Lithium borohydride/ alkali metal aluminum hydride/calcium carbide composite hydrogen storage material and preparation method thereof
A technology of aluminum hydride and lithium borohydride, applied in chemical instruments and methods, non-metallic elements, hydrogen, etc., can solve problems such as poor reversible hydrogen absorption performance, loss of effective hydrogen storage capacity, and reduced activation energy of hydrogen desorption. Achieve the effects of increasing surface defects, reducing thermodynamic stability and hydrogen release temperature, and improving hydrogen storage performance
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
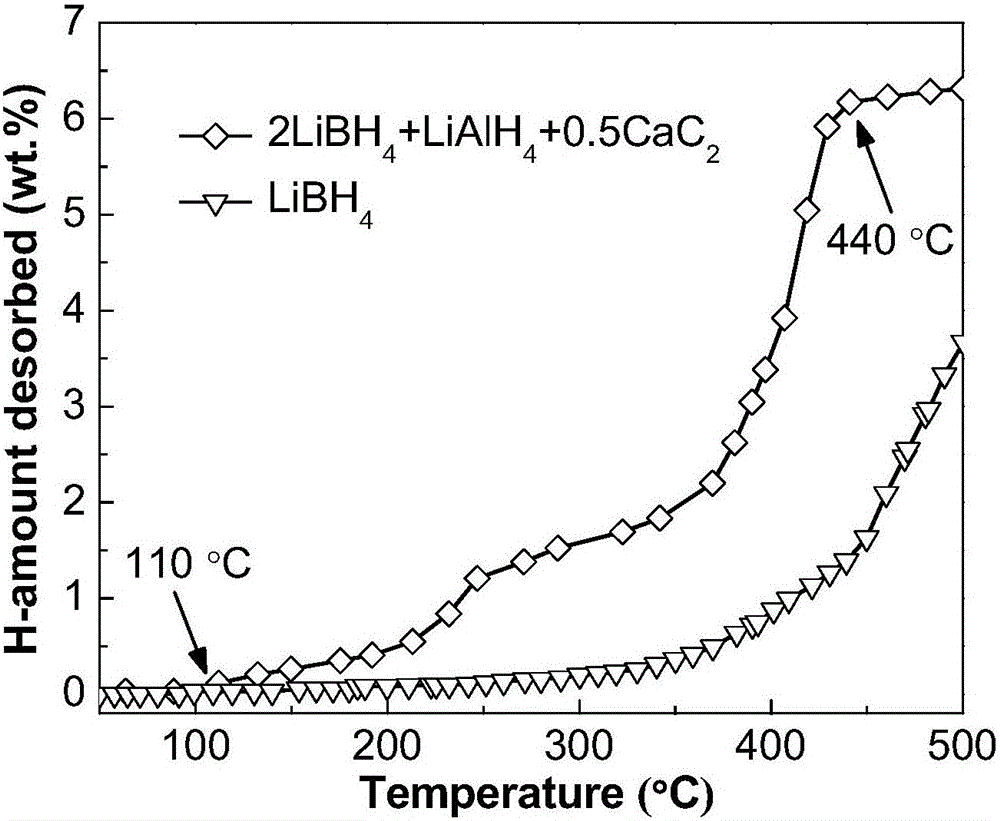
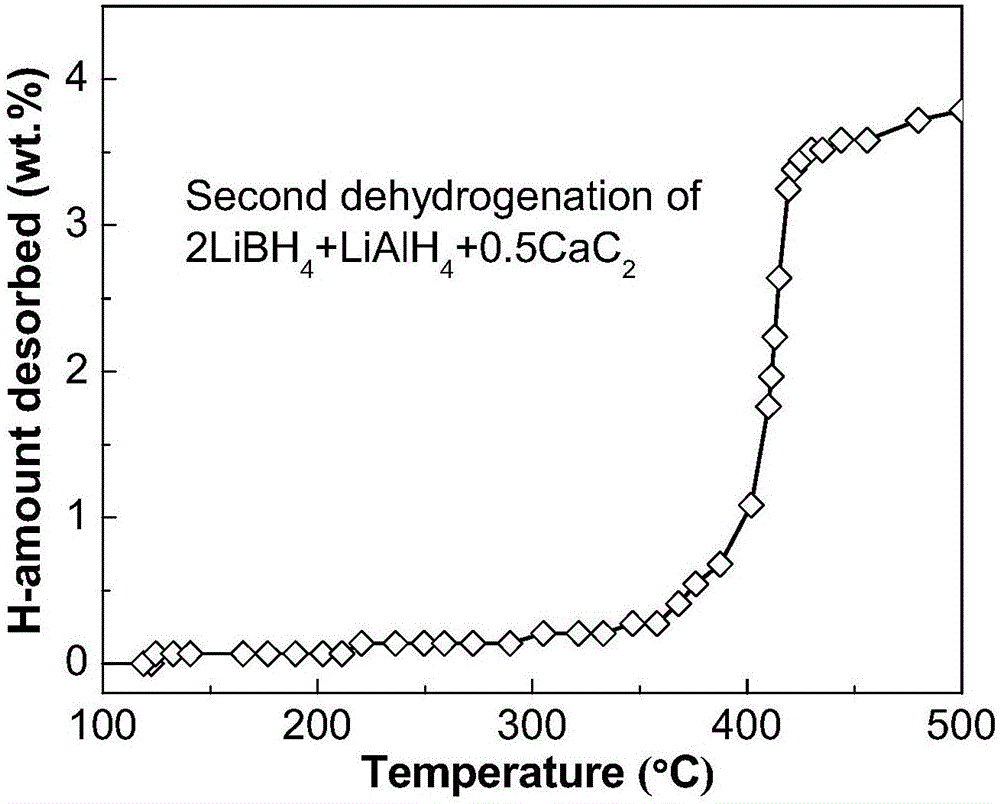
[0024] Commercially available irregular small calcium carbide (CaC 2 , purity not less than 97%) mechanically pulverized into a powder with a particle size of less than 500 μm; weigh lithium borohydride (LiBH 4 ), lithium aluminum hydride (LiAlH 4 ) and calcium carbide powder and mixed; the LiBH 4 , LiAlH 4 and CaC 2 The mixed powder is poured into a 250mL stainless steel ball mill tank, and 5 atm argon is filled into the ball mill tank; the mixed powder is milled for 10 hours with a planetary ball mill (ball-to-material ratio 20:1, speed 400rpm), namely The 2LiBH can be obtained 4 +LiAlH 4 +0.5CaC 2 Composite hydrogen storage materials. Depend on figure 1 It can be seen that the resulting 2LiBH 4 +LiAlH 4 +0.5CaC 2 The composite hydrogen storage material begins to release hydrogen at 110°C, and basically ends at 440°C, with a hydrogen release amount of 6.3wt.%. Compared to pure LiBH 4 The hydrogen is released slowly from 320°C, and the amount of hydrogen released...
Embodiment 2
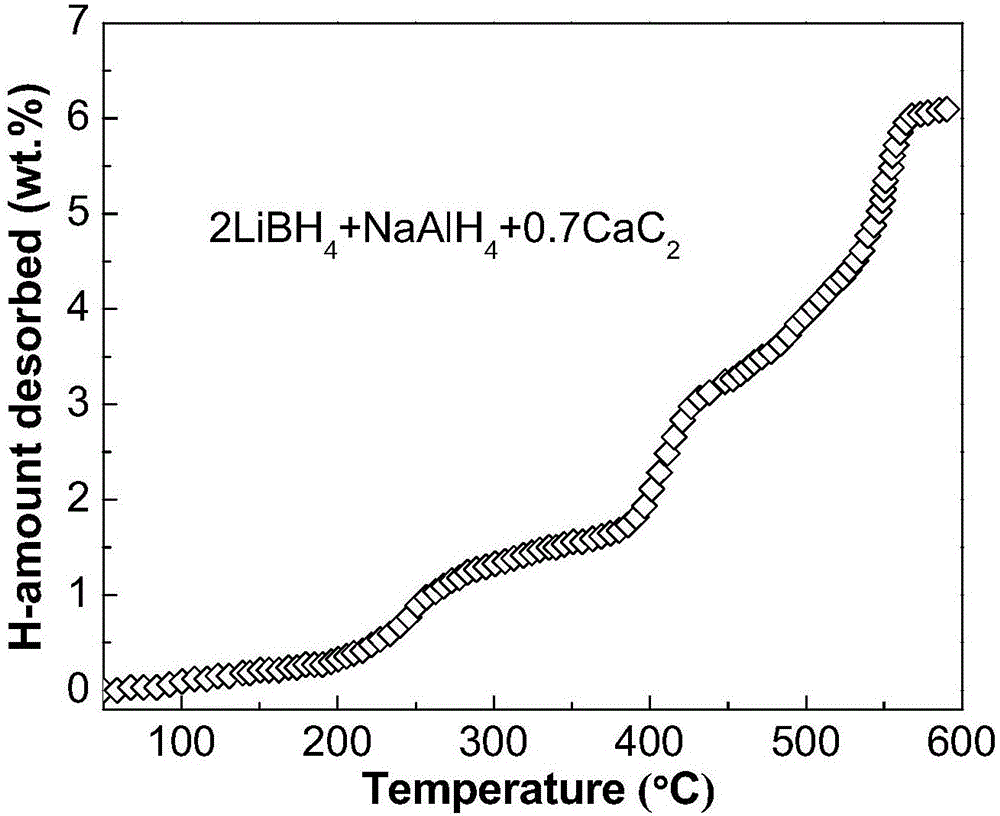
[0026] Commercially available irregular small calcium carbide (CaC 2 , purity not less than 97%) mechanically pulverized into a powder with a particle size of less than 500 μm; weigh LiBH respectively according to the molar ratio of 2:1:0.7 4 , sodium aluminum hydride (NaAlH 4 ) and CaC 2 powder and mix; LiBH 4 , NaAlH 4 and CaC 2 The mixed powder is poured into a stainless steel ball mill tank with a volume of 250 mL, and 1 atm argon gas is filled into the ball mill tank; the mixed powder is ball milled for 8 hours using a planetary ball mill (ball-to-material ratio 30:1, speed 400 rpm), namely The 2LiBH can be obtained 4 +NaAlH 4 +0.7CaC 2 Composite hydrogen storage materials. Depend on image 3 It can be seen that the resulting 2LiBH 4 +NaAlH 4 +0.7CaC 2 The composite hydrogen storage material has obvious hydrogen desorption phenomenon at 200°C, and the hydrogen desorption is basically completed at 570°C, with a hydrogen desorption amount of 6.1wt.%.
Embodiment 3
[0028] Commercially available irregular small calcium carbide (CaC 2 , purity not less than 97%) mechanically pulverized into powders with a particle size of less than 500 μm; weigh LiBH respectively according to the molar ratio of 2:1:1 4 , LiAlH 4 and CaC 2 powder and mix; LiBH 4 , LiAlH 4 and CaC 2 The mixed powder is poured into a stainless steel ball mill tank with a volume of 250 mL, and 1 atm argon gas is filled into the ball mill tank; the mixed powder is ball milled for 8 hours using a planetary ball mill (ball-to-material ratio 30:1, speed 400 rpm), namely The 2LiBH can be obtained 4 +LiAlH 4 +CaC 2 Composite hydrogen storage materials. Depend on Figure 4 It can be seen that the resulting 2LiBH 4 +LiAlH 4 +CaC 2 The composite hydrogen storage material begins to release hydrogen at 100°C, and basically ends at 425°C, with a hydrogen release amount of 5.3wt.%.
PUM
| Property | Measurement | Unit |
|---|---|---|
| particle size | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com