Patents
Literature
174 results about "Lithium borohydride" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
Lithium borohydride (LiBH₄) is a tetrahydroborate and known in organic synthesis as a reducing agent for esters. Although less common than the related sodium borohydride, the lithium salt offers some advantages, being a stronger reducing agent and highly soluble in ethers, whilst remaining safer to handle than lithium aluminium hydride.
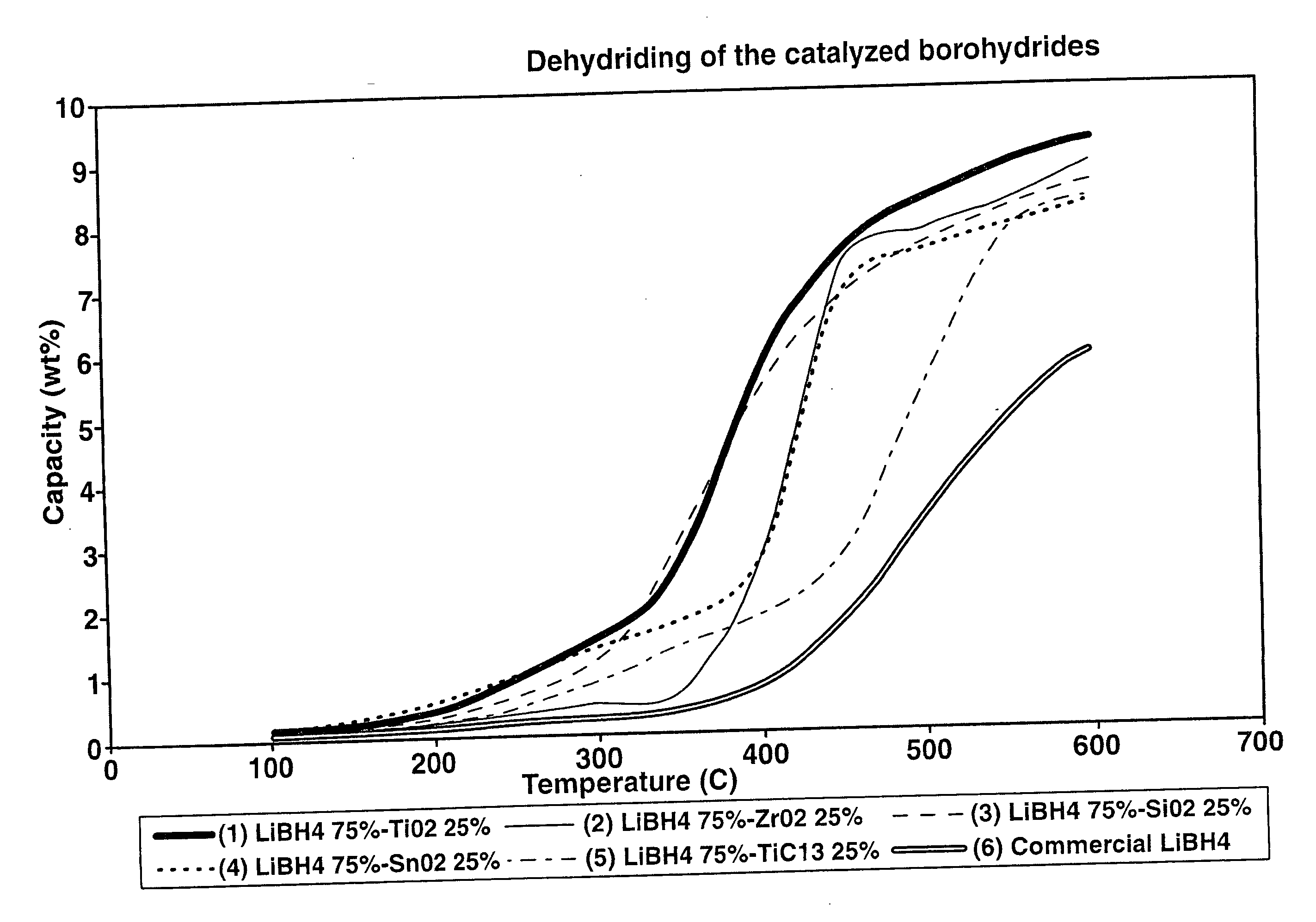
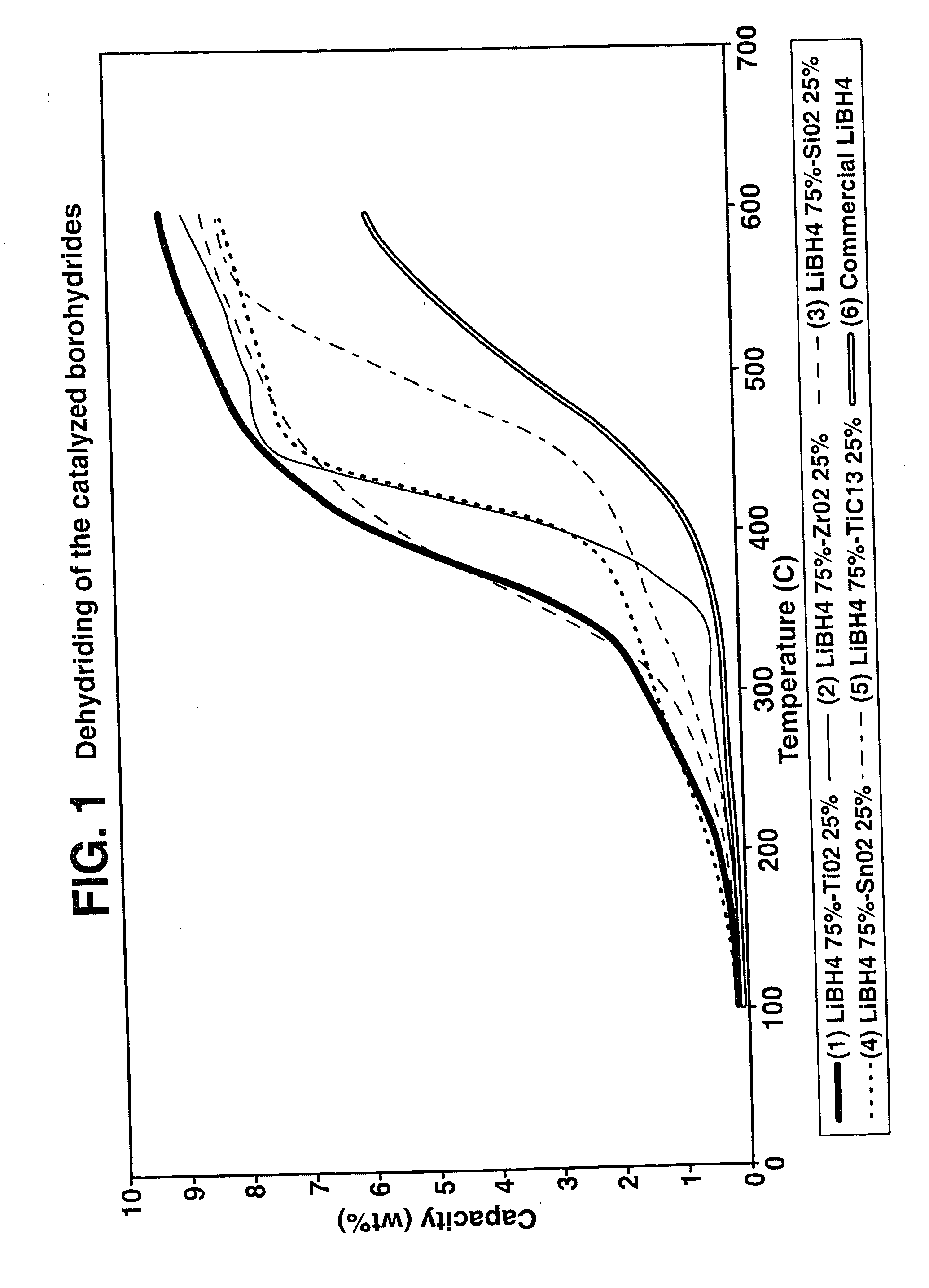
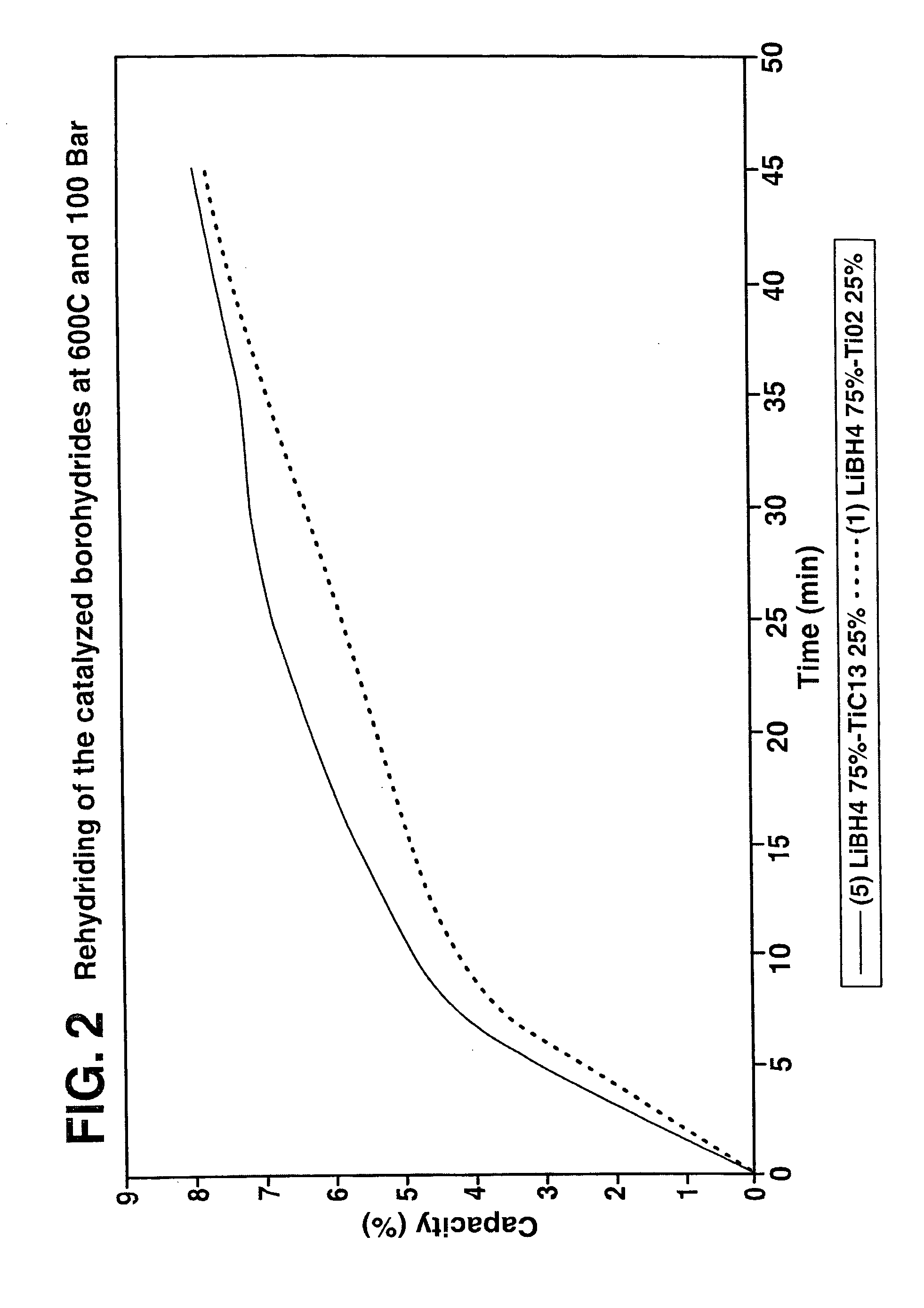
Destabilized and catalyzed borohydrided for reversible hydrogen storage
InactiveUS20060194695A1Reduce the temperatureImproved hydrogen binding/release kineticsHydrogenPhysical/chemical process catalystsIndiumCobalt
A hydrogen storage material and process is provided in which catalyzed alkali borohydride materials and partially substituted borohydride materials are created and which may contain effective amounts of catalyst(s) which include transition metal oxides, halides, and chlorides of titanium, zirconium, tin, vanadium, iron, cobalt and combinations of the various catalysts and the destabilization agents which include metals, metal hydrides, metal chlorides and complex hydrides of magnesium, calcium, strontium, barium, aluminum, gallium, indium, thallium and combinations of the various destabilization agents. When the catalysts and destabilization agents are added to an alkali borodydride such as a lithium borohydride, the initial hydrogen release point of the resulting mixture is substantially lowered. Additionally, the hydrogen storage material may be rehydrided with weight percent values of hydrogen of at least about nine percent.
Owner:SAVANNAH RIVER NUCLEAR SOLUTIONS
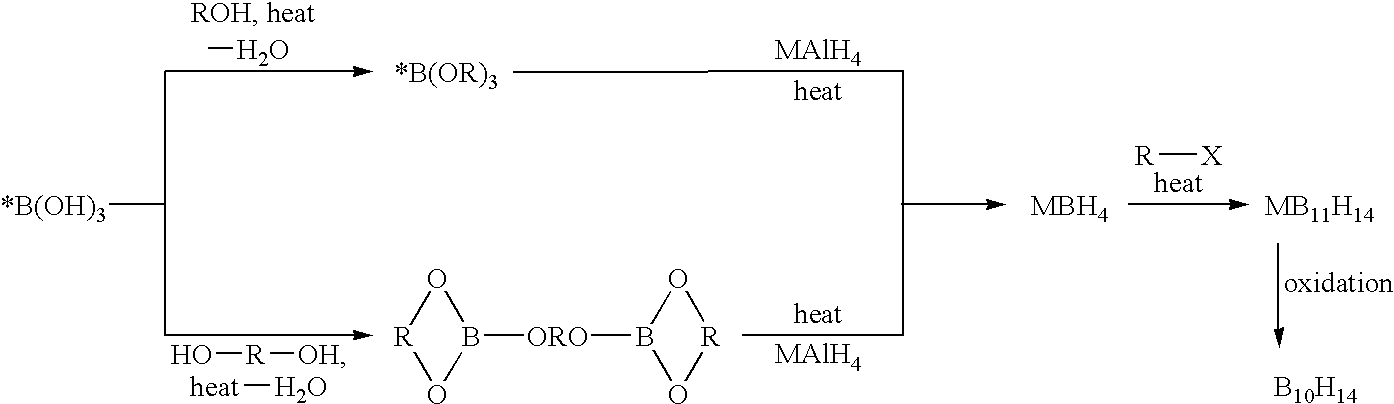
Methods of synthesis of isotopically enriched borohydride and methods of synthesis of isotopically enriched boranes
InactiveUS7641879B2High yieldPeroxides/peroxyhydrates/peroxyacids/superoxides/ozonidesMonoborane/diborane hydridesIsotopeBoric acid
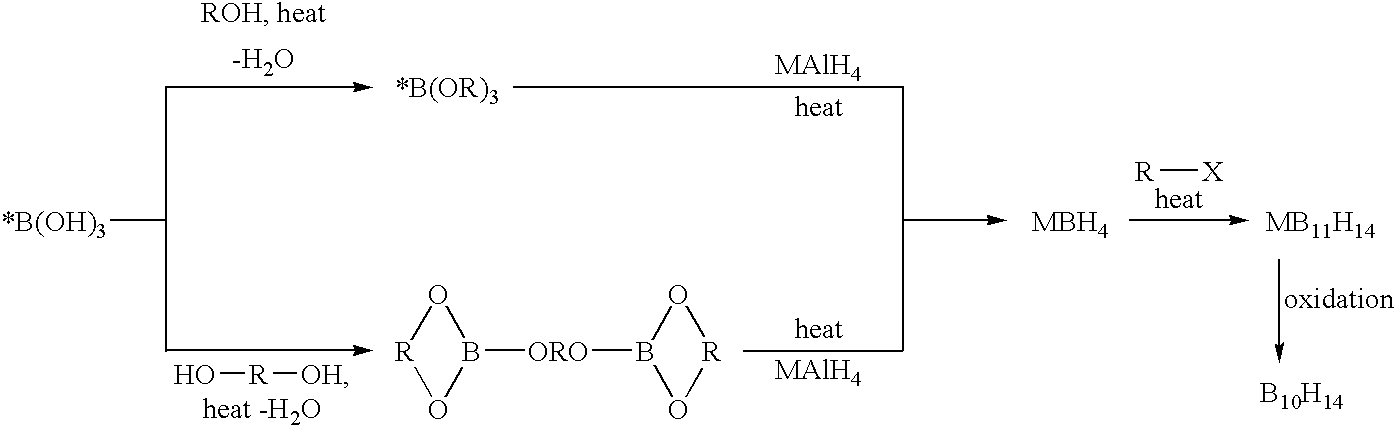
The invention provides new methods for the synthesis of isotopically enriched metal borohydrides, metal tetrahydroundecaborate salts, and decaborane from isotopically enriched 10B-boric acid or 11B-boric acid. The invention is particularly useful for synthesis of isotopically enriched sodium or lithium borohydride, MB11H14 (where M is Li, Na, K, or alkylammonium), and decaborane.
Owner:SEMEQUIP
Synthetic method of hydroxytyrosol
InactiveCN103664536AMild reaction conditionsLower reaction costOrganic chemistryOrganic compound preparationChemical synthesisHydroxytyrosol
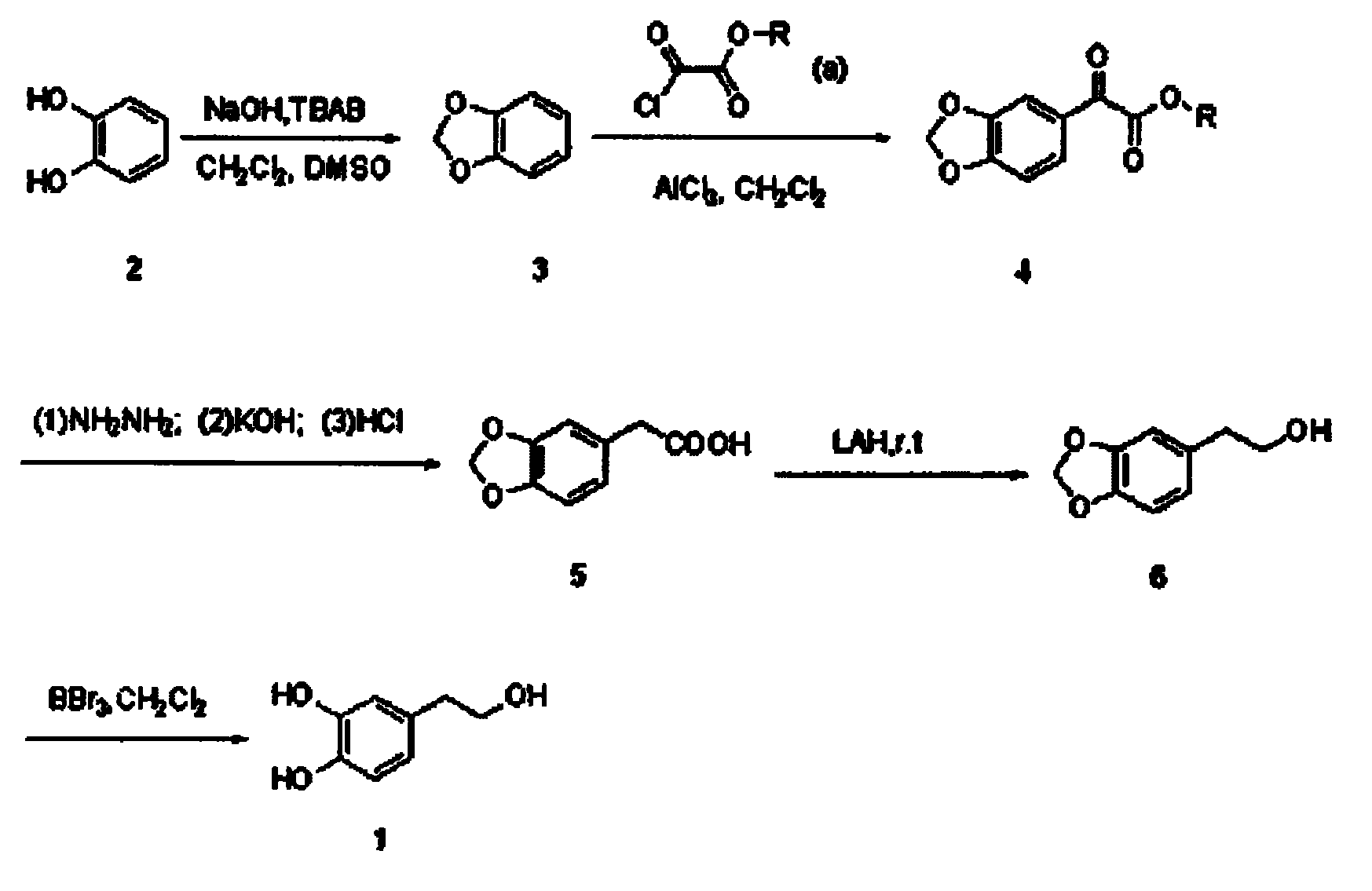
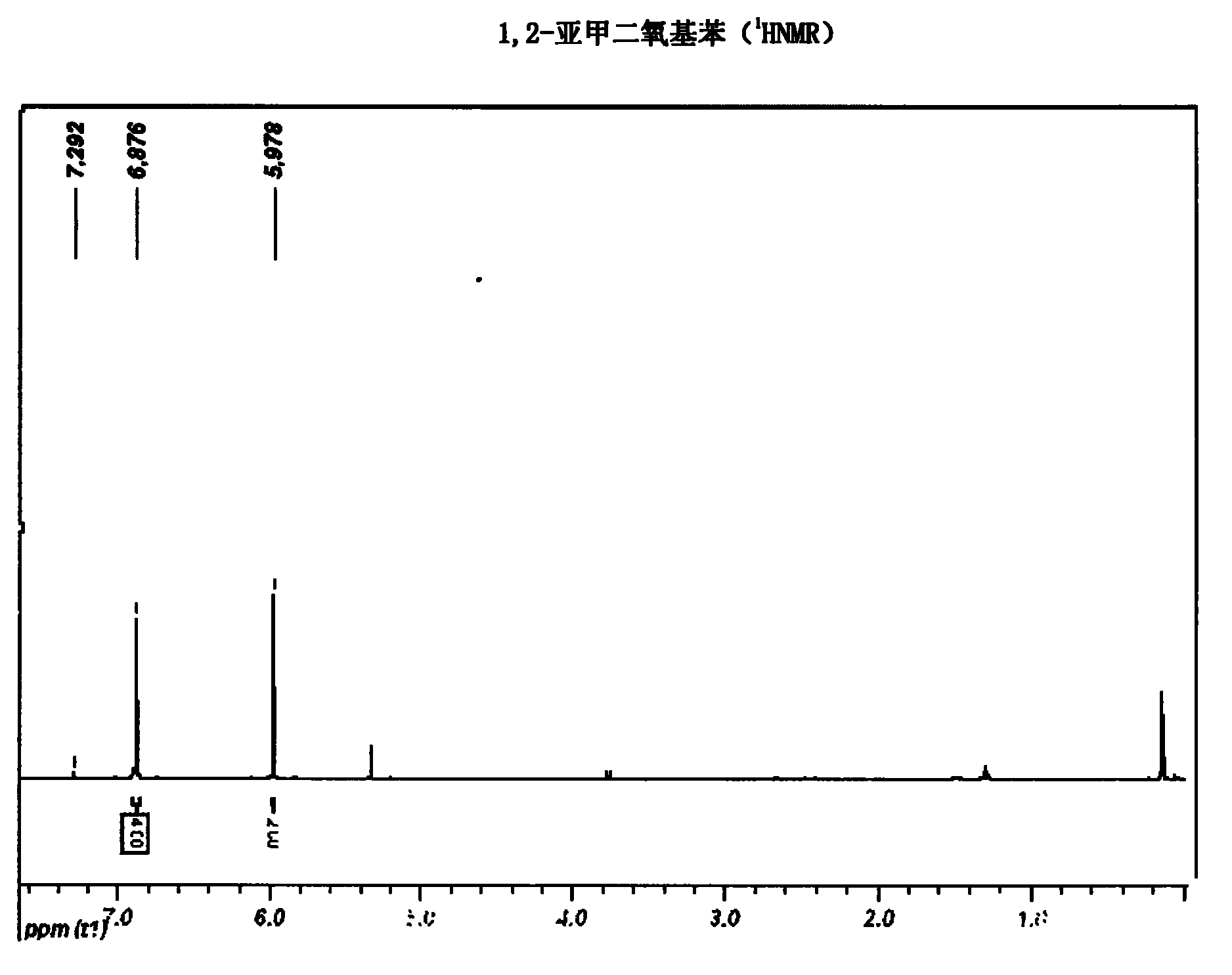
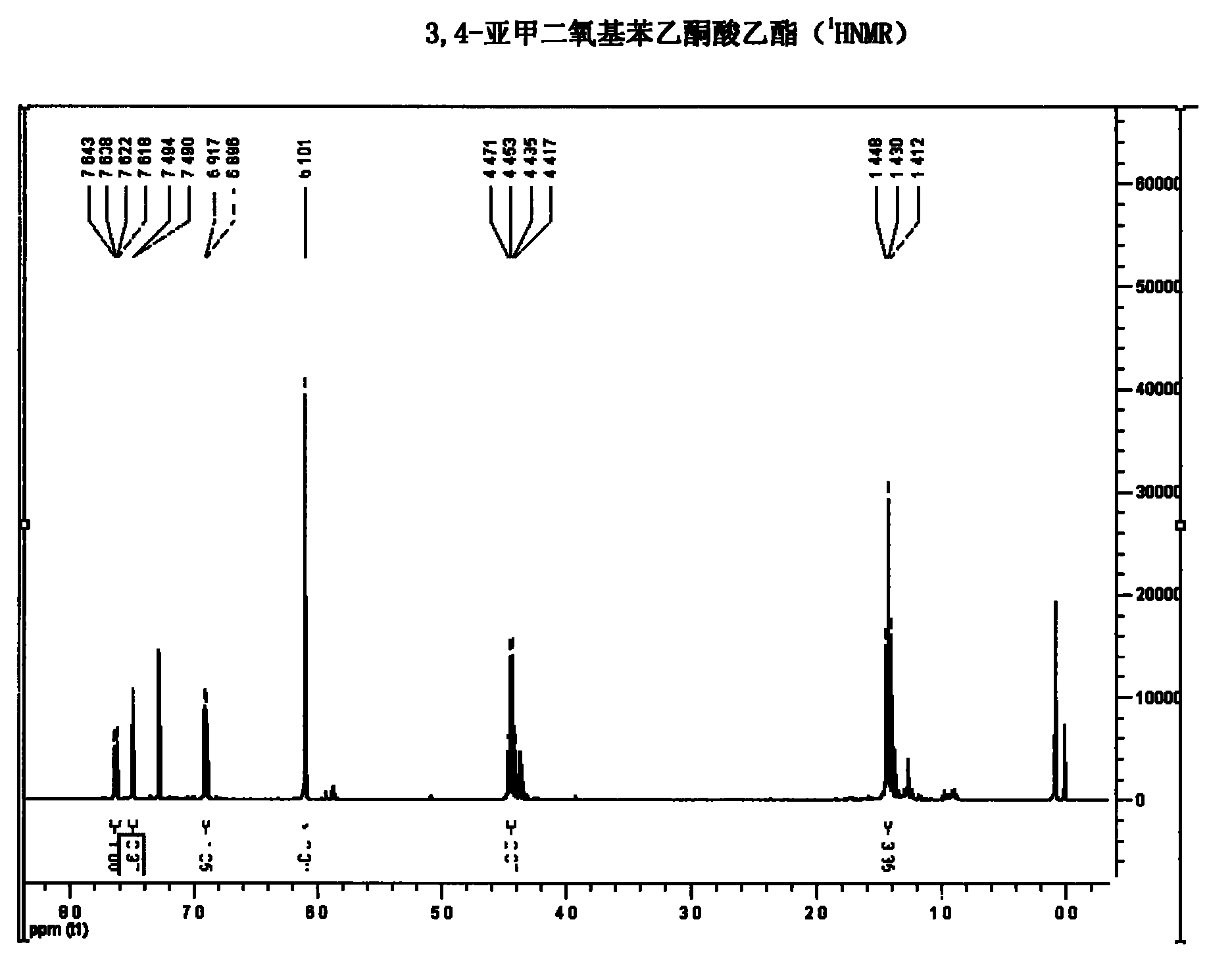
The invention belongs to the technical field of medicament synthesis, and in particular relates to a chemical synthetic method of hydroxytyrosol. The chemical synthetic method comprises the steps of (1) protecting two phenolic hydroxyl groups of catechol by using dichloromethane, and enabling catechol to react with dichloromethane to prepare 1,2-methylenedioxybenzene; (2) enabling 1,2-methylenedioxybenzene to react with various monoesters of oxalyl chloride to prepare 3,4-methylenedioxy phenylglyoxylic acid ester; (3) preparing 3,4-methylenedioxy phenylacetic acid by using 3,4-methylenedioxy phenylglyoxylic acid ester through a Wollff-kishner-Huang Minglong reduction reaction; and (4) reducing the 3,4-methylenedioxy phenylacetic acid by using lithium aluminum hydride, lithium borohydride or sodium borohydride to prepare 3,4-methylenedioxy phenethyl alcohol, and then removing methylene protection of the 3,4-methylenedioxy phenethyl alcohol by using boron tribromide or palladium on activated carbon to prepare hydroxytyrosol. A reactive reagent used in the synthetic method disclosed by the invention is easy to obtain and low in price, the reaction condition is mild, and the final yield of the whole reaction is 23%.
Owner:TIANJIN UNIVERSITY OF SCIENCE AND TECHNOLOGY
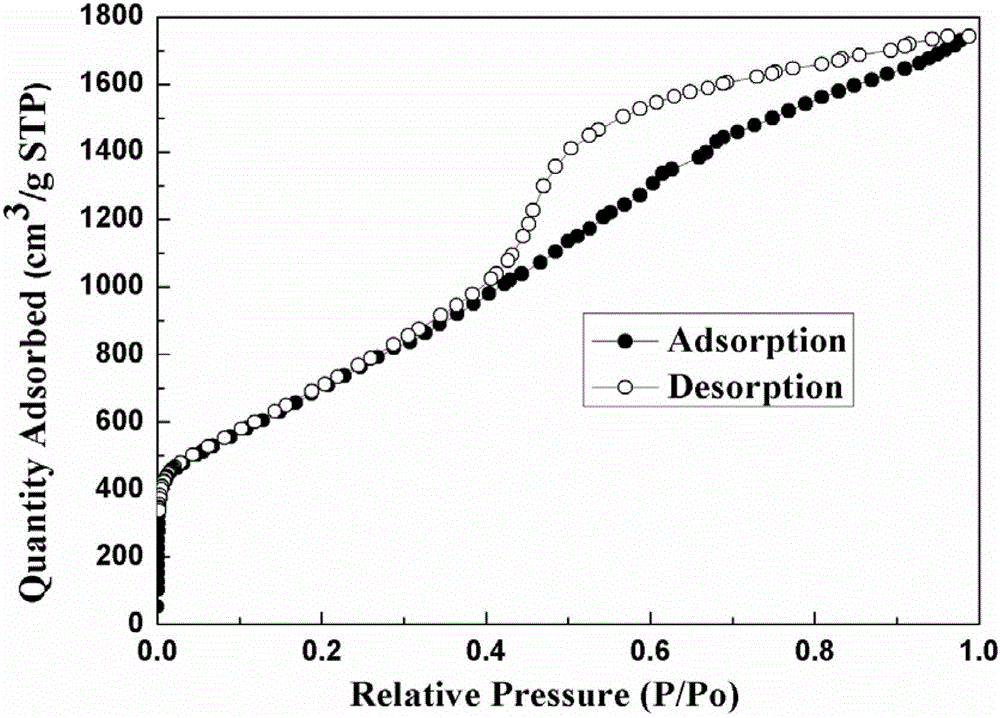
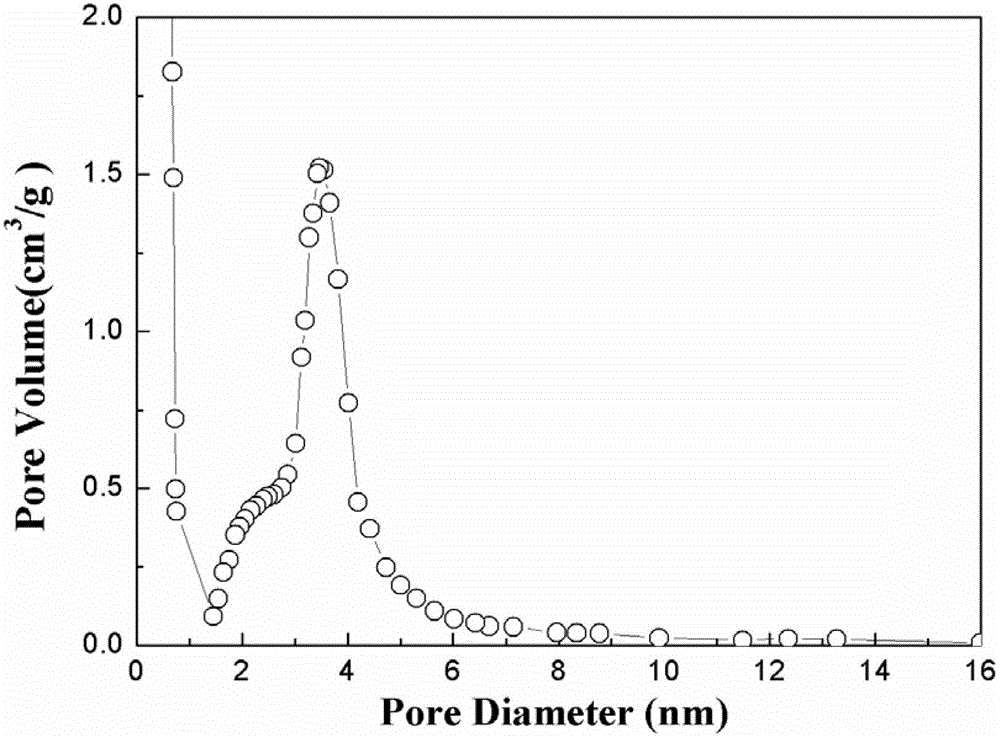
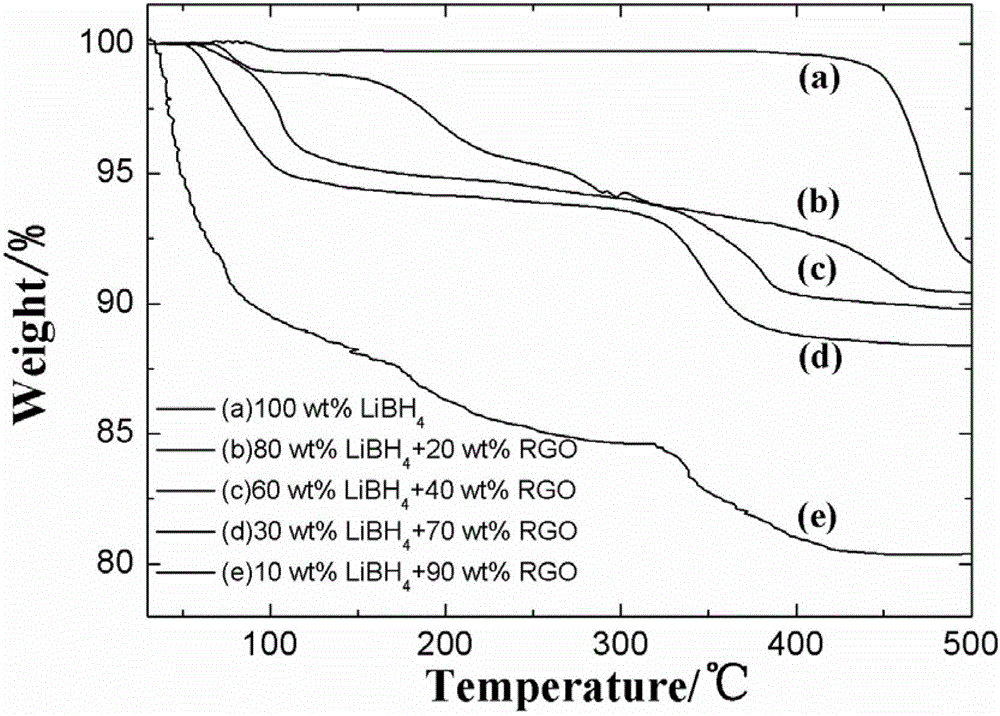
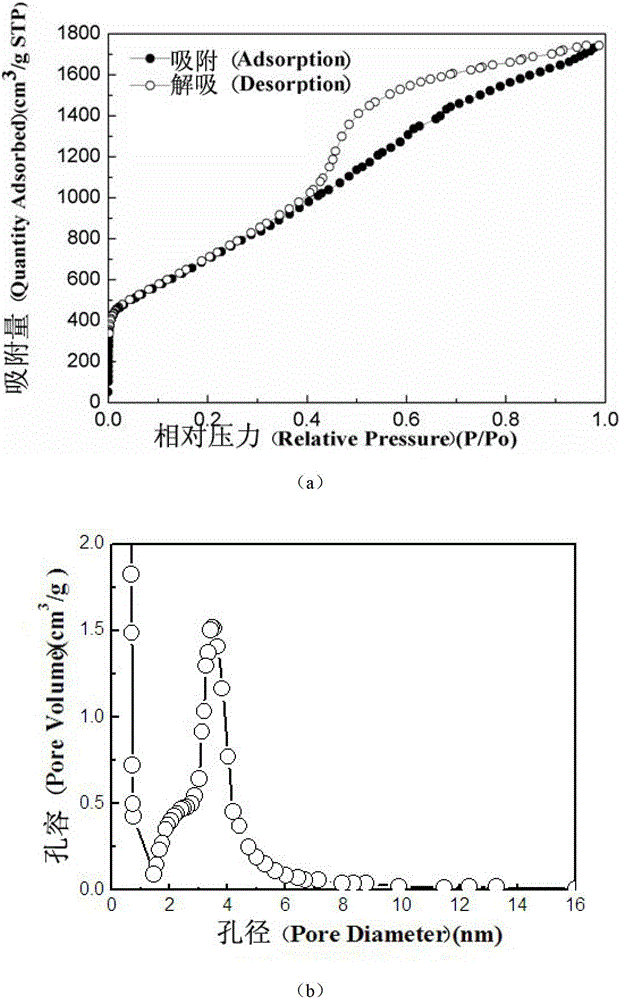
High-hydrogen-storage-capacity lithium borohydride/graphene (LiBH4/RGO) composite hydrogen storage material and preparation method thereof
InactiveCN102718183AMake up for the shortcomings of high hydrogen desorption temperatureHydrogen productionRedoxPore diameter
The invention relates to the field of the modification of hydrogen storage materials and provides a high-hydrogen-storage-capacity lithium borohydride / graphene (LiBH4 / RGO) composite hydrogen storage material and a preparation method thereof. According to the preparation method, the LiBH4 is uniformly dispersed into pore channels of the RGO which is prepared through a chemical redox method by adopting a melt infiltration method or a high-speed ball milling method under the protective atmosphere of inert gas. In the composite material, the RGO which is prepared through the chemical redox method has the pore diameter of 2-10 nm, the pore volume of 0.08-1.9 cm<3> / g and the specific surface area of 800-2540 m<2> / g, and the mass percent of the LiBH4 is 10-80 wt%. According to the LiBH4 / RGO composite hydrogen storage material provided by the invention, the initial hydrogen generation temperature is lower than 100 DEG C, the hydrogen generation volume at the temperature below 500 DEG C is 5-18 wt%, and the LiBH4 / RGO composite hydrogen storage material can be applied to the fields of hydrogen supply sources of fuel cells, hydrogen energy source vehicles and the like.
Owner:CHANGZHOU UNIV +1
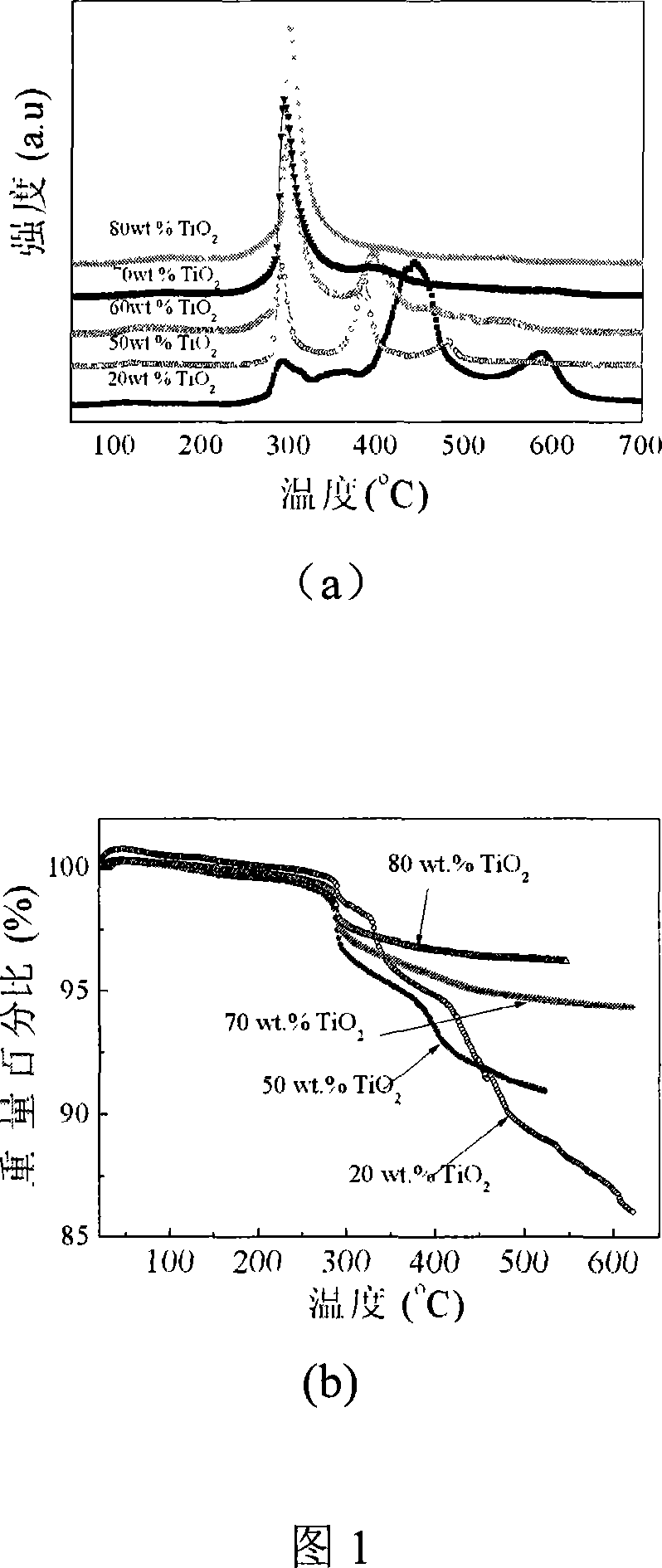
Lithium borohydride hydrogen storage material decorated by oxide and preparation method thereof
InactiveCN101054162ASimple methodLow hydrogen release temperatureOther chemical processesMonoborane/diborane hydridesBall millAtmosphere
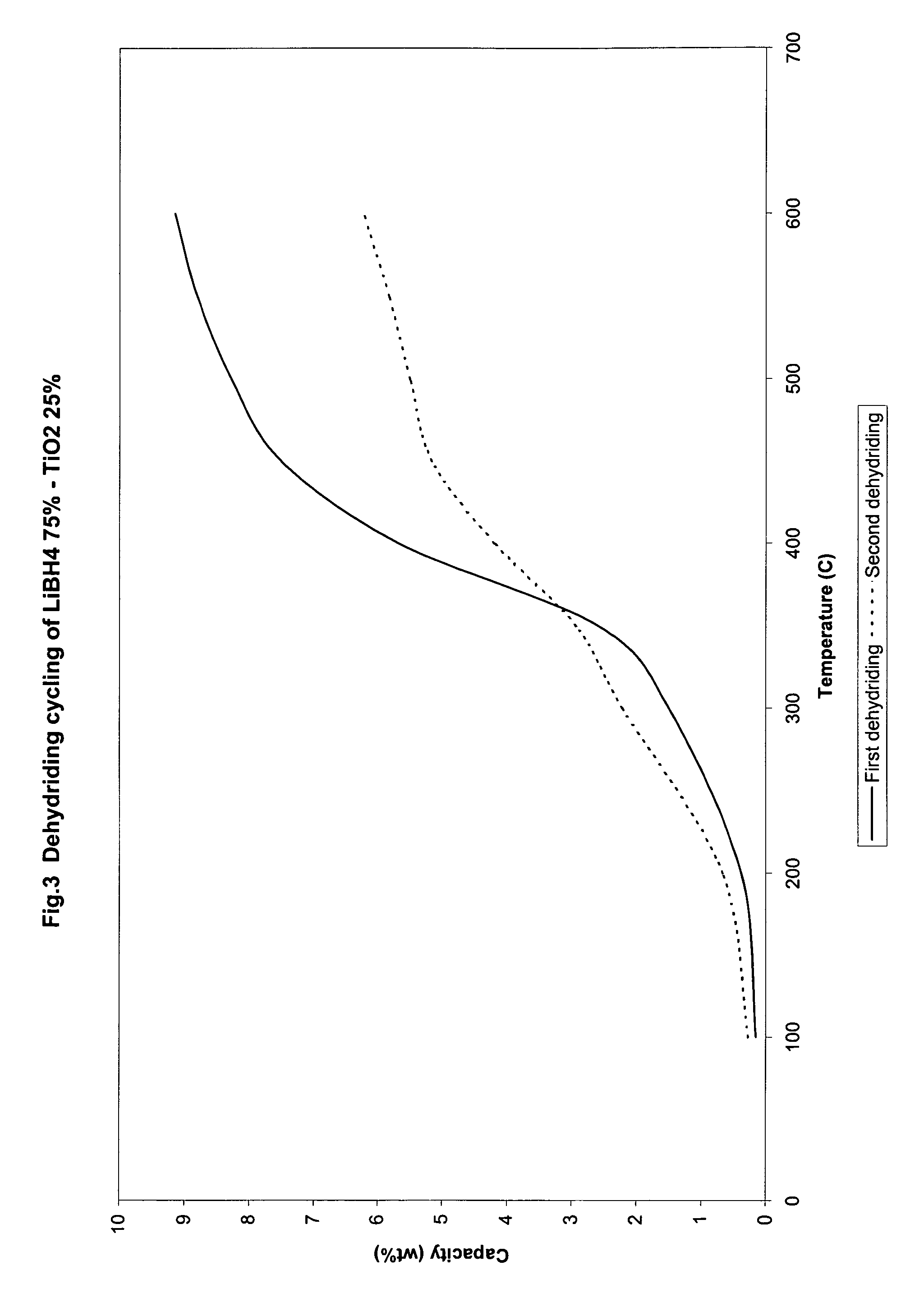
The invention relates to liborohydride hydrogen storage material and a preparation method thereof, which is characterized in that the formula of hydrogen storage material is (100-x)LiBH4+XMeO, wherein MeO is a adorning oxide, weight percent of X is 10-80%. After blending LiBH4 and the oxide according to the formula, the mixture is ball milled in protection of inert atmosphere to process surface treatment. The oxide is optional one of TiO2, Fe2O3, ZrO2, V2O5, SiO2, Al2O3, Al2O3-SiO2 or TiO2-SiO2. The intial hydrogen temperature of hydrogen storage material of the invention is lower than 100 DEG C, hydrogen amount is 3-6.5% at lower than 300 DEG C.
Owner:SHANGHAI INST OF MICROSYSTEM & INFORMATION TECH CHINESE ACAD OF SCI
Catalyzed borohydrides for hydrogen storage
InactiveUS20060046930A1Reduce the temperatureOther chemical processesMonoborane/diborane hydridesChlorideTitanium
A hydrogen storage material and process is provided in which alkali borohydride materials are created which contain effective amounts of catalyst(s) which include transition metal oxides, halides, and chlorides of titanium, zirconium, tin, and combinations of the various catalysts. When the catalysts are added to an alkali borodydride such as a lithium borohydride, the initial hydrogen release point of the resulting mixture is substantially lowered. Additionally, the hydrogen storage material may be rehydrided with weight percent values of hydrogen at least about 9 percent.
Owner:SAVANNAH RIVER NUCLEAR SOLUTIONS
Method of controlled alcoholysis and regeneration of a borohydride
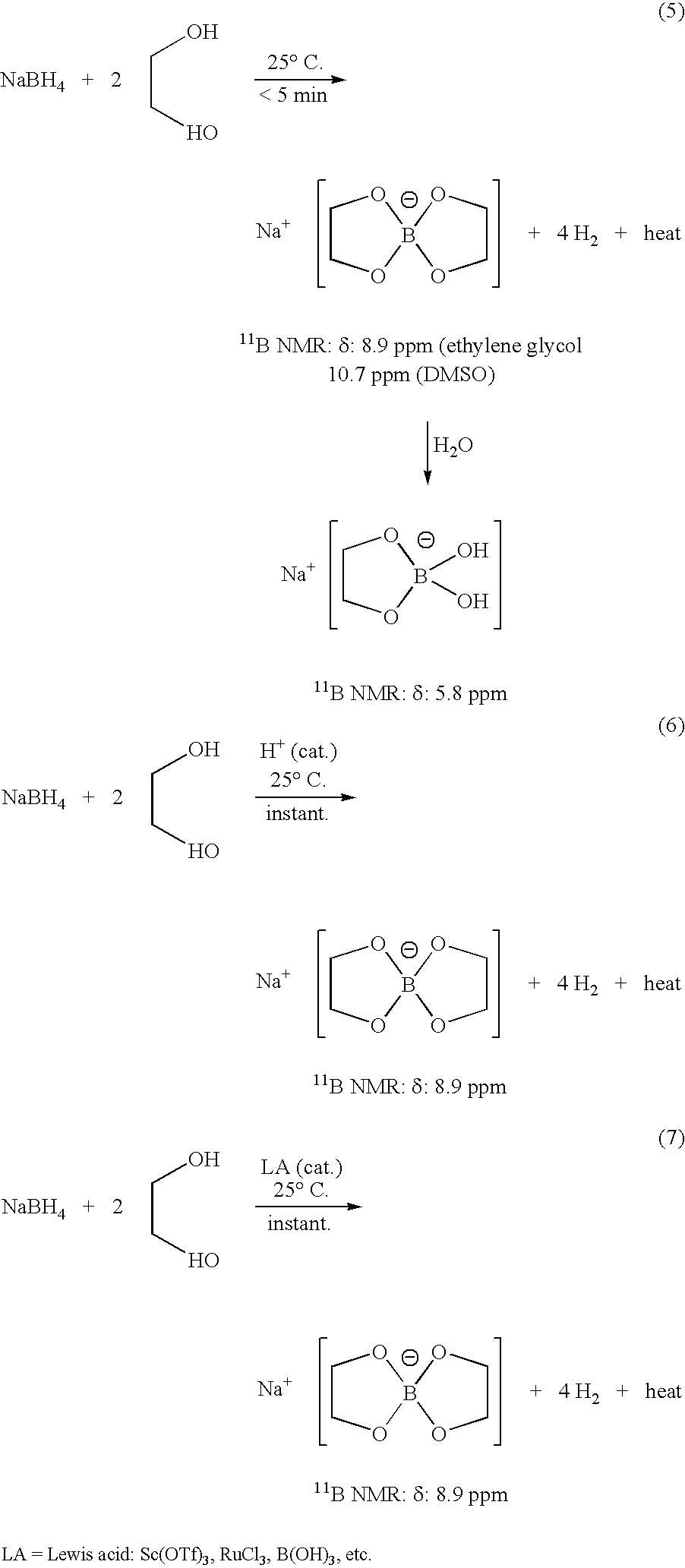
InactiveUS20050255024A1Efficient procedureEffective protocolMonoborane/diborane hydridesBoron/boridesSodium borohydrideBoric acid
Methods of controlled hydrolysis / alcoholysis and regeneration of a borohydride are disclosed. Examples of the present invention show that hydrolysis of sodium borohydride or lithium borohydride with dilute acid provides simultaneous generation of H2 and boric acid for recycling. Other examples of the present invention show methods for regenerating a borohydride by reacting an aluminum hydride to a borate compound to provide a regenerated borohydride.
Owner:PURDUE RES FOUND INC
Me-RGO (Reduced Graphene Oxide)/LiBH4 hydrogen storage material with high hydrogen storage capacity and preparation methods of Me-RGO/LiBH4 hydrogen storage material
InactiveCN102910581AThe initial hydrogen release temperature decreasesIncrease profitHydrogen productionEvaporationIon exchange
The invention provides a Me-RGO (Reduced Graphene Oxide) modified LiBH4 hydrogen storage material with high hydrogen storage capacity and preparation methods of the Me-RGO modified LiBH4 hydrogen storage material. A uniformly-dispersed Me-RGO catalyst with a high special surface is compounded on the surface of the hydrogen storage material LiBH4 at the inert gas shielding atmosphere by respectively using a solid-phase high-speed ball-milling method, a melt infiltration method and a liquid-phase dispersion method to prepare the hydrogen storage material with high hydrogen storage capacity. The preparation methods of the Me-RGO catalyst with high dispensability include an aqueous phase complex reduction method, a vapour phase reduction method, an ion exchange method, a sol-gel method, a hydrothermal method, a vacuum sputtering method and a gas evaporation method. Even if the content of the used Me-RGO catalyst is as low as 3wt.%, the hydrogen discharging temperature of LiBH4 can also be greatly reduced, and meanwhile, the low-temperature hydrogen discharging capacity and the circulated hydrogen absorption and discharging properties of LiBH4 can be improved.
Owner:CHANGZHOU UNIV +1
Preparation method of 3-(4-chlorobutyl)-5-cyanoindole
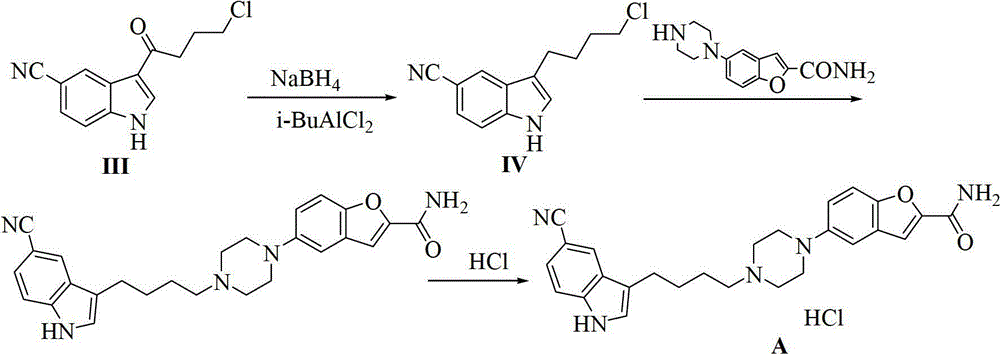
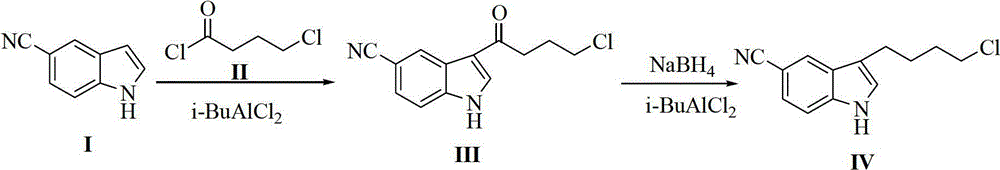
ActiveCN102875440AReduce usageOperational securityOrganic chemistryPotassium borohydrideLewis acid catalysis
The invention discloses a preparation method of 3-(4-chlorobutyl)-5-cyanoindole which is shown as the formula IV. The preparation method comprises the steps as follows: carrying out following carbonyl reduction reaction on a compound III and a hydroboron reducing agent in the solvent under the catalyzing of Lewis acid to obtain the product, wherein Lewis acid is one or more of aluminium trichloride, magnesium chloride, zinc chloride and ferric chloride; and the hydroboron reducing agent is one or more of sodium borohydride, potassium borohydride, lithium borohydride and borane. The preparation method disclosed by the invention is safe in operation, low in requirement on equipment, low in cost, simple in post-processing steps, high in yield of product, high in purity, and is suitable for industrialization.
Owner:CHIRAL QUEST (SUZHOU) CO LTD +1
Method for preparing hydroxytyrosol
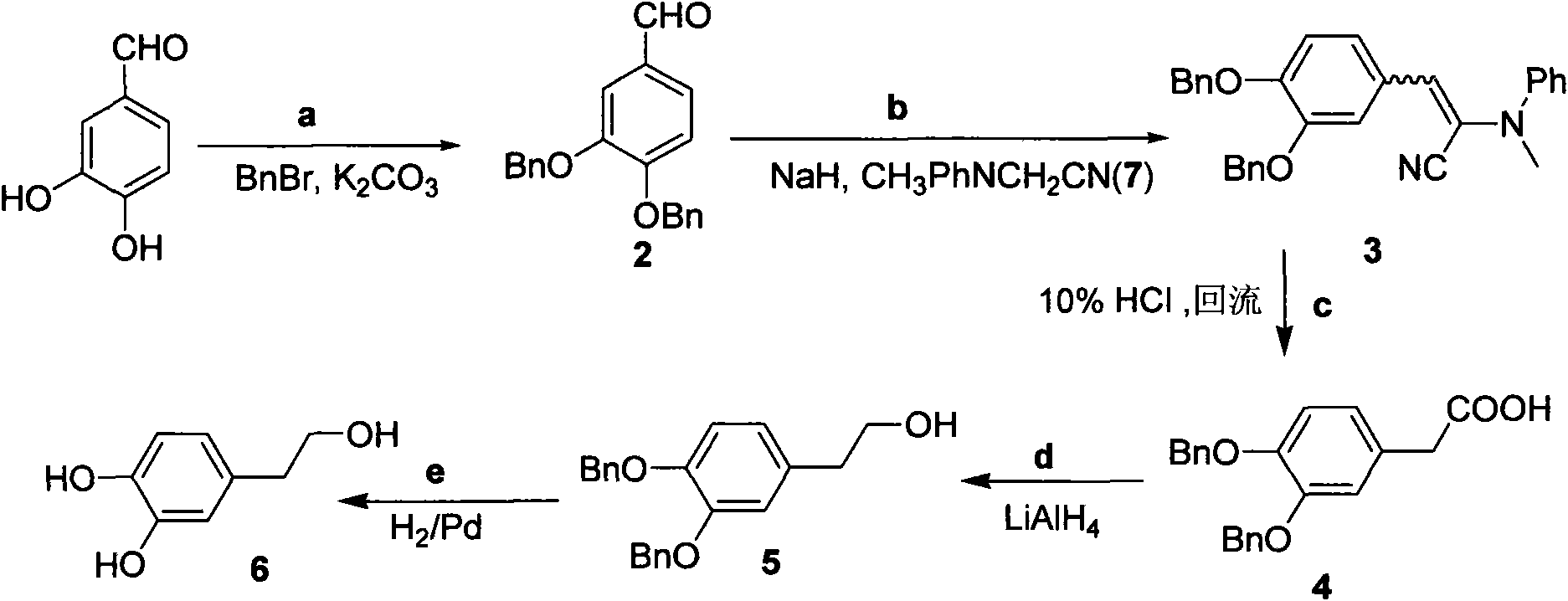
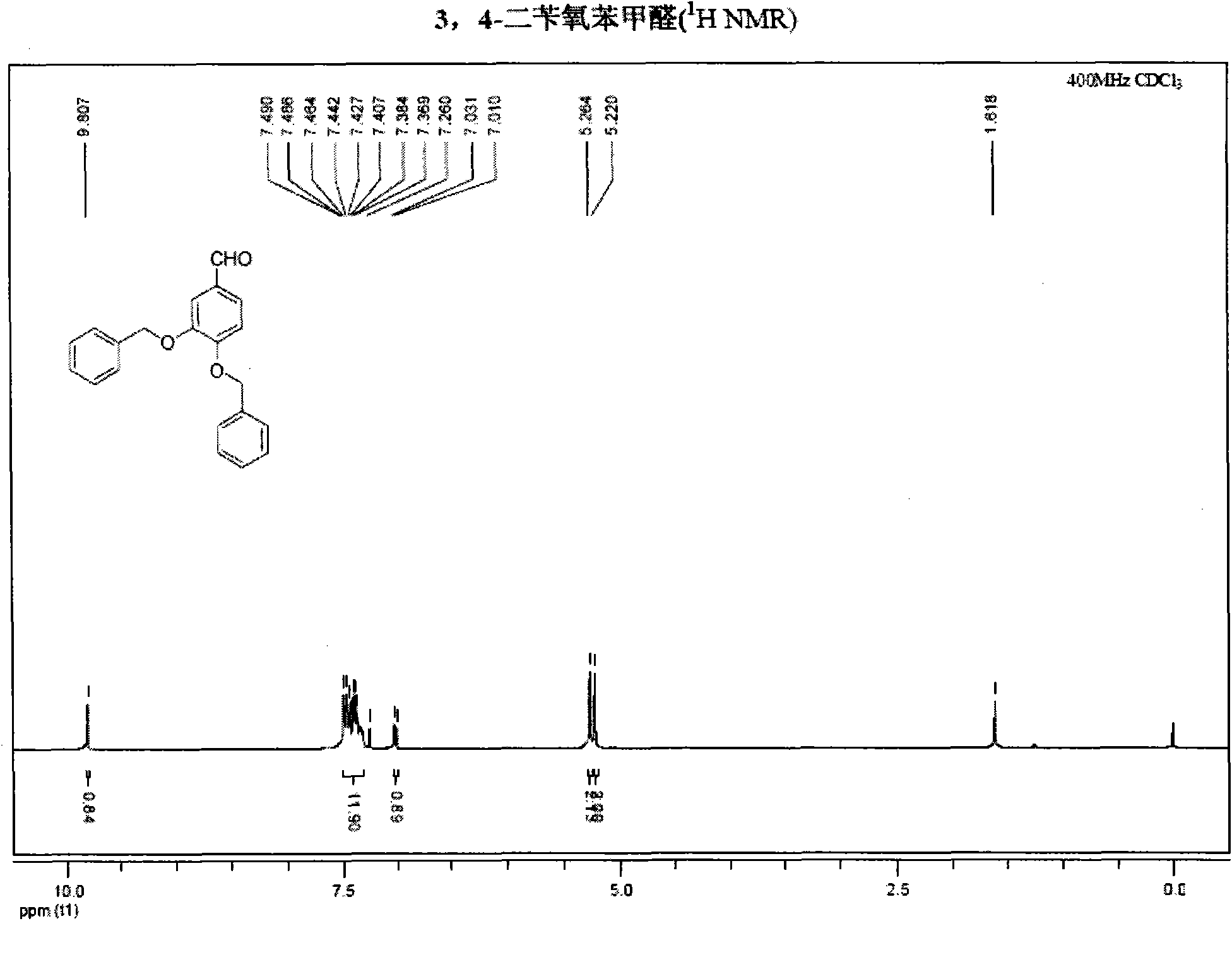
InactiveCN101891595AMild reaction conditionsFew reaction stepsOrganic chemistryOrganic compound preparationHydroxytyrosolBenzoyl bromide
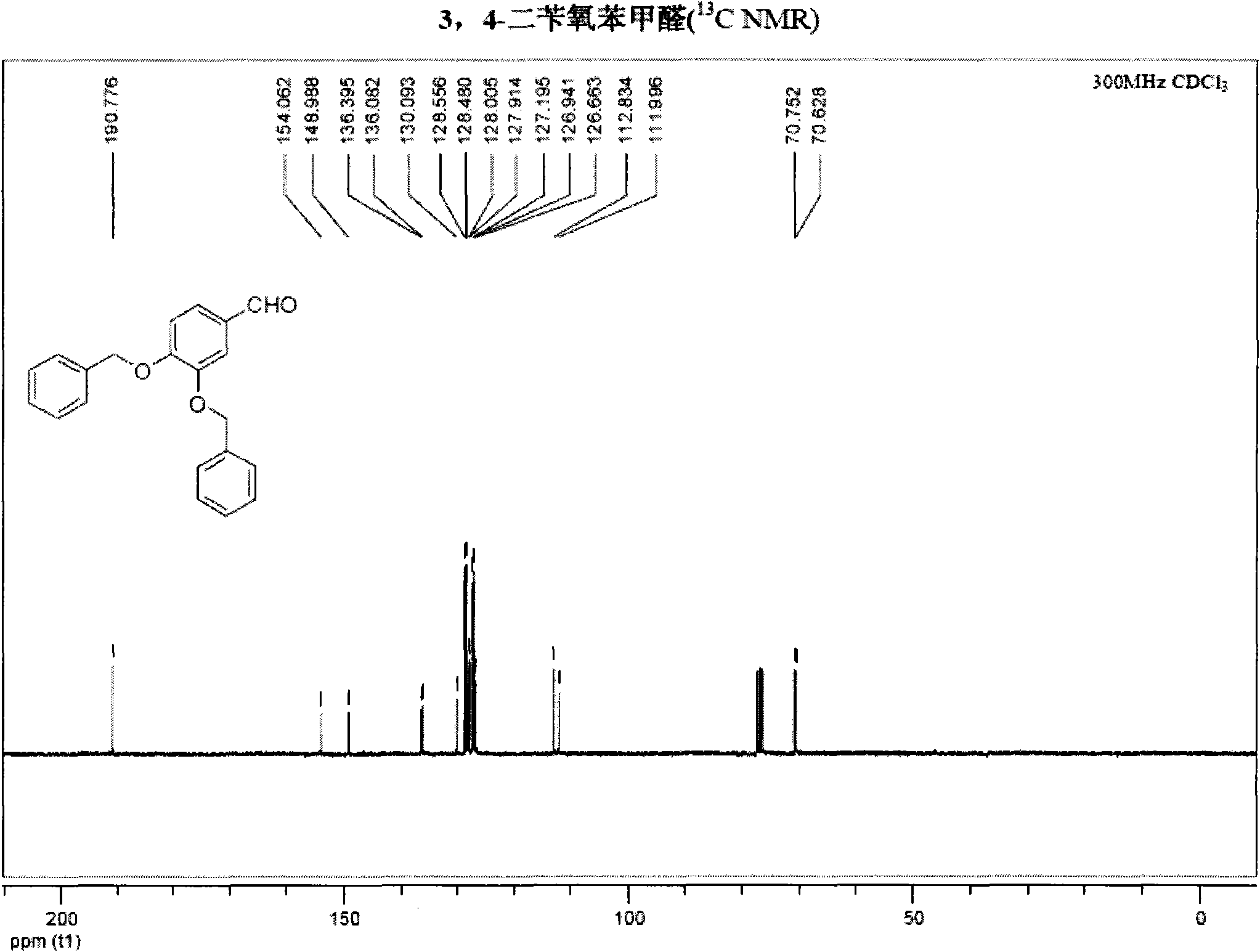
The invention belongs to the field of medicinal synthesis, and particularly relates to a method for preparing hydroxytyrosol, which comprises the following steps of: (1) protecting free hydroxyl groups at 3 and 4 positions, on 3,4-dihydroxy benzaldehyde with benzyl, namely, reacting the 3,4-dihydroxy benzaldehyde with benzyl bromide to prepare 3,4-dibenzyloxybenzaldehyde; (2) reacting N-methylaniline acetonitrile with the 3,4-dibenzyloxybenzaldehyde to prepare 3-(3,4-dibenzyloxyphenyl)-2-(methylphenylamino) acrylonitrile, and hydrolyzing the 3-(3,4-dibenzyloxyphenyl)-2-(methylphenylamino) acrylonitrile under acidic condition to prepare a 3,4-dibenzylosyphenylacetic acid; (3) reducing a carboxyl group of the 3,4-dibenzylosyphenylacetic acid with lithium borohydride, lithium aluminum hydride or sodium borohydride to prepare 3,4-dibenzyloxyphenethyl alcohol; and (4) catalyzing the 3,4-dibenzyloxyphenethyl alcohol with a catalyst palladium / carbon to prepare the hydroxytyrosol. The reagents used in the method are easily obtainable and low in cost, reaction conditions are mild, and the final overall yield of the whole reaction reaches 50 to 60 percent.
Owner:SUZHOU UNIV
Ceramic metal material
The invention discloses a ceramic metal material which is composed of following raw materials including 10-15 parts of ferric oxide, 10-15 parts of aluminium oxide, 5-12 parts of nano silicon dioxide, 100-150 parts of kaolin, 80-100 parts of quartz sand, 5-10 parts of zinc oxide, 50-60 parts of zircon, 5-10 parts of silicon nitride, 5-8 parts of aluminium nitride, 20-25 parts of hydroxyapatite, 10-15 parts of paraffin, 5-10 parts of lithium borohydride, 5-10 parts of potassium borohydride and 3-8 parts of sodium triacetoxyborohyride. In the ceramic metal material, the raw materials are reasonably blended. The ceramic metal material is excellent in performance, is good in stability, is high-temperature-resistant and corrosion-resistant, is good in water-proof performance, is high in strength and can be applied in various fields.
Owner:黄惠娟
Method for synthesizing lithium borohydride
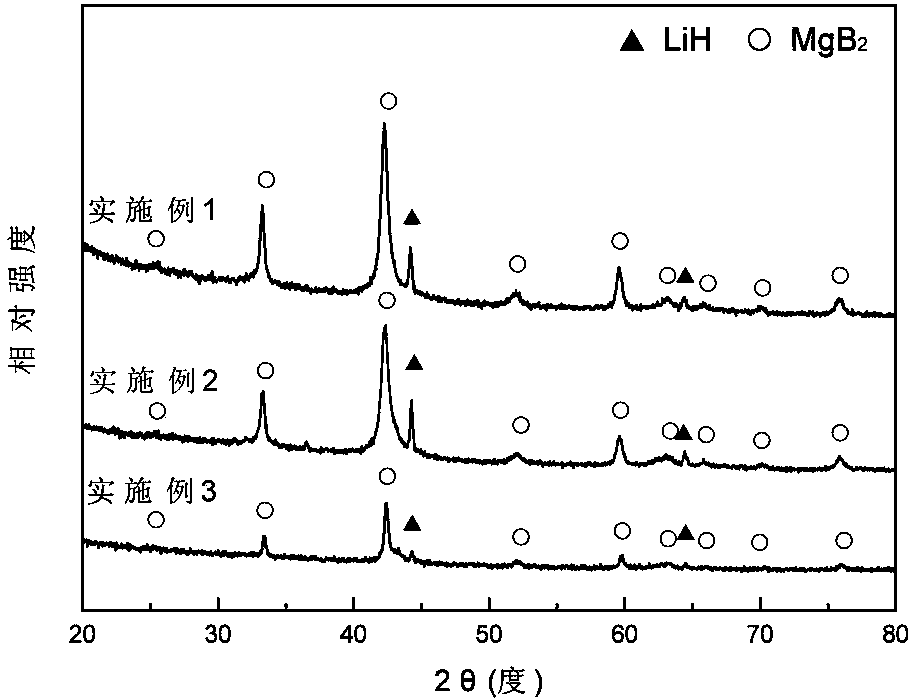
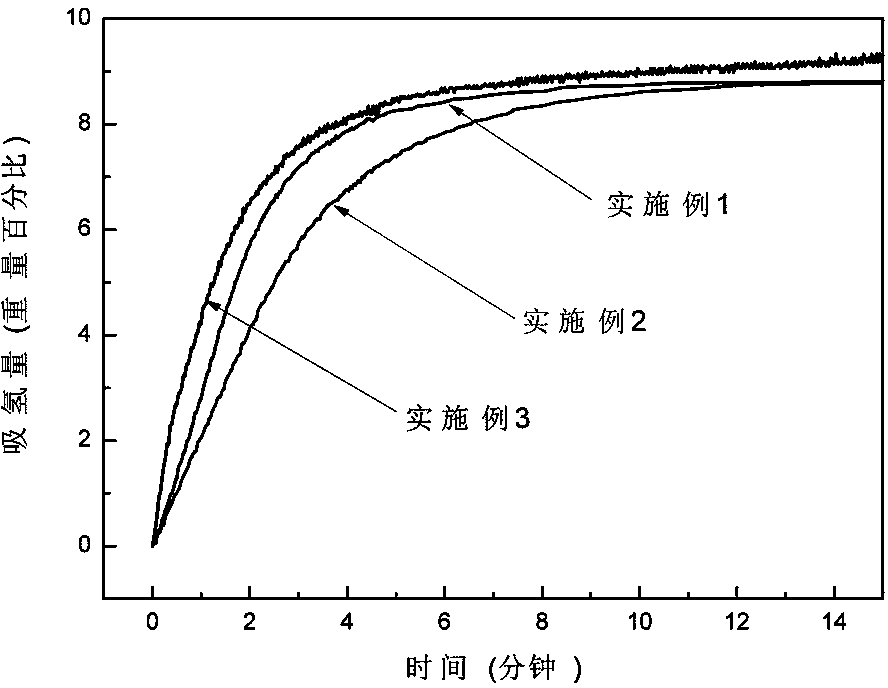
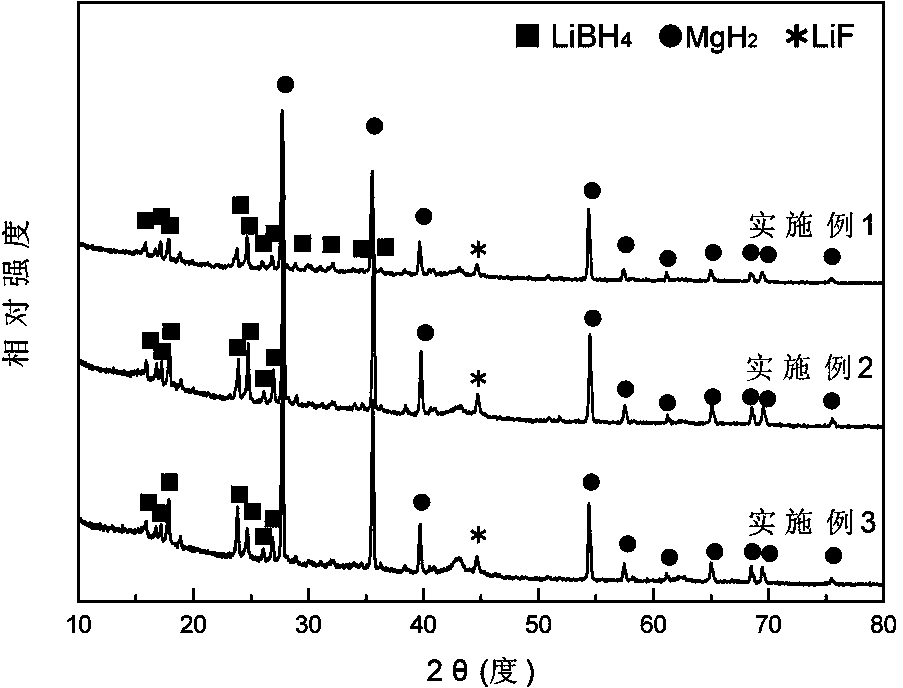
ActiveCN103922285ASimple reaction conditionsShort reaction timeMonoborane/diborane hydridesMagnesium diborideHydrogen absorption
The invention discloses a method for synthesizing lithium borohydride. The method aims at solving the problems that an existing method for preparing lithium borohydride is complex in technology, strict in synthesis condition and the like. The method comprises the following steps that in a protective atmosphere, after lithium hydride, magnesium diboride and a catalyst are mixed according to a ratio, ball-milling treatment is conducted, and a product is obtained after ball-milling treatment; a hydrogen absorption reaction is conducted on the product which is obtained after ball-milling treatment, so that a mixture of the lithium borohydride and magnesium hydride is obtained and is recorded as a reaction product; the magnesium hydride and the lithium borohydride in the reaction product are separated, so that the lithium borohydride is obtained. The method overcomes the shortages that a traditional method for preparing the lithium borohydride is complex in technology and strict in synthesis condition and the purity of the product is low and the like. The method is simple, gentle in reaction condition and short in reaction time. Synthesized LiBH4 is high in yield and purity. Meanwhile, through the method, the production period of the LiBH4 can be shortened, the production cost is reduced, the lithium borohydride can be produced in a large scale industrially, and application of the lithium borohydride is facilitated.
Owner:SICHUAN INST OF MATERIALS & TECH
Methods of synthesis of isotopically enriched borohydride and methods of synthesis of isotopically enriched boranes
InactiveUS20050169827A1High yieldMonoborane/diborane hydridesPeroxides/peroxyhydrates/peroxyacids/superoxides/ozonidesIsotopeBoric acid
The invention provides new methods for the synthesis of isotopically enriched metal borohydrides, metal tetrahydroundecaborate salts, and decaborane from isotopically enriched 10B-boric acid or 11B-boric acid. The invention is particularly useful for synthesis of isotopically enriched sodium or lithium borohydride, MB11H14 (where M is Li, Na, K, or alkylammonium), and decaborane.
Owner:SEMEQUIP
Composite hydrogen storage material and preparation method thereof
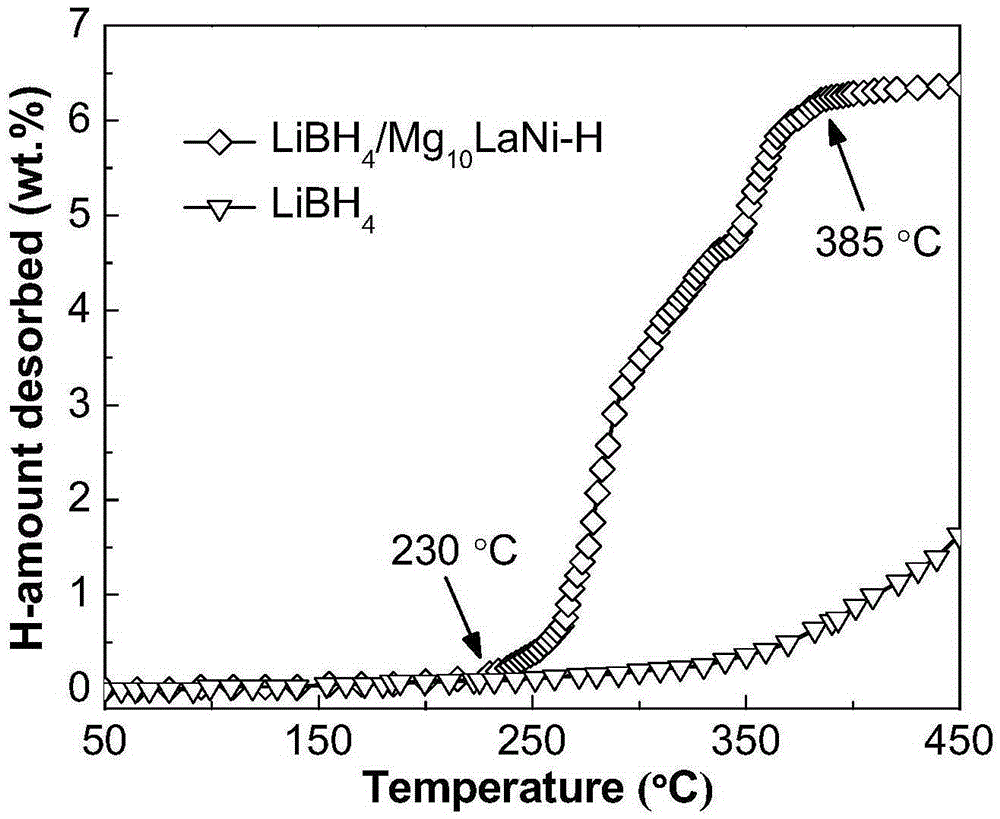
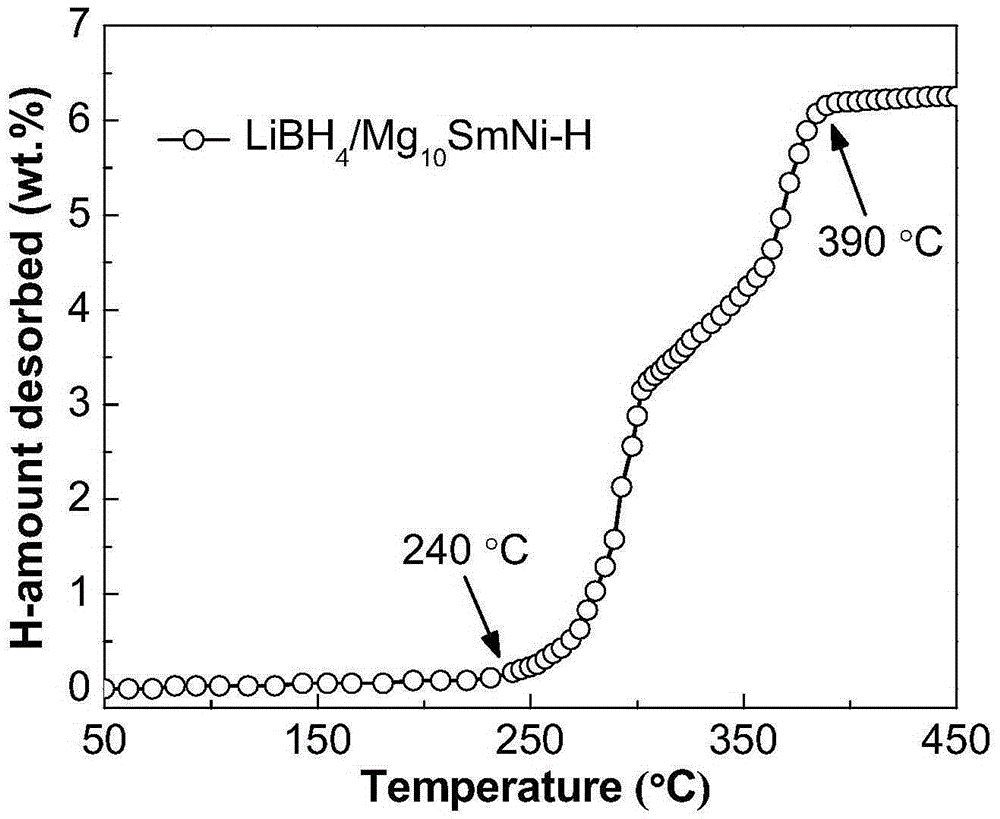
InactiveCN105271113AImprove hydrogen storage performanceShorten the diffusion distanceHydrogen productionRare earthHydrogen desorption
The invention discloses a composite hydrogen storage material and a preparation method thereof and belongs to the technical field of hydrogen storage materials. The composite hydrogen storage material comprises lithium borohydride and amorphous-state magnesium-rare earth-nickel alloy hydride in the mole ratio being (6-7):1, wherein a magnesium-rare earth-nickel alloy is Mg10LaNi or Mg10SmNi. During preparation, elementary magnesium, rare earth and nickel are taken as raw materials, an amorphous-state magnesium-rare earth-nickel alloy is obtained through a smelting and melt-spinning technology, amorphous-state magnesium-rare earth-nickel alloy hydride is obtained through ball milling and low-temperature hydrogenation, and finally, lithium borohydride and amorphous-state magnesium-rare earth-nickel alloy hydride are mixed. The provided composite hydrogen storage material has low hydrogen desorption temperature and high hydrogen desorption capacity, and a preparation process is simple, safe and reliable.
Owner:ANHUI UNIVERSITY OF TECHNOLOGY
Electrolyte separators including lithium borohydride and composite electrolyte separators of lithium-stuffed garnet and lithium borohydride
ActiveUS20190319240A1Solid electrolytesMonoborane/diborane hydridesComposite electrolyteElectrolysis
Set forth herein are compositions comprising A.(LiBH4).B.(LiX).C.(LiNH2), wherein X is fluorine, bromine, chloride, iodine, or a combination thereof, and wherein 0.1≤A≤3, 0.1≤B≤4, and 0≤C≤9 that are suitable for use as solid electrolyte separators in lithium electrochemical devices. Also set forth herein are methods of making A.(LiBH4).B.(LiX).C.(LiNH2) compositions. Also disclosed herein are electrochemical devices which incorporate A.(LiBH4).B.(LiX).C.(LiNH2) compositions and other materials.
Owner:QUANTUMSCAPE BATTERY INC
Lithium borohydride compound fast ion conductor and preparation method thereof
The invention discloses a lithium borohydride compound fast ion conductor and a preparation method thereof. The material system of LiBH4-NaX compound comprises a combination of two phases of LiBH4 andNaCl, or LiBH4 and NaI, wherein LiBH4-NaX (X refers to CI<-> and I<-> ions) is formed through composition compounding according to the molar ratio range (1-10):1. The LiBH4 compound fast ion conductor is prepared under the air-free (H2O is less than 1ppm, and O2 is less than 1ppm) situation. The LiBH4-NaX (X refers to Cl<-> and I<-> ions) compound fast ion conductor is a lithium-ion conductor with the excellent low-temperature performance, and has the probability of serving as solid electrolytes of full solid state lithium-ion batteries.
Owner:SOUTHEAST UNIV
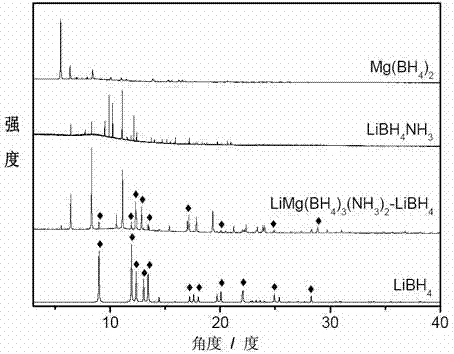
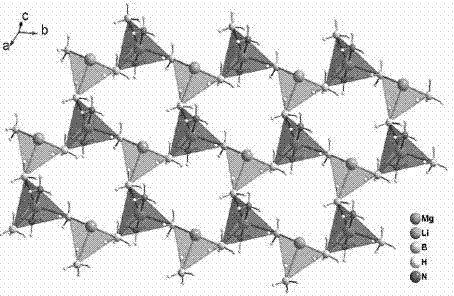
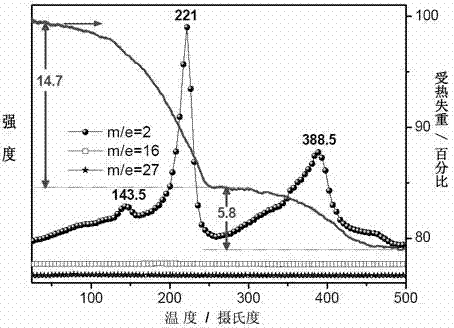
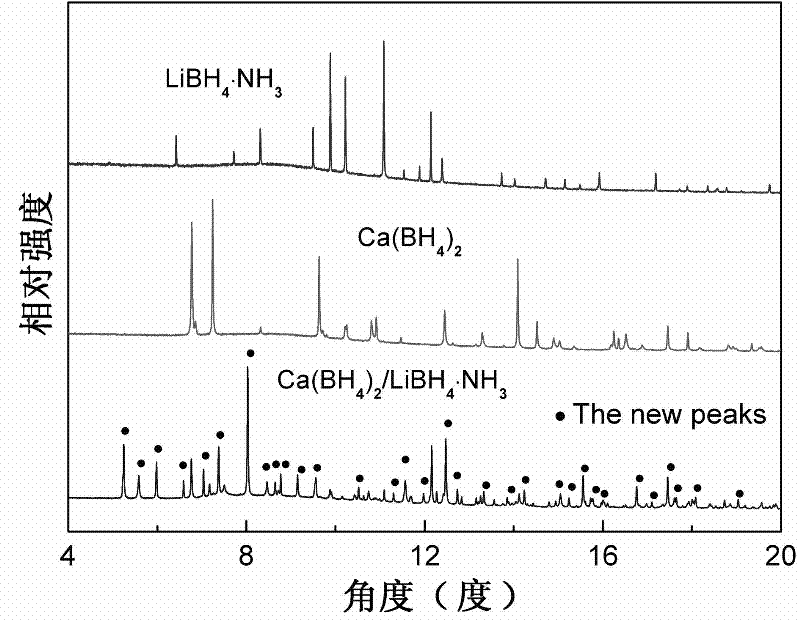
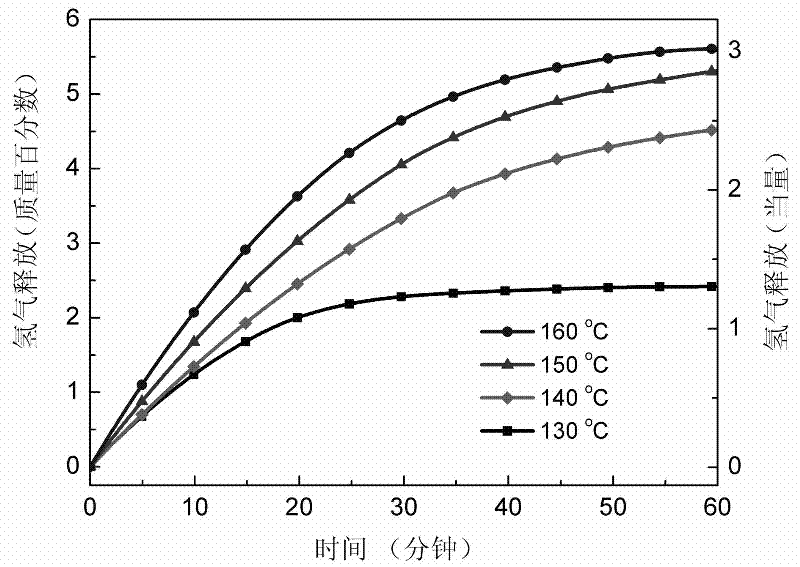
Method for preparing high-capacity composite hydrogen storage material calcium borohydride/lithium borohydride ammine
InactiveCN102198933ASimple preparation processEasy to implementHydrogen productionCalcium borohydrideAmmonia
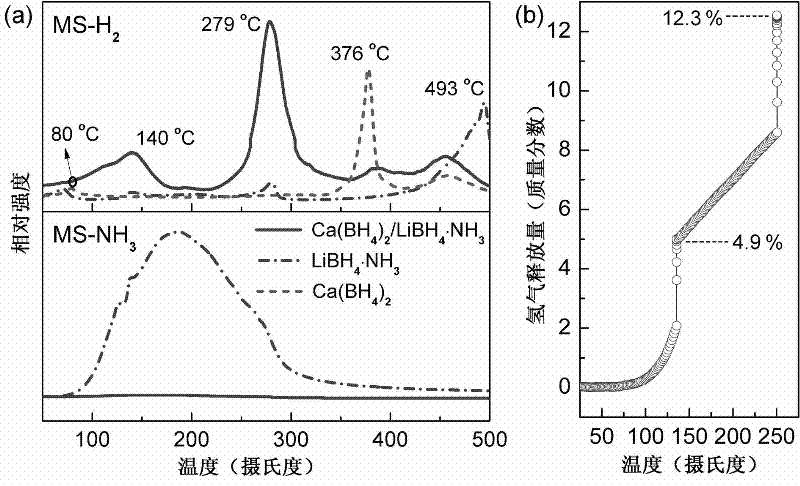
The invention relates to a method for preparing a high-capacity composite hydrogen storage material calcium borohydride / lithium borohydride ammine (Ca(BH4)2 / LiBH4.NH3), which comprises the following steps of: mixing calcium borohydride and lithium borohydride ammine in a molar ratio of 1:1, and grinding or ball-milling in inert gas to obtain the required product. The Ca(BH4)2 / LiBH4.NH3 serving asa novel high-efficiency hydrogen storage material has high hydrogen release property, can slowly release hydrogen when heated to the temperature of 80 DEG C, and can release 12.3 weight percent of high-purity hydrogen before being heated to the temperature of 250 DEG C.
Owner:FUDAN UNIV
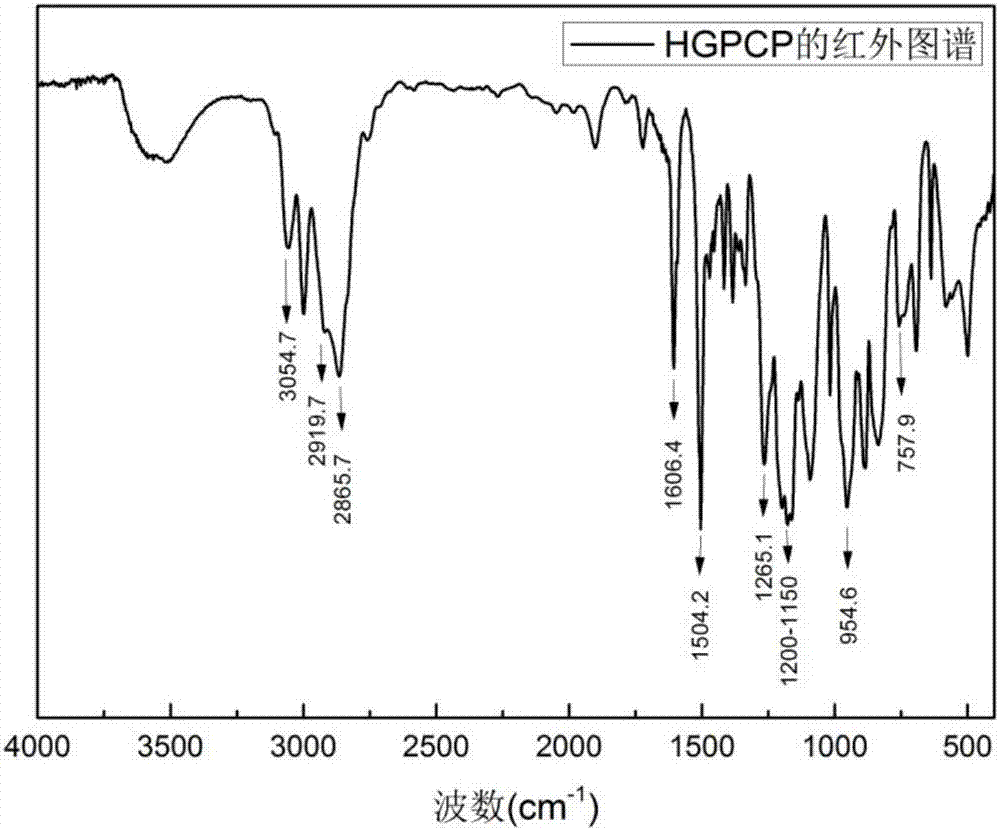
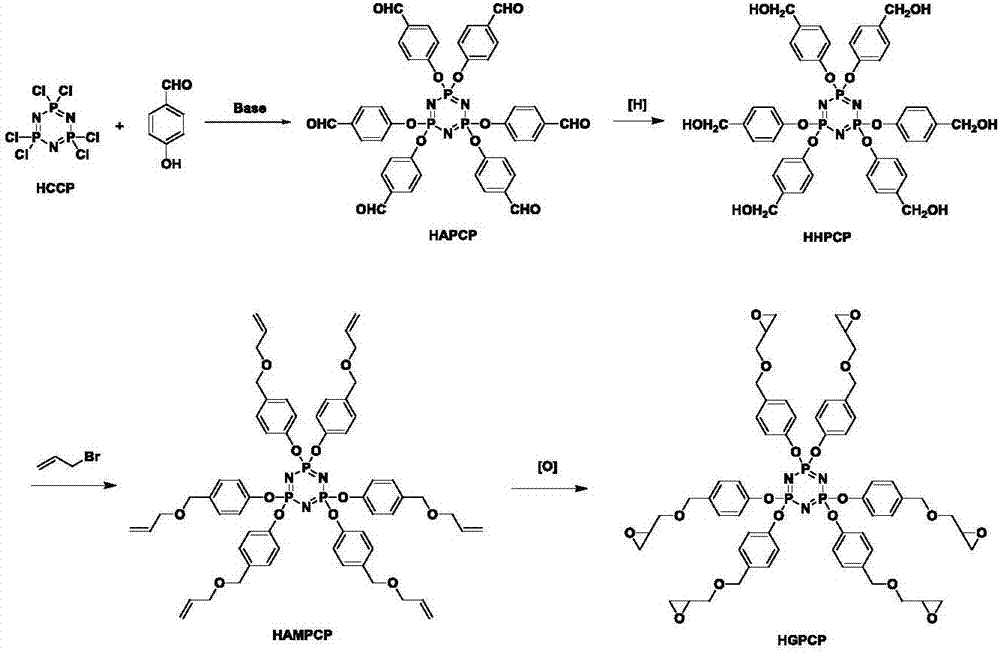
Preparation method of six-functionality epoxy resin based on cyclotriphosphazene
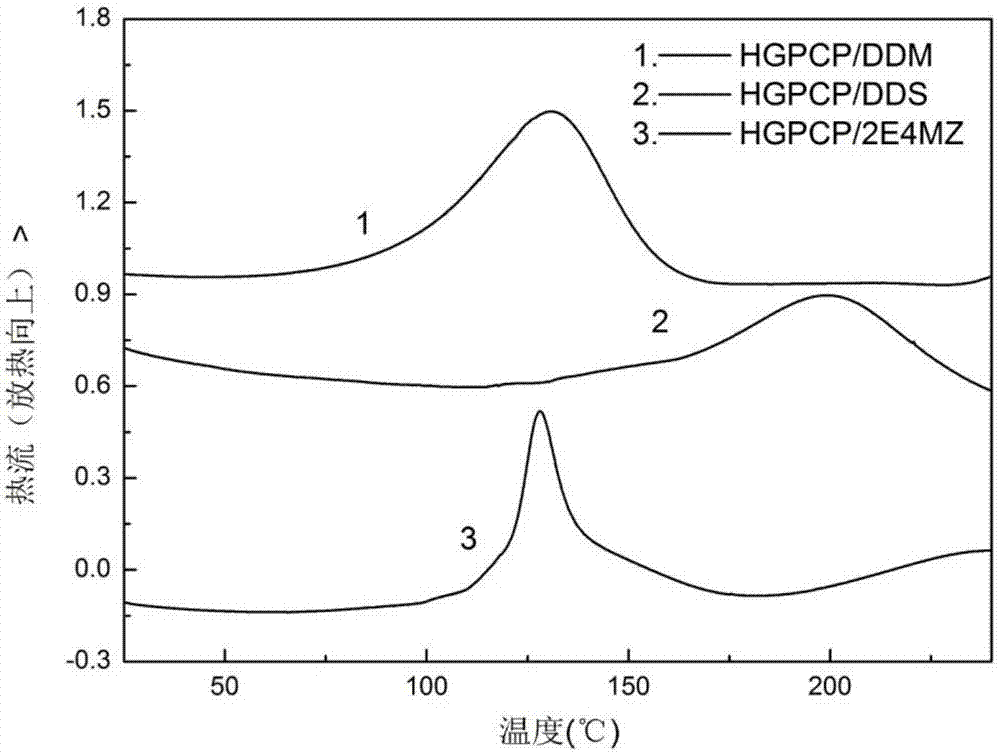
ActiveCN107216354AStable flame retardant propertiesImprove mechanical propertiesGroup 5/15 element organic compoundsEpoxyOxygen
The invention discloses a preparation method of six-functionality epoxy resin based on cyclotriphosphazene, which aims at solving the technical problem of poor comprehensive property in the existing prepared epoxy resin flame-retardant system. The preparation method adopts the technical scheme with the following steps of firstly, performing nucleophilic substitution on p-hydroxybenzaldehyde and phosphonitrilic chloride trimer under the condition of alkaline existence, so as to obtain a hexaaldehyde compound HAPCP; using sodium borohydride or lithium borohydride to reduce the HAPCP, so as to obtain a hexahydroxy intermediate HHPCP; dissolving the HHPCP, bromopropylene and alkaline into an organic solvent, so as to obtain HAMPCP; oxidizing a hexaalkenyl-containing compound HAMPCM by metachloroperbenzoic acid in an organic solvent, so as to obtain a hexaepoxy target product HGPCP. The preparation method has the advantages that because the molecular structure of HGPCP contains six epoxy functional groups, the mechanical property and heat-resistant property are improved; by containing the cyclotriphosphazene structure with the flame-retardant property, the flame-retardant property is very excellent, the limited oxygen index is greater than 30%, and the UL-94 reaches V-0 level.
Owner:NORTHWESTERN POLYTECHNICAL UNIV
Method for improving hydrogen storage property of lithium borohydride
InactiveCN102502488AImprove hydrogen storage performanceImproved hydrogen releaseHydrogen productionAlkaline earth metalHydrogen fuel cell
The invention provides a method for improving a hydrogen storage property of lithium borohydride, and belongs to the technical field of hydrogen storage materials. The method comprises the following steps of: mixing the lithium borohydride and alkaline-earth metal alanate according to the molar ratio of 2:1-10:1 under the protection of vacuum or inert gases, and heating mixed powder of the lithium borohydride and the alkaline-earth metal alanate to a certain temperature to ensure that the alkaline-earth metal alanate is decomposed into alkaline-earth metal hydride, aluminum or aluminum alloy in advance. By the method, double effects of in situ and concerted catalysis in the process of discharging and absorbing hydrogen of the lithium borohydride by using the alkaline-earth metal alanate are achieved, so that a hydrogen discharging temperature of the lithium borohydride is greatly reduced, and the hydrogen absorbing and discharging dynamic properties of the lithium borohydride are improved. The method is suitable for storing the hydrogen safely and efficiently and is particularly applied in fields of hydrogen fuel cells and the like.
Owner:ANHUI UNIVERSITY OF TECHNOLOGY
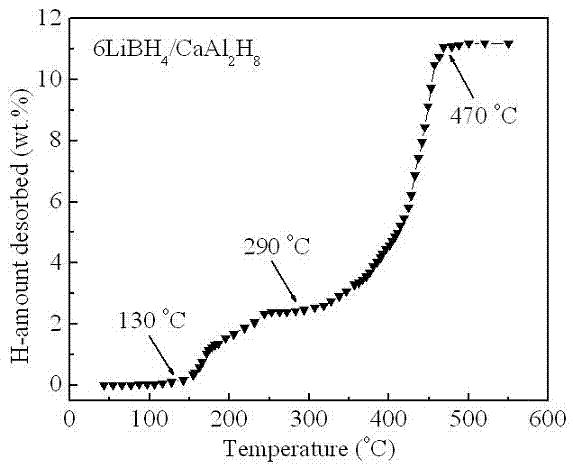
Lithium borohydride/rare earth magnesium base alloy composite hydrogen storage material and preparation method thereof
ActiveCN103101880AHigh quality hydrogen storage densitySimple manufacturing methodHydrogen productionFreeze-dryingAlloy composite
The invention discloses a lithium borohydride / rare earth magnesium base alloy composite hydrogen storage material which consists of lithium borohydride and rare earth magnesium base alloy, wherein the general formula is LiBH4 / La(1-x)MgxNiaCobMncAld; the rare earth magnesium base alloy accounts for 10-80% of the composite material by mass; and x is 0.1-0.8, a is 2.7-3.2, b is 0.1-0.8, c is 0.1-0.4 and d is 0.05-0.5. The lithium borohydride / rare earth magnesium base alloy composite hydrogen storage material is prepared by the following steps of: smelting according to the proportion of the components and tossing to obtain an alloy sheet; after heat treatment and cooling, performing ball milling and sieving to obtain tossing-state alloy powder; performing hydrogen treatment of the tossing-state alloy powder to obtain hydrogenated-state alloy powder; mixing LiBH4 and alloy powder according to a mass proportion; adding heptane, hexane or tetrahydrofuran, and performing ball milling; and freeze-drying to obtain the lithium borohydride / rare earth magnesium base alloy composite hydrogen storage material. The lithium borohydride / rare earth magnesium base alloy composite hydrogen storage material disclosed by the invention has high mass hydrogen storage density, and the preparation method is simple and easy to implement. The composite material can be widely applied to the fields such as large-scale transportation of hydrogen, hydrogen supply source of fuel cell, purification of hydrogen and the like.
Owner:GUANGDONG INST OF RARE METALS
Lithium ion conductor with nanoscale and preparation method thereof
ActiveCN103762346AEasy to prepareLow costMaterial nanotechnologySolid electrolytesElectrical conductorDiffusion network
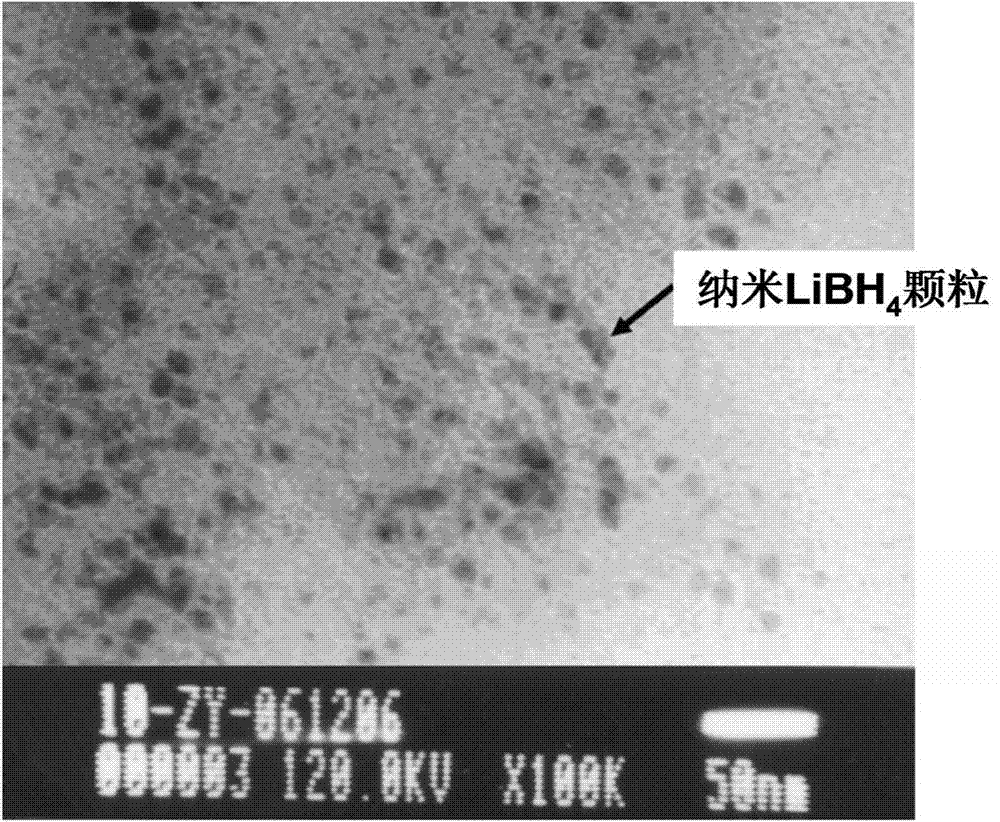
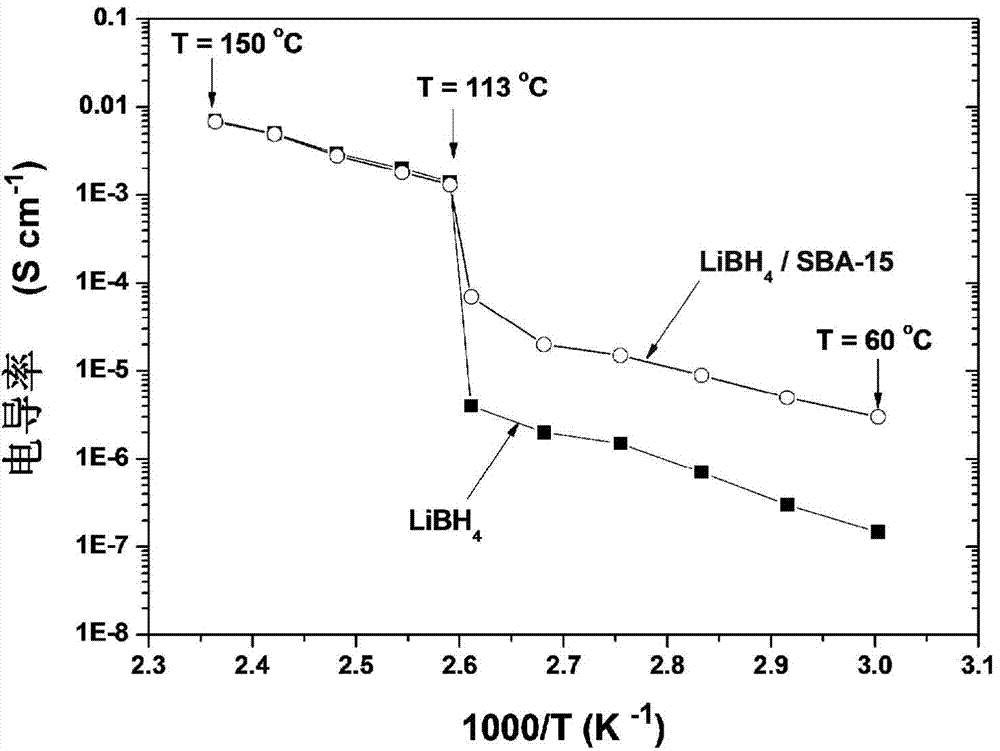
The invention provides a lithium ion conductor with a nanoscale. The lithium ion conductor comprises a mesoporous silicon material and solid solution phase particles dispersed in the mesoporous silicon material, wherein the solid solution phase particles are selected from one or more of LiBH4 solid solution phase particles, 4LiBH4-LiF solid solution phase particles, 4LiBH4-LiCl solid solution phase particles, 4LiBH4-LiBr solid solution phase particles and 4LiBH4-LiI solid solution phase particles; the mesoporous silicon material is SBA-15. The lithium ion conductor has the beneficial effects that a preparation method of the lithium ion conductor is simple and is relatively low in cost; by dispersing lithium borohydride and lithium halide in the mesoporous silicon material, the diffusion path of the ions is greatly shortened, the diffusion network channel of the ions is widened, and the phase inversion temperature of the lithium ion conductor is reduced, thus greatly improving the electrical conductivity of the lithium ion conductor.
Owner:SOUTHEAST UNIV
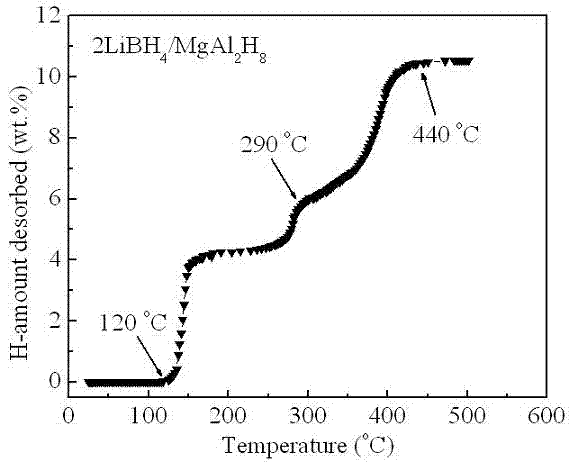
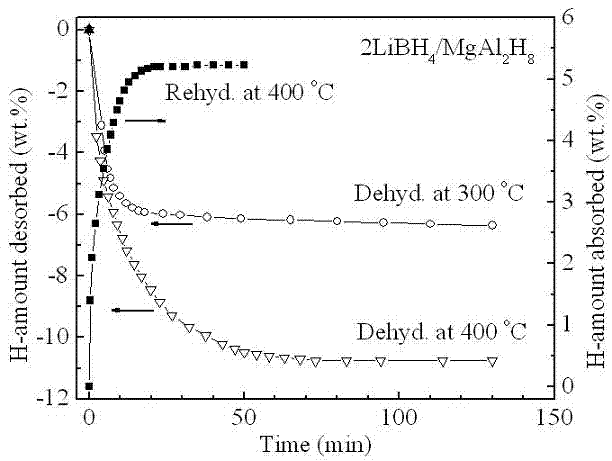
Light-metal and high-capacity composite hydrogen storage material and preparation method thereof
InactiveCN102556963AComposite uniformSmall particle sizeMultiple metal hydridesHydrogen productionChemical reactionPtru catalyst
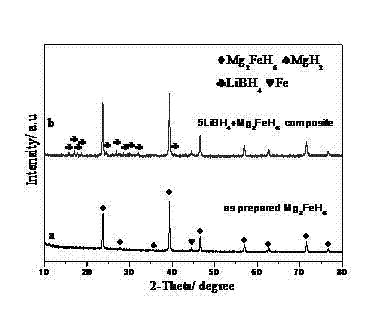
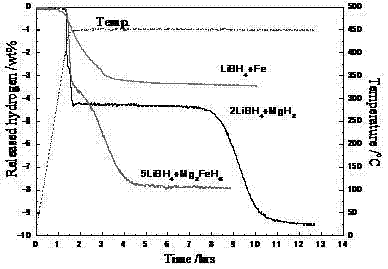
The invention belongs to the field of hydrogen storage and discloses a light-metal and high-capacity composite hydrogen storage material and a preparation method thereof. The light-metal and high-capacity composite hydrogen storage material consists of lithium borohydride and magnesium-based hydride in stoichiometric ratio of (4:1)-(5:1), wherein a chemical general formula of the magnesium-based hydride is Mg2MHx and M is Fe or Co. The preparation method of the light-metal and high-capacity composite hydrogen storage material comprises a high-energy ball milling method and a high-pressure hydrogenation method and is simple; no specific catalyst is needed to be doped in the obtained lithium borohydride and magnesium-based hydride composite hydrogen storage material; and the obtained lithium borohydride and magnesium-based hydride composite hydrogen storage material has a favorable hydrogen discharge performance. With favorable dispersibility and nano-grade size, the M generated in situ in the reaction process promotes a mutual chemical reaction of the lithium borohydride and the M. The invention can provide a method which can be used for better increasing the dispersibility and enhancing the mutual chemical action for in-situ modification of complex hydrides.
Owner:ZHEJIANG UNIV
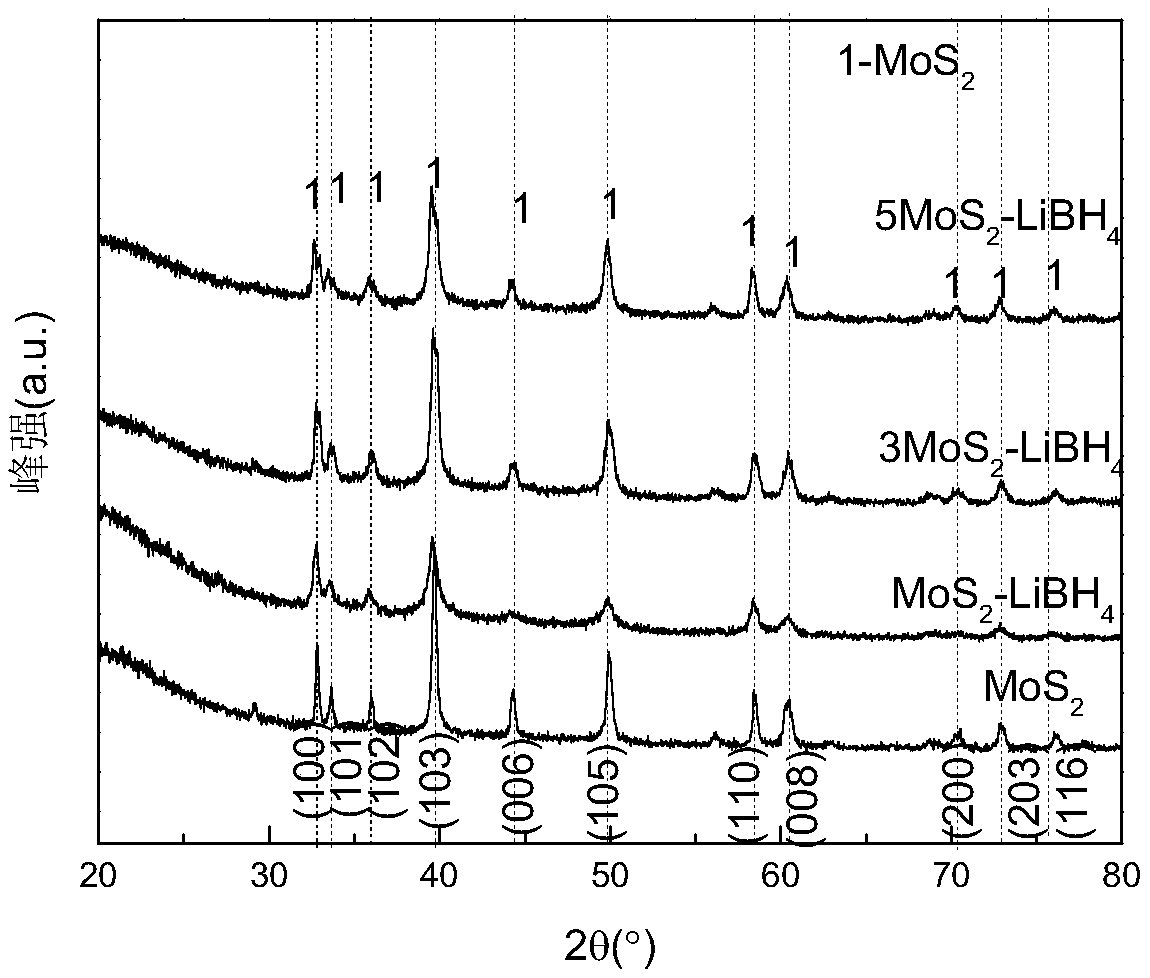
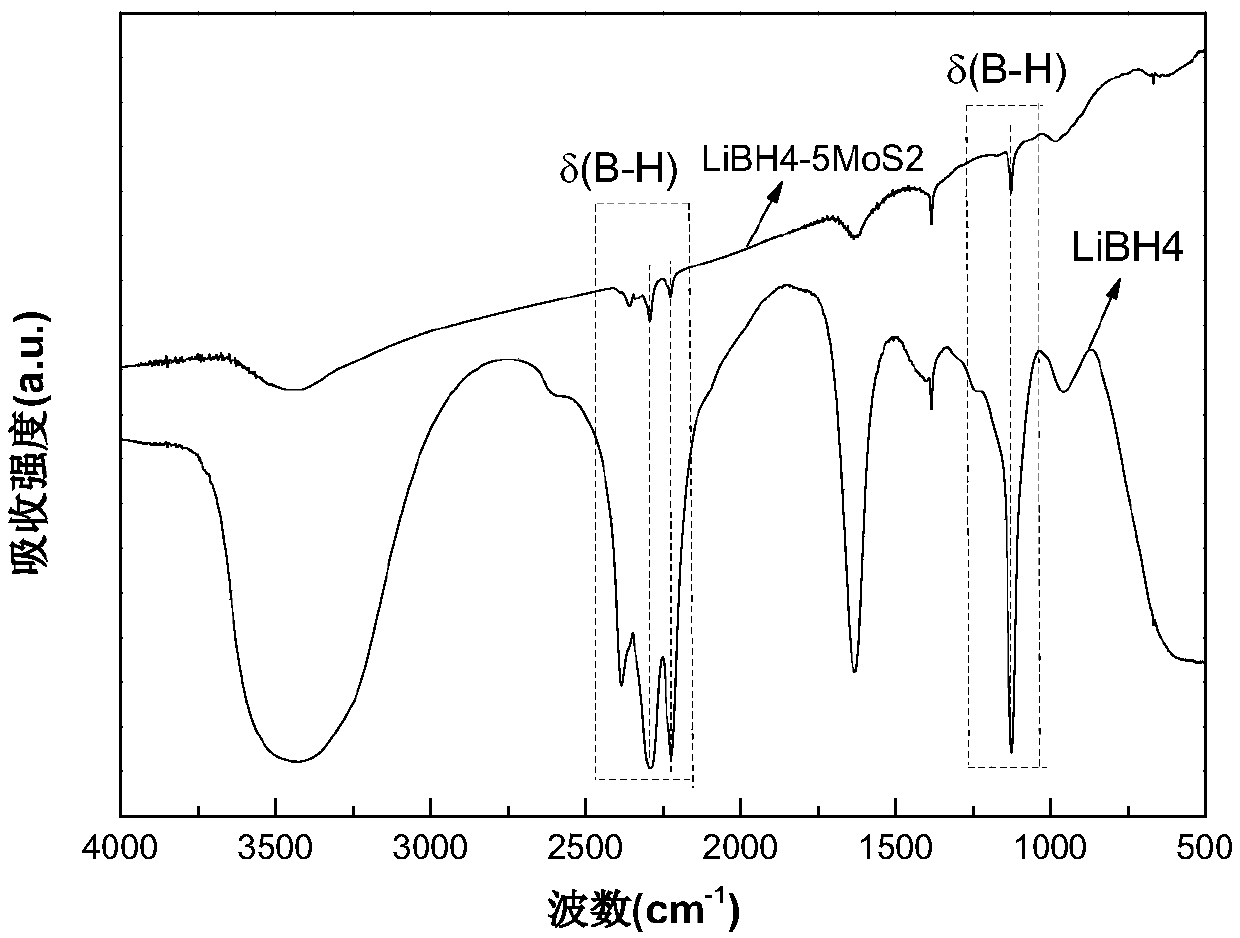
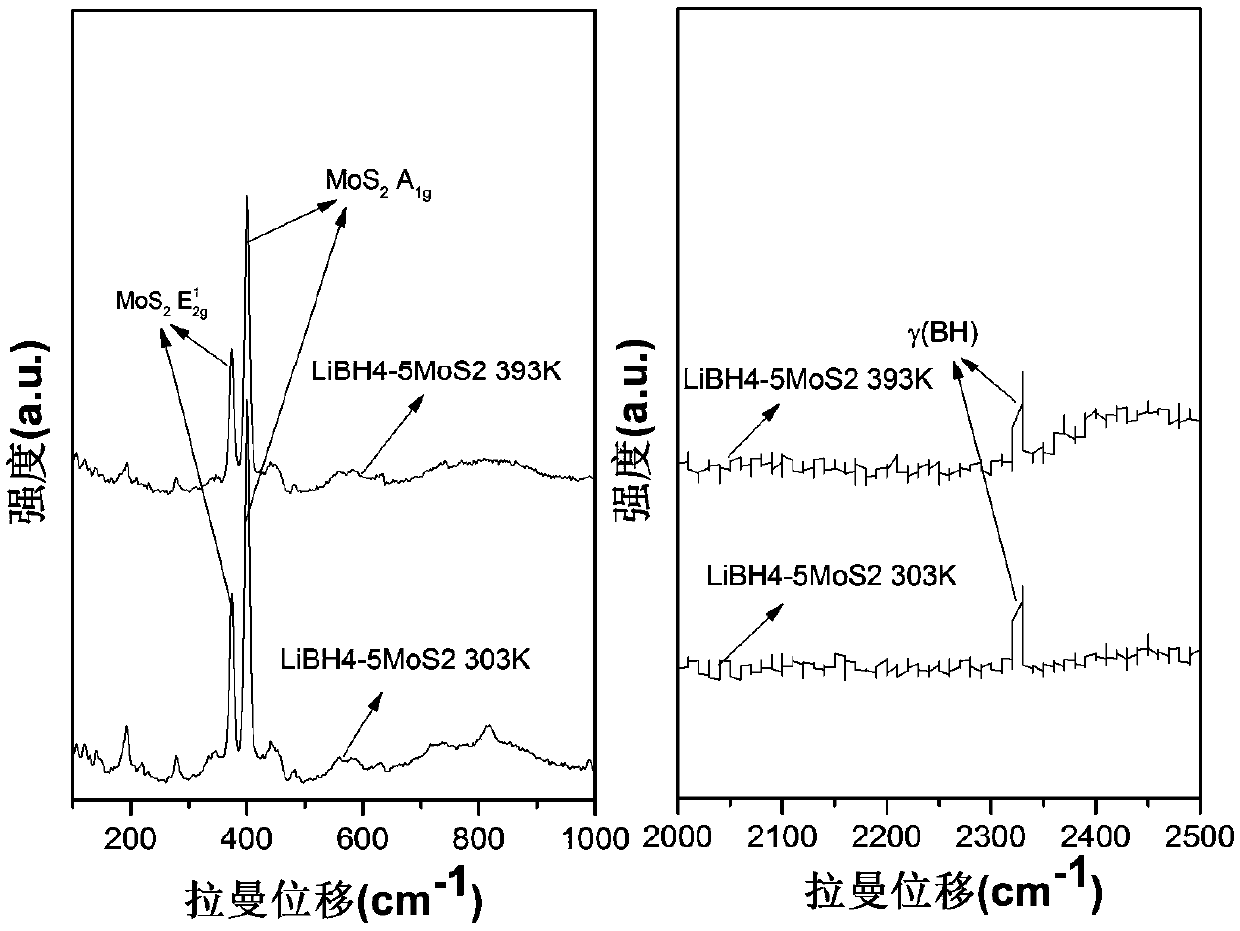
Lithium borohydride and molybdenum disulfide composite system solid electrolyte material, and preparation method and application thereof
The invention discloses a lithium borohydride and molybdenum disulfide composite system solid electrolyte material, and a preparation method and application thereof. The composite system solid electrolyte material is an xLiBH4-yMoS2 ball-milling compound or a high-temperature dehydrogenation reaction product thereof, wherein x is 1 to 10 and y is 1 to 10. The preparation method comprises the following steps: mixing the LiBH4 and the MoS2 according to the above molar ratio in an inert gas atmosphere and then performing ball milling, or further loading a ball-milled product into a Sievert-type gas-solid reaction closed device for a temperature-programmed dehydrogenation reaction at a dehydrogenation temperature from the room temperature to 500 degrees centigrade and at a temperature rise rate from 1-10 degrees centigrade per minute. The material of the invention is a lithium ion conductor with excellent room temperature (T < 100 degrees centigrade) performance, is about 3-4 orders of magnitude higher than LiBH4 at room temperature, and can be used for preparing a solid electrolyte of an all-solid lithium ion battery.
Owner:SOUTHEAST UNIV
Method for preparing lithium borohydride by room-temperature solid-phase ball milling
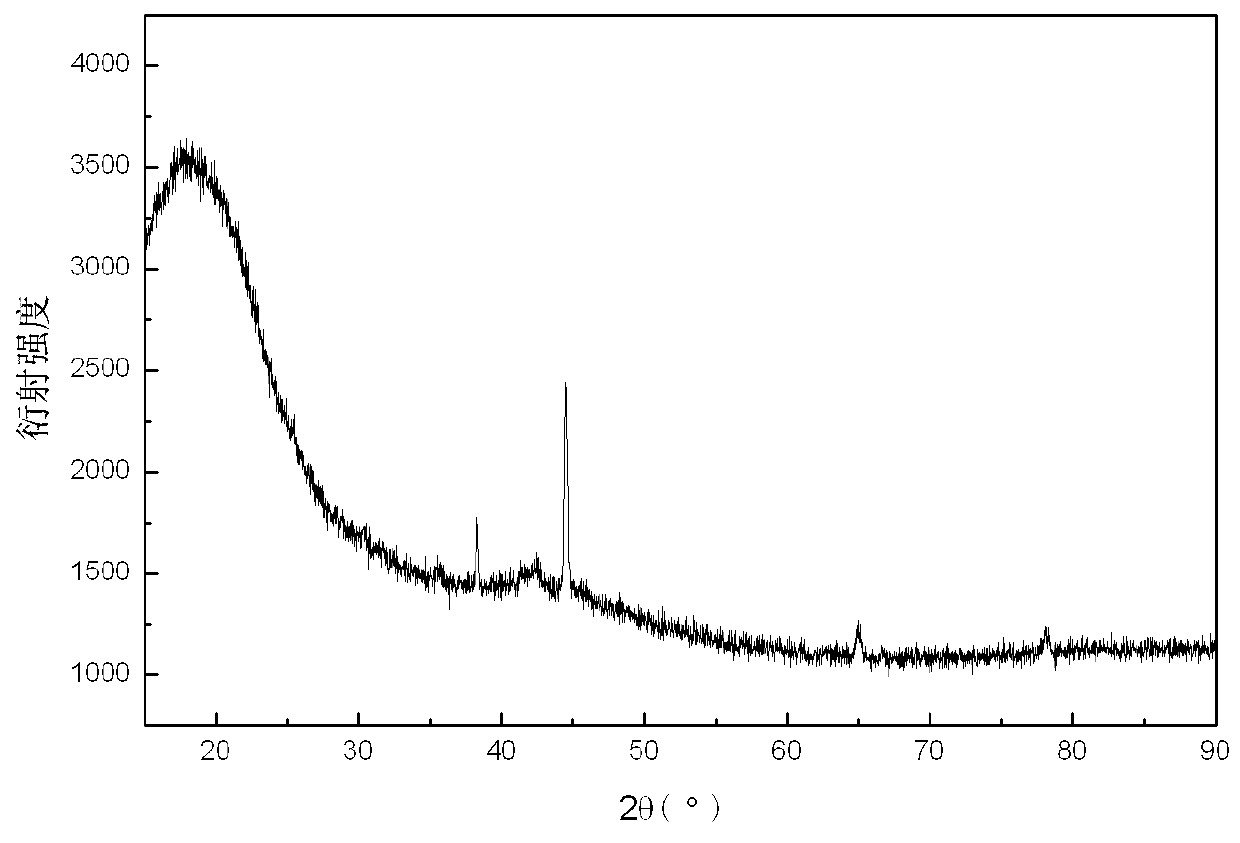
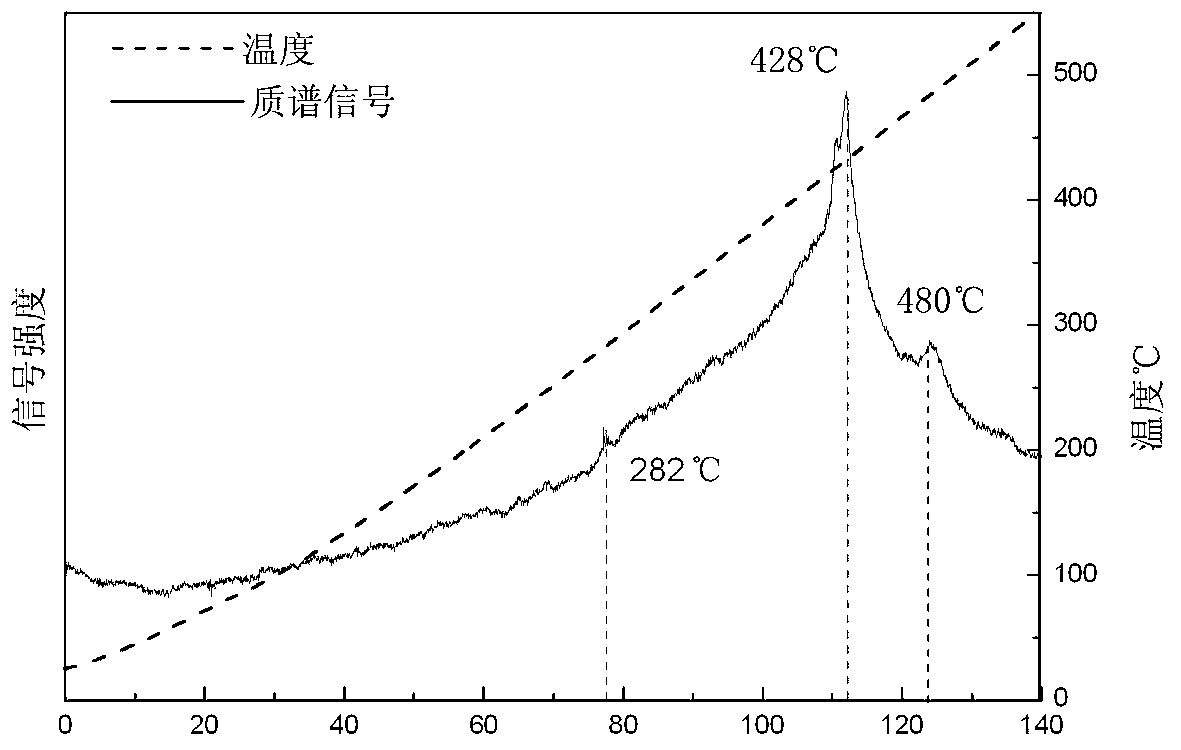
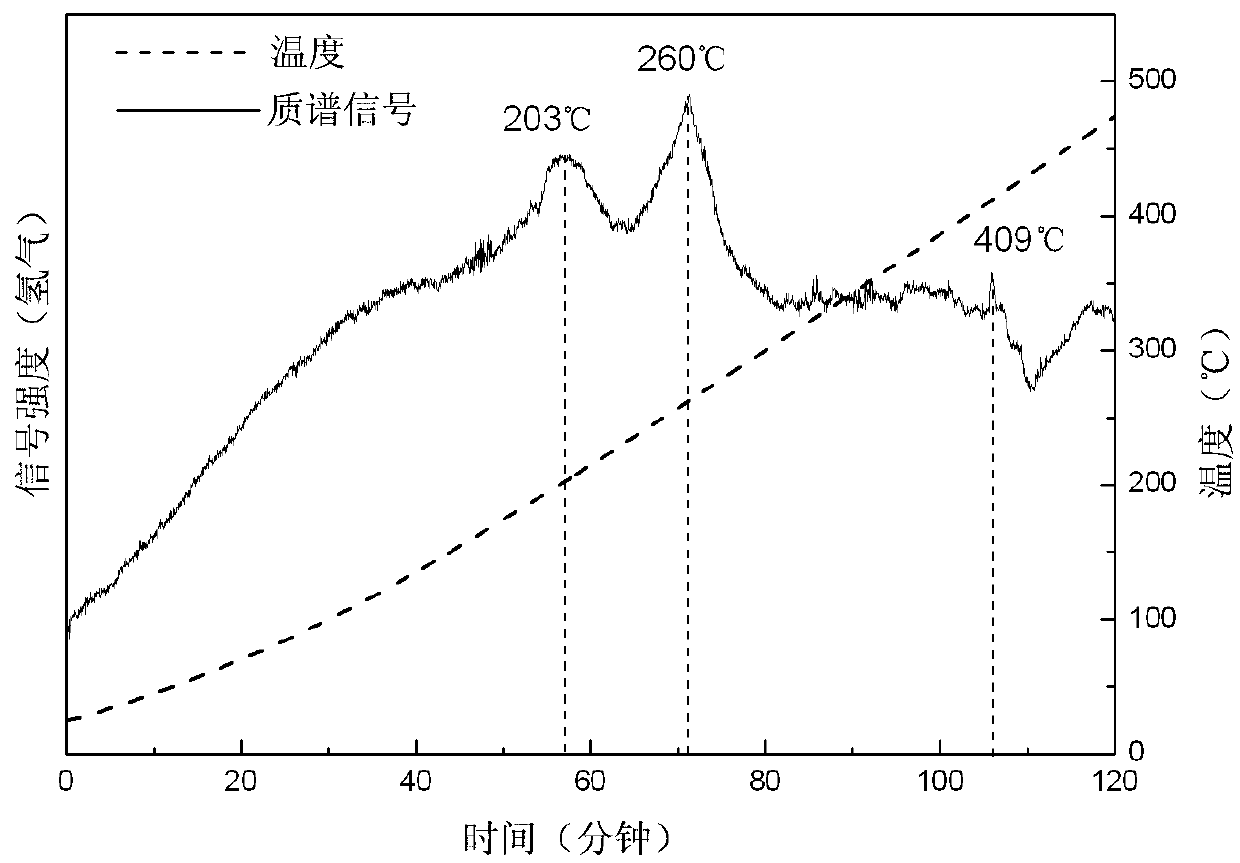
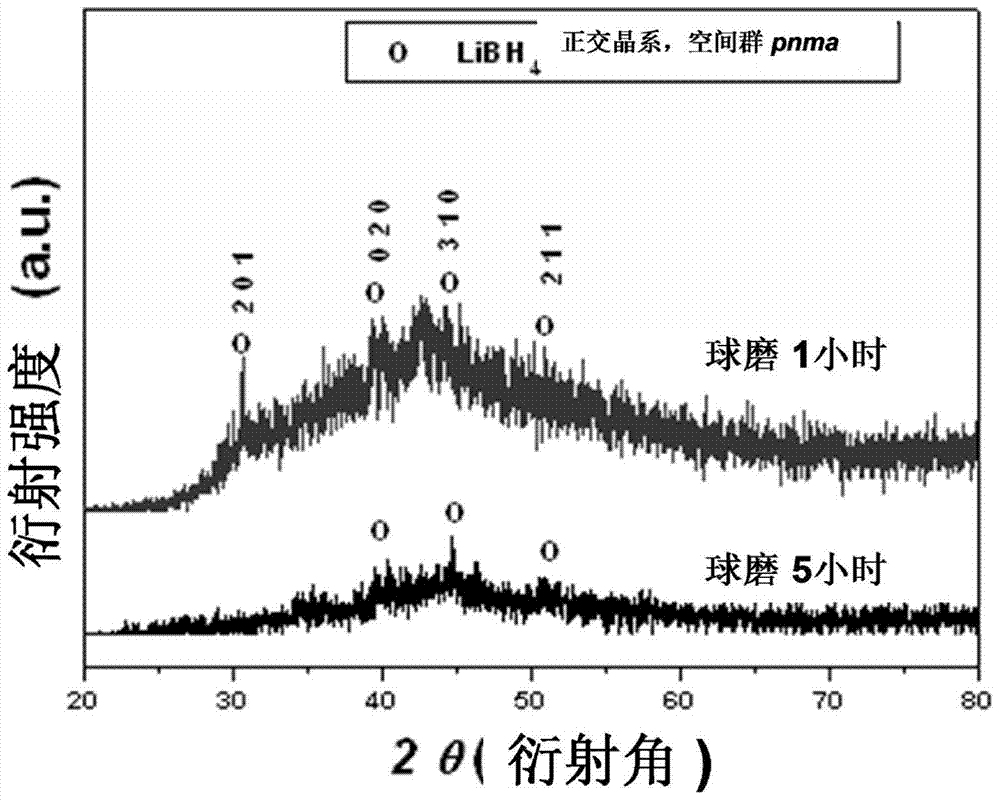
ActiveCN108285131AWide variety of sourcesAvoid high temperature and pressureReversible hydrogen uptakeMonoborane/diborane hydridesLithium metaborateRoom temperature
The invention discloses a method for preparing lithium borohydride by room-temperature solid-phase ball milling. The method comprises the following steps: under a non-oxidizing atmosphere at room temperature, uniformly mixing a magnesium-containing reducing agent with a lithium metaborate-containing reducing material, carrying out solid-phase ball milling, and carrying out separation and purification to obtain the lithium borohydride. The preparation method provided by the invention has the advantages of simple process, controllable and adjustable reaction process, mild reaction conditions, low energy consumption, low cost, high yield, no pollution, good safety and realization of cyclic utilization of boron resources, and has important practical significance.
Owner:SOUTH CHINA UNIV OF TECH
Preparation method for 3-(trifluoromethyl)benzenepropanol
ActiveCN104892356AMild reaction conditionsEasy to operateOrganic compound preparationHydroxy compound preparationPotassium borohydrideDihydrocinnamic acid
The invention discloses a preparation method for 3-(trifluoromethyl)benzenepropanol. According to the method, in an inert solvent, 3-(trifluoromethyl)hydrocinnamic acid is reduced to generate 3-(trifluoromethyl)benzenepropanol under the presence of boron hydride as a reducing agent and Lewis acid as a cocatalyst. The inert solvent is composed of one or two components selected from methyl tert-butyl ether, isopropyl ether, methyl tetrahydrofuran and tetrahydrofuran. The molar ratio of boron hydride to 3-(trifluoromethyl)hydrocinnamic acid is 1:1 to 3:1. The boron hydride is in the form of sodium borohydride, potassium borohydride, lithium borohydride or zinc borohydride. The molar ratio of Lewis acid to 3-(trifluoromethyl)hydrocinnamic acid is 1:1 to 2:1. The Lewis acid is in the form of methanesulfonic acid, concentrated sulfuric acid, trifluoroacetic acid, zinc chloride, aluminium trichloride or zirconium chloride. The method is mild in reaction condition, simple in operation and post-treatment, and especially high in safety, thus being suitable for industrial mass production.
Owner:CHANGZHOU SUNLIGHT PHARMA
Carbon fiber surface modified treatment method
InactiveCN106192407AImprove surface propertiesImprove monofilament tensile strengthCarbon fibresFiberCarbon fibers
The invention provides a carbon fiber surface modified treatment method. The method comprises the following steps that firstly, carbon fiber is steeped into a compatilizer solution, then, under the vacuum condition, a sulfuric acid solution of potassium permanganate is used for performing oxidation etching treatment on CF-1, after treatment is completed, natural cooling is performed to reach room temperature, washing is performed till pH is 6-8, drying is performed, CF-2 is obtained, then, lithium borohydride is used for reduction in diethyl ether, an alcohol-water solution of the silane coupling agent for the reduced CF-3 is subjected to treatment to obtain CF-4. According to the method, the surface activity of the fiber is improved, and meanwhile the fiber body is not damaged.
Owner:郑州峰泰纳米材料有限公司
Niobium-based coordination hydroboron composite hydrogen storage material and preparation method and applications
InactiveCN102219181AReduce energy consumptionImprove securityMonoborane/diborane hydridesHydrogen productionNiobiumUnit mass
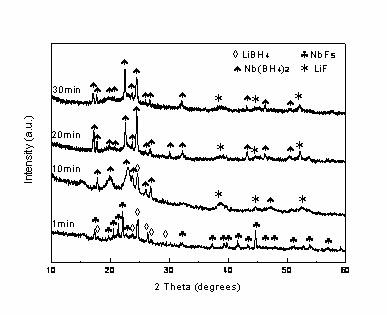
The invention relates to the field of hydrogen storage material, and discloses a niobium-based coordination hydroboron composite hydrogen storage material and a preparation method. The hydrogen storage material is mainly used for the fields of fuel cell hydrogen supply source, hydrogen vehicles and the like. The basic material of the composite hydrogen storage material-niobium-based coordination hydroboron has the following chemical formula: Nb(BH4)5, and the hydrogen storage density in unit mass is 12wt%. The preparation method of composite hydrogen storage material is as follows: under the protective atmosphere of a room temperature and inert gas, grinding lithium borohydride and halogenate niobium in an agate mortar according to the molar ratio being 5:1, wherein white LiBH4 is gradually changed into the rufous Nb(BH4)5 hydroboron during grinding, thus directly obtaining Nb(BH4)5 / 5LiM composite hydrogen storage material. According to the method, the needed niobium-based coordination hydroboron can be synthesizing by simple manual grinding under normal temperature and pressure, the operation requirement is simple, the safety is high, and the invention is particularly suitable for large-scale industry production.
Owner:ZHEJIANG UNIV
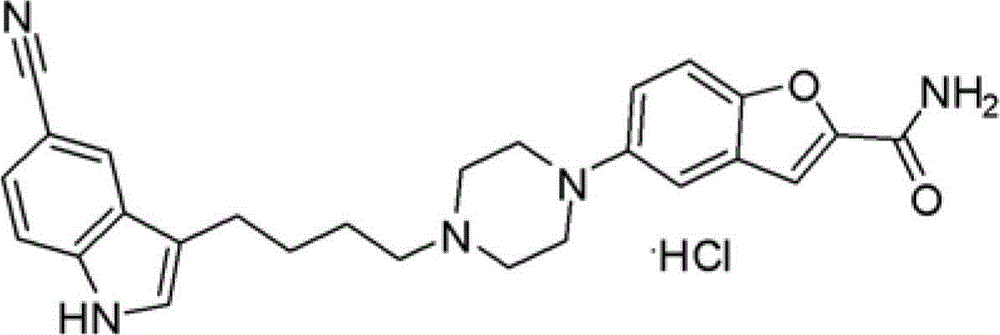
Synthesis method of tert-butyl-1, 7-diazaspiro [3.5] nonane-1-formate
ActiveCN111620869AReasonable reaction process designMethod route shortOrganic chemistryBulk chemical productionPalladium on carbonNonane
The invention relates to a synthesis method of tert-butyl-1, 7-diazaspiro [3.5] nonane-1-formate, and mainly solves the technical problem that no suitable industrial synthesis method exists at present. The method comprises the following seven steps: 1, reacting a compound 1 with ethyl malonate added into a solvent ethanol to obtain a compound 2; 2, reacting the compound 2 with lithium borohydridein tetrahydrofuran to obtain a compound 3; 3, reacting the compound 3 with p-toluenesulfonyl chloride in dichloromethane to obtain a compound 4; 4, adding cesium carbonate into the compound 4 in acetonitrile serving as a solvent for cyclization to obtain a compound 5; 5, adding magnesium chips into the compound 5 in methanol serving as a solvent for reduction to obtain a compound 6, 6, reacting the compound 6 with Boc anhydride in dichloromethane to obtain a compound 7, and 7, reacting the compound 7 with palladium on carbon in methanol to obtain a final compound 8.
Owner:SHANGHAI STA PHARMA R&D CO LTD +1
Long-acting ceramic whitening glaze
A long-acting ceramic whitening glaze is prepared from raw materials in parts by weight as follows: 3-10 parts of strontium carbonate, 4-9 parts of cobalt acetate, 6-10 parts of polymethylsiloxane, 2-7 parts of zirconium silicate, 6-11 parts of tetraethoxysilane, 3-10 parts of metakaolin, 3-8 parts of polyvinyl alcohol, 2-6 parts of tertiary calcium phosphate, 3-11 parts of titanium dioxide, 3-9 parts of forsterite, 2-8 parts of quartz powder, 1.2-4 parts of zirconium silicate, 4-7 parts of zinc oxide, 6-8.5 parts of aluminum oxide, 1.2-3 parts of quartz, 1-3 parts of Sc2O3 and 4-7 parts of lithium borohydride. The long-acting ceramic whitening glaze has the benefits as follows: the brightness and the glossiness of the ceramic glaze can be improved very well, meanwhile, long action is realized, and the use cost is low.
Owner:青岛海蓝海洋复合功能材料科技有限公司
Preparation method of ammonia-containing composite ionic hydrogen storage material
InactiveCN102225741AEasy to manufactureEasy to implementMonoborane/diborane hydridesHydrogen productionDecompositionStructural formula
The invention relates to a preparation method of an ammonia-containing composite ionic hydrogen storage material. The structural formula of the hydrogen storage material is Mg (BH4) 2 / n LiBH4.NH3, wherein n has a value range of 1 to 2. The Mg (BH4) 2 / n LiBH4.NH3 system has an excellent hydrogen release performance. The Mg (BH4) 2 / n LiBH4.NH3 system starts to release hydrogen when being heated to a temperature of 100 DEG C and desorbs 10% of hydrogen with a high purity when a temperature is lower than 260 DEG C. A release of ammonia gas is controlled effectively during the above decomposition process thus a conversion of ammonia gas to hydrogen is realized successfully. The Mg (BH4) 2 / n LiBH4.NH3 compound is prepared from magnesium borohydride and lithium borohydride-monoammonium complex according to a mol ratio of (1: 1) to (1: 2) through a mixing process and a simple ball milling process in an inert gas atmosphere. Raw materials of the preparation method are easy to be prepared and the preparation method is simple and is easy to be realized. An Mg (BH4) 2 / n LiBH4.NH3 system prepared by the preparation method can release a mass of hydrogen with a high purity at a low temperature and realizes an effective conversion of ammonia gas to hydrogen. A cost of the preparation method is proper.
Owner:FUDAN UNIV
Popular searches
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com
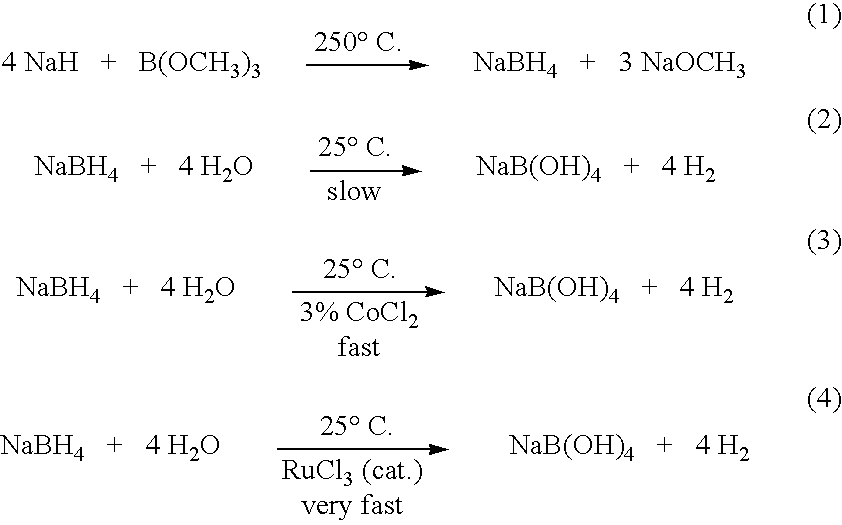
![Synthesis method of tert-butyl-1, 7-diazaspiro [3.5] nonane-1-formate Synthesis method of tert-butyl-1, 7-diazaspiro [3.5] nonane-1-formate](https://images-eureka.patsnap.com/patent_img/c419c075-256f-4485-9366-6c39e9d8594c/204216DEST_PATH_IMAGE004.png)
![Synthesis method of tert-butyl-1, 7-diazaspiro [3.5] nonane-1-formate Synthesis method of tert-butyl-1, 7-diazaspiro [3.5] nonane-1-formate](https://images-eureka.patsnap.com/patent_img/c419c075-256f-4485-9366-6c39e9d8594c/349392DEST_PATH_IMAGE002.png)