Aluminum hydride hydrogen storage material and preparation method thereof
A hydrogen storage material, aluminum hydride technology, applied in chemical instruments and methods, hydrogen, inorganic chemistry and other directions, can solve the problems of unsatisfactory hydrogen release amount, reduce the hydrogen release temperature of aluminum hydride, etc. simple craftsmanship
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0029] (1) Weigh 0.6820g of AlH respectively 3 and 0.3180g of Nb 2 o 5 , namely the formula AlH 3 +5mol%Nb 2 o 5 The ratio stated, AlH 3 with Nb 2 o 5 The molar ratio of 100:5, a total of 1g.
[0030] (2) Seal the weighed sample together with 50 g of stainless steel balls in a ball mill jar for mechanical ball milling, and obtain an aluminum hydride hydrogen storage material after ball milling. The ball milling parameters are set as follows: the ball milling speed is 250 rpm, the ball milling atmosphere is argon, and the ball milling time is 2 h.
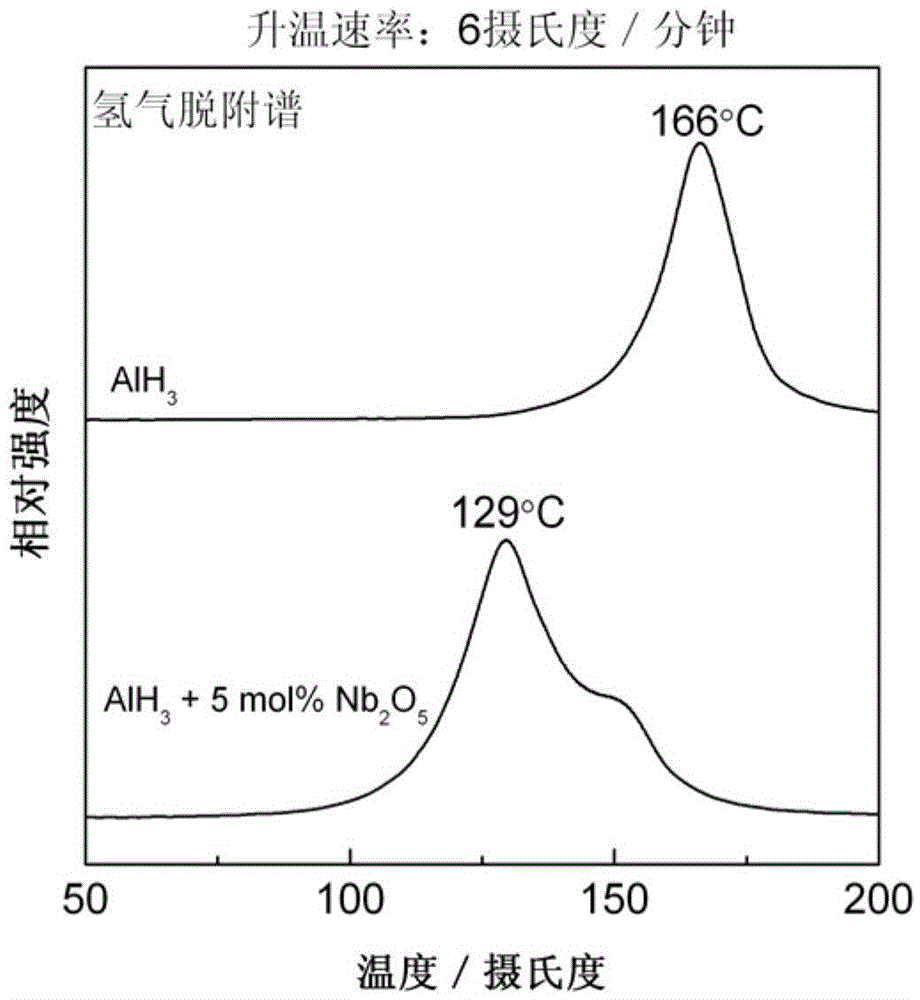
[0031] (3) The sample after ball milling was subjected to a constant rate temperature rise hydrogen desorption test, and its hydrogen desorption spectrum was as attached figure 1 shown. For comparison, pure AlH 3 The hydrogen desorption spectrum of the constant rate heating process is also drawn in the attached figure 1 middle. It can be seen that adding 5mol% Nb 2 o 5 Significantly reduced AlH 3 The hydrogen desorpt...
Embodiment 2
[0033] (1) According to formula AlH 3 +1mol%Nb 2 o 5 Said ratio weighs 0.9179gAlH 3 and 0.0821gNb 2 o 5 , AlH 3 with Nb 2 o 5 The molar ratio of 100:1, a total of 1g.
[0034](2) Seal the sample weighed in step (1) together with 50 g of stainless steel balls in a ball mill jar for mechanical ball milling, and obtain an aluminum hydride hydrogen storage material after ball milling. The ball milling parameters are set as follows: the ball milling speed is 250rpm, the ball milling atmosphere is argon, and the ball milling time is 2h.
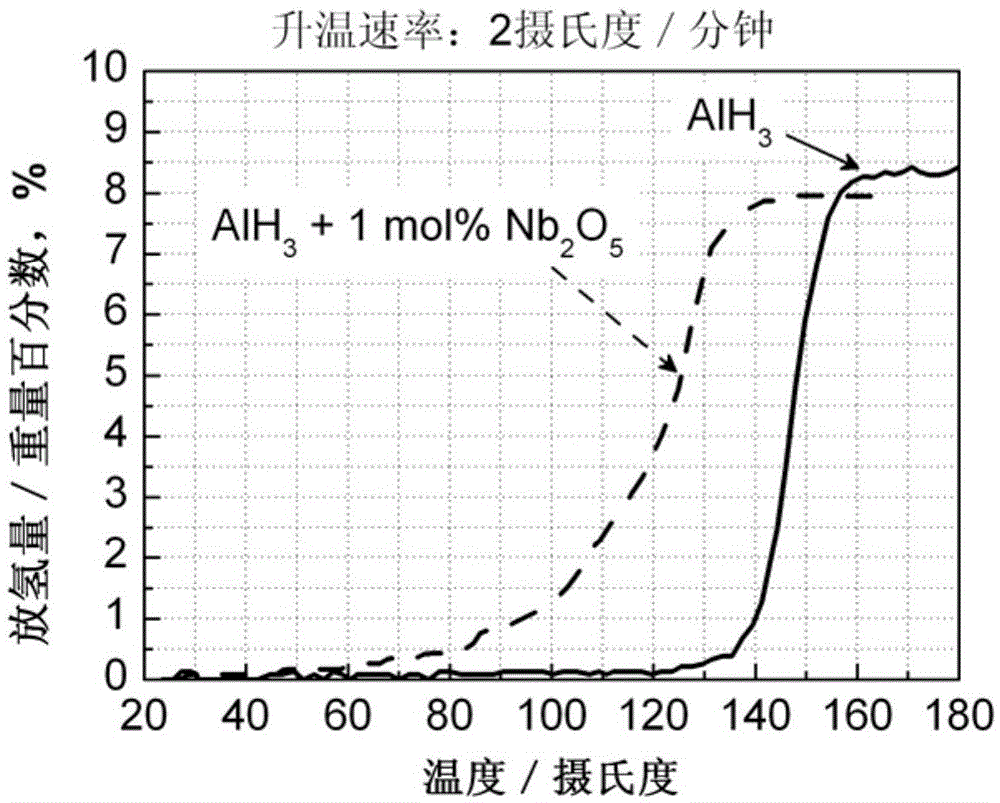
[0035] (3) The sample after ball milling was subjected to a constant rate temperature rise hydrogen desorption test, and the dehydrogenation curve is as attached figure 2 shown. It can be seen that adding 1mol% Nb 2 o 5 AlH 3 The initial hydrogen desorption temperature dropped from 120°C to 60°C, and the drop range reached 60°C. This initial hydrogen desorption temperature is much lower than the operating temperature (85°C) of hydrog...
Embodiment 3
[0037] (1) According to formula AlH 3 Weigh 0.4247gAlH according to the ratio of +10mol%Ti 3 and 0.0753gTi, AlH 3 The molar ratio with Ti is 100:10, 1g in total.
[0038] (2) Seal the sample weighed in step (1) together with 25 g of stainless steel balls in a ball mill jar for mechanical ball milling, and obtain an aluminum hydride hydrogen storage material after ball milling. The ball milling parameters are set as follows: the ball milling speed is 150 rpm, the ball milling atmosphere is argon, and the ball milling time is 2 h.
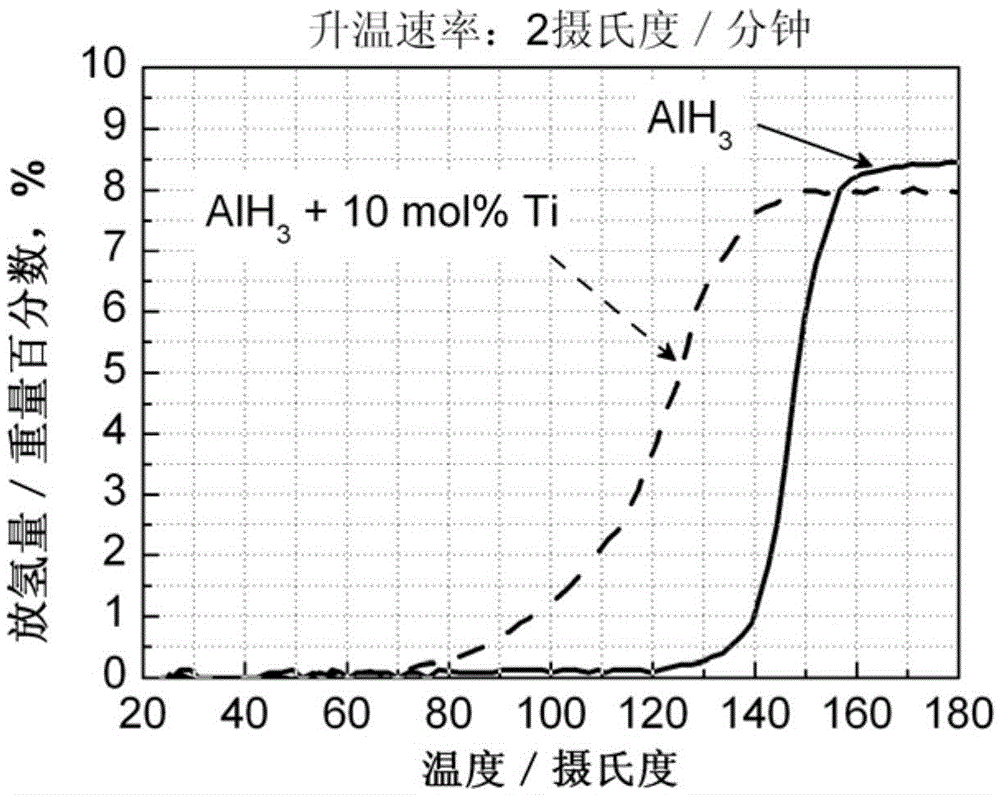
[0039] (3) The sample after ball milling was subjected to a constant rate temperature rise hydrogen desorption test, and the dehydrogenation curve is as attached image 3 shown. It can be seen that the addition of 10mol% Ti in AlH 3 The initial hydrogen desorption temperature dropped from 120°C to 80°C, with a decrease of 40°C. This initial hydrogen desorption temperature is much lower than the operating temperature (85°C) of hydrogen fuel cell...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com