErF3, 34.75 to 90 percent of NaBH4 and less than 0.01 percent of impurity element. The preparation method comprises the following steps of: mixing 10 to 65.25 mass percent of ErF3 and 34.75 to 90 mass percent of NaBH4 to obtain mixed powder, putting into a ball mill, setting a ball-milling rotating speed, a ball-milling period and ball-milling time and performing ball milling to obtain the ErF3-containing rare earth composite reversible hydrogen storage material. The preparation method of the hydrogen storage material is relatively simple, and the hydrogen storage material has reversibility and dynamics property which cannot be achieved by a pure NaBH4 hydrogen storage material.
Owner:SHANGHAI JIAO TONG UNIV
High-volume composite hydrogen storage material, and synthetic method and hydrogen desorption method thereof
InactiveCN102951608AThe synthesis process is simpleHigh hydrogen contentMonoborane/diborane hydridesHydrogen productionThermal decomposition methodDecomposition
The invention relates to a high-volume composite hydrogen storage material, and a synthetic method and a hydrogen desorption method thereof. The composite hydrogen storage material is synthesized through interacting a substance containing H<delta-> with a substance containing H<delta+>, wherein the ratio of the substance containing H<delta-> to the substance containing H<delta+> is 20:1-1:20. Dehydrogenation is carried out through mainly utilizing a thermal decomposition or catalytic thermal decomposition method, wherein the temperature of the thermal decomposition or the catalytic heat dehydrogenation decomposition is 0-300DEG C, and the application amount of a catalyst is 0.01-20mol%.
Owner:DALIAN INST OF CHEM PHYSICS CHINESE ACAD OF SCI
Lithium borohydride/ alkali metal aluminum hydride/calcium carbide composite hydrogen storage material and preparation method thereof
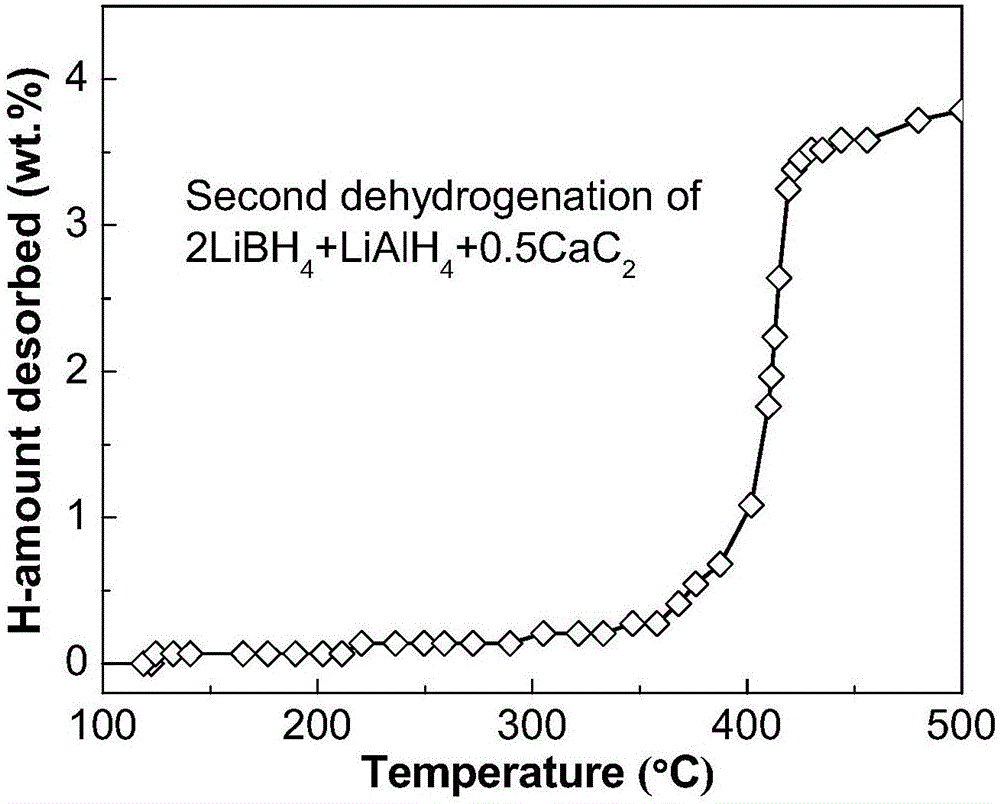
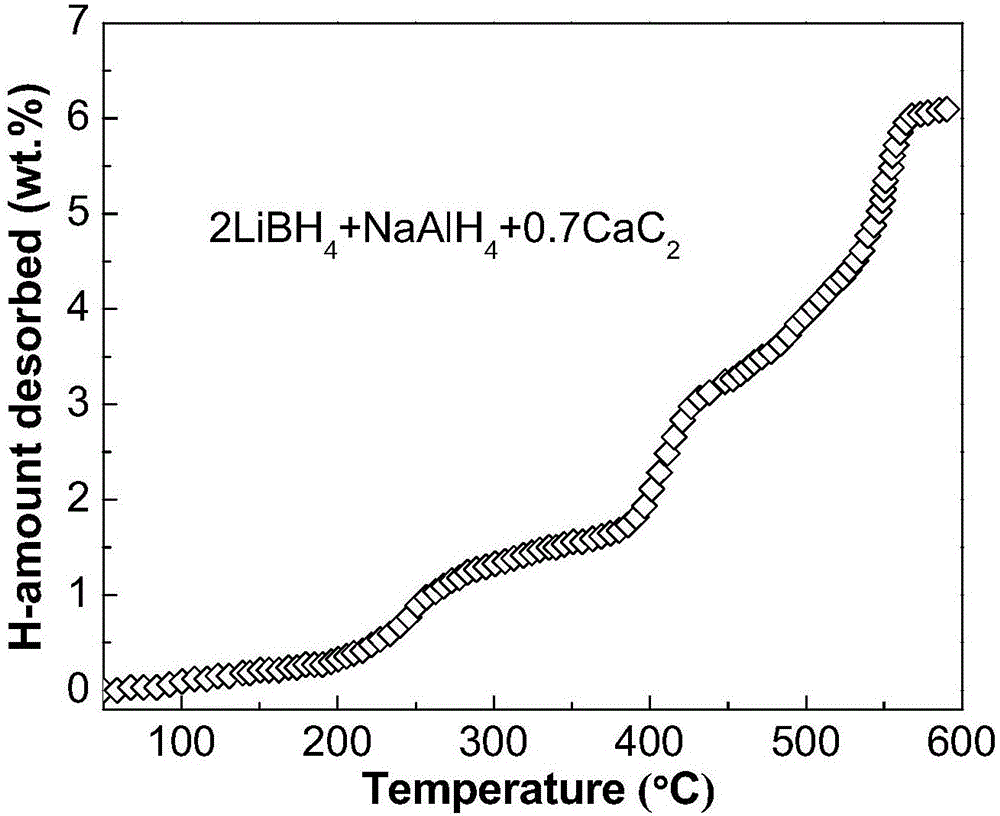
ActiveCN106517089ALow hydrogen release temperatureHigh hydrogen releaseReversible hydrogen uptakeHydrogen desorptionALUMINUM HYDRIDE
The invention discloses a lithium borohydride / alkali metal aluminum hydride / calcium carbide composite hydrogen storage material and a preparation method thereof and belongs to the technical field of hydrogen storage materials. The composite hydrogen storage material is prepared from lithium borohydride, alkali metal aluminum hydride and calcium carbide, wherein the mole ratio of lithium borohydride to alkali metal aluminum hydride is 2:1, the additive amount of calcium carbide is 12-25mol%, and alkali metal aluminum hydride is lithium aluminum hydride or sodium aluminum hydride. During preparation, calcium carbide with the purity not lower than 97% is ground into powder with the granularity smaller than 500 mu m, lithium borohydride, alkali metal aluminum hydride and the calcium carbide powder are weighed in proportion and mixed, and finally, the mixed powder is subjected to ball-milling treatment through a planetary ball mill. The composite hydrogen storage material has the advantages as follows: a preparation process of the composite hydrogen storage material is simple, safe and reliable, the composite hydrogen storage material has low hydrogen desorption temperature, high hydrogen desorption and good reversible hydrogen reabsorption performance, the hydrogen storage performance of the material is improved through calcium carbide, raw materials are widely sourced, and the cost is low.
Owner:ANHUI UNIVERSITY OF TECHNOLOGY
Metal amino borane composite hydrogen storage material
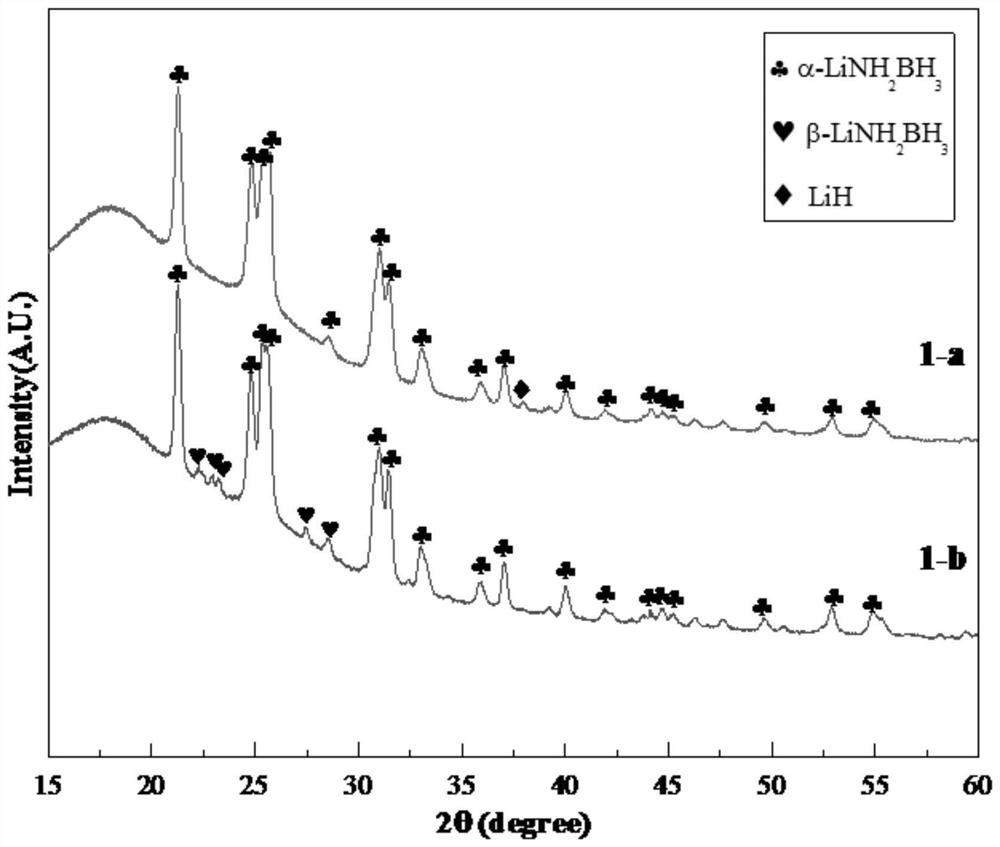
The invention discloses a metal amino borane composite hydrogen storage material, and belongs to the technical field of solid hydrogen storage materials. The metal amino borane composite hydrogen storage material is prepared from an alpha-LiNH2BH3 phase, a LiH phase and a hydrogen storage alloy hydride phase, LiH, NH3BH3 and a hydrogen storage alloy are used as raw materials and subjected to in-situ metallization composite ball milling, and the molar ratio of the NH3BH3 to the LiH to the hydrogen storage alloy is 1: (1.01-1.05): (0.1-0.5). Compared with ammonia borane, the metal ammonia boranecomposite hydrogen storage material has the advantages that the metal ammonia borane can rapidly release hydrogen near the room temperature, the hydrogen release kinetics is faster, no foreign gas isgenerated, the preparation process is simple, the efficiency is high, and the metal ammonia borane composite hydrogen storage material can be used for a high-safety high-density solid hydrogen sourceof a fuel cell.
Owner:GRIMAT ENG INST CO LTD
Light-metal composite hydrogen storage material and preparation method thereof
InactiveCN102167284BLow hydrogen release temperatureHigh hydrogen storage capacityMultiple metal hydridesHydrogen productionOrganic solventHydrogen atmosphere
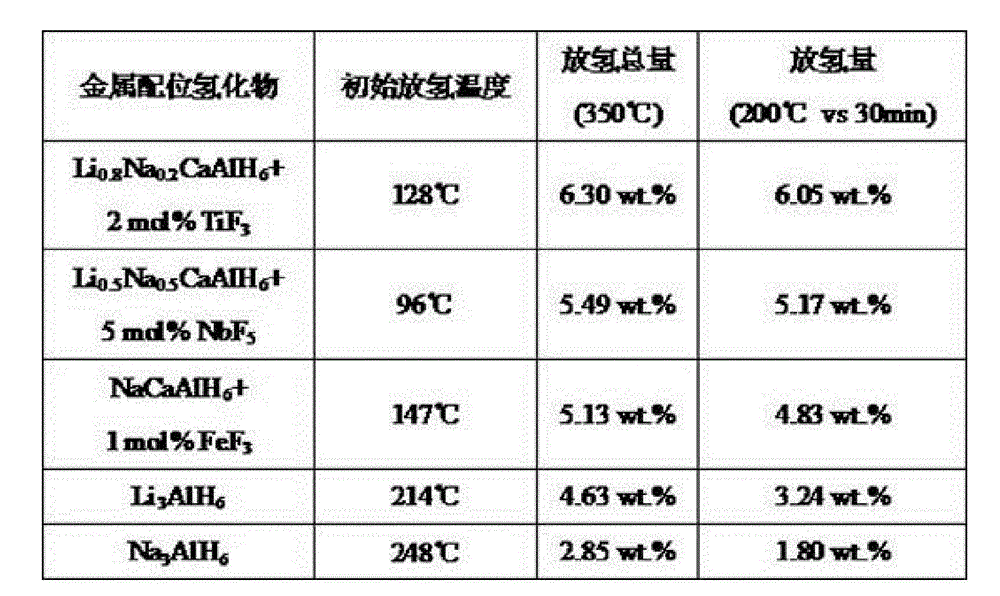
The invention discloses a light-metal composite hydrogen storage material and a preparation method thereof, relating to the technical field of hydrogen storage materials. The light-metal composite hydrogen storage material comprises Li1-xNaxCaAlH6 (x is not less than 0 and not more than 1) metal hydride and a fluoride catalyst. The preparation method of the composite hydrogen storage material comprises the following steps of: with LiH, NaH and Al as raw materials, preparing Li1-xNaxCaAlH6 intermediate hydride in an organic solvent by adopting a reaction ball-milling method; carrying out high-energy ball-milling on the Li1-xNaxCaAlH6 and CaCl2 under an argon atmosphere to prepare Li1-xNaxCaAlH6 metal hydride; and directly doping the fluoride catalyst to the Li1-xNaxCaAlH6 metal hydride in the ball-milling process carried out under a hydrogen atmosphere to prepare the composite hydrogen storage material. The metal composite hydrogen storage material provided by the invention is high in hydrogen storage capacity and rapid in hydrogen release characteristic, and can be synthesized at room temperature with high yield. The preparation method of the light-metal composite hydrogen storage material is simple to operate, safe, reliable and beneficial to scale production.
Owner:ZHEJIANG UNIV
A lightweight solid-state hydrogen storage power system for fuel cell exhaust water reuse
ActiveCN110165262BReduce weightImprove quality hydrogen storage capacityFuel cellsElectrochemical responseWater vapor
The invention discloses a light solid-state hydrogen storage power system for water reuse of fuel cell exhaust gas. The system is characterized in that hydrogen released from a hydrogen source systementers a hydrogen fuel cell, the exhaust gas generated by the hydrogen fuel cell and a part of the air enter a water vapor cooling device, and the cooling water condensed by the water vapor cooling device is injected into the hydrogen source system through a water outlet pipe and a water inlet pipe. The system is advantaged in that light element hydride (a light element hydride bed) is utilized, the water produced after hydrogen electrochemical reaction is recovered and returned to the hydrogen source system for hydrolysis reaction with the light element hydride to produce hydrogen, the weightof the reactant water is eliminated to increase the mass hydrogen storage amount of the system, under the same hydrogen release effect, the system mass is reduced.
Owner:XI AN JIAOTONG UNIV
Lithium borohydride hydrogen storage material modified by oxide and preparation method thereof
InactiveCN100581991CSimple methodLow hydrogen release temperatureOther chemical processesMonoborane/diborane hydridesBall millAtmosphere
The invention relates to liborohydride hydrogen storage material and a preparation method thereof, which is characterized in that the formula of hydrogen storage material is (100-x)LiBH4+XMeO, wherein MeO is a adorning oxide, weight percent of X is 10-80%. After blending LiBH4 and the oxide according to the formula, the mixture is ball milled in protection of inert atmosphere to process surface treatment. The oxide is optional one of TiO2, Fe2O3, ZrO2, V2O5, SiO2, Al2O3, Al2O3-SiO2 or TiO2-SiO2. The intial hydrogen temperature of hydrogen storage material of the invention is lower than 100 DEG C, hydrogen amount is 3-6.5% at lower than 300 DEG C.
Owner:SHANGHAI INST OF MICROSYSTEM & INFORMATION TECH CHINESE ACAD OF SCI
Preparation method of magnesium-based hydrogen storage material coated with rare earth oxide and nano boron nickel
ActiveCN112609102AProcess stabilityImprove performanceHydrogenReactant parameters controlMetallic materialsHydrogen desorption
The invention relates to a metal material hydrogen storage technology, and provides a preparation method of a magnesium-based hydrogen storage material coated by rare earth oxide and nano boron nickel. The preparation method comprises the following steps of dropwise adding sodium borohydride alkali liquor into a mixed solution containing rare earth and nickel, reducing the nickel to form nano amorphous boron-nickel, meanwhile, enabling the pH value of the solution to rise by the alkali liquor to generate rare earth hydroxide colloidal precipitate, then forming nano amorphous boron-nickel doped rare earth hydroxide gel, carrying out vacuum drying treatment on the gel, carrying out high-temperature drying dehydration to obtain a rare earth oxide supported nano boron-nickel composite material, mixing and ball-milling the rare earth oxide supported nano boron-nickel composite material with magnesium hydride, and dehydrogenating the magnesium hydride into magnesium metal, and obtaining the magnesium-based hydrogen storage material. Formation of magnesium-nickel alloy is avoided, and rare earth oxide remains stable, so that performance stability is kept; the hydrogen desorption temperature is reduced, and the hydrogen desorption speed is increased; the hydrogen absorption temperature of rare earth magnesium alloy is reduced and the hydrogen absorption speed is accelerated; the material can be used as a high-capacity hydrogen storage medium for manufacturing a portable power supply for commercial application.
Owner:ZHEJIANG UNIV
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com