Method for preparing hydroxytyrosol
A technology of hydroxytyrosol and dihydroxybenzaldehyde, which is applied in the field of preparation of hydroxytyrosol, can solve the problems of difficulty in obtaining raw material tyrosol, increase production cost and high market price, and achieve the effects of few reaction steps and mild reaction conditions.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
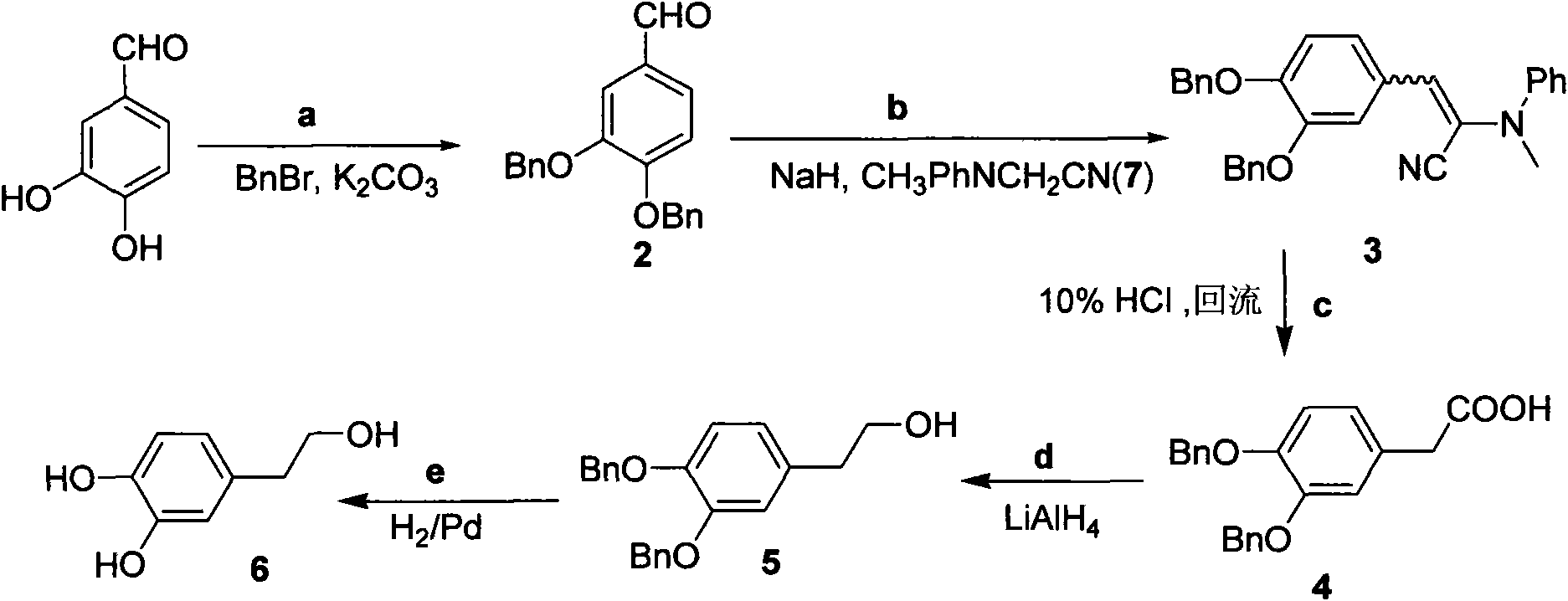
[0042] Embodiment one: according to figure 1 The synthetic route shown prepares 3,4-dibenzyloxyphenethyl alcohol
[0043] 1,3, the synthesis of 4-benzyloxybenzaldehyde (2)
[0044]Weigh 3,4-dihydroxybenzaldehyde (138.0mg, 1mmol, 1equiv) and potassium carbonate (288.0mg, 2.1mmol, 2.1equiv) and dissolve in 10mL of acetone, then add bromine (0.24mL, 2mmol, 2equiv), After heating to reflux for 22 hours, TLC traced the end of the reaction. Acetone was removed under reduced pressure, extracted with diethyl ether (10 mL×3), and the combined organic phases were dried over anhydrous sodium sulfate. The desiccant was removed by suction filtration, and concentrated under reduced pressure to obtain a white solid (292.0 mg, 92.0%).
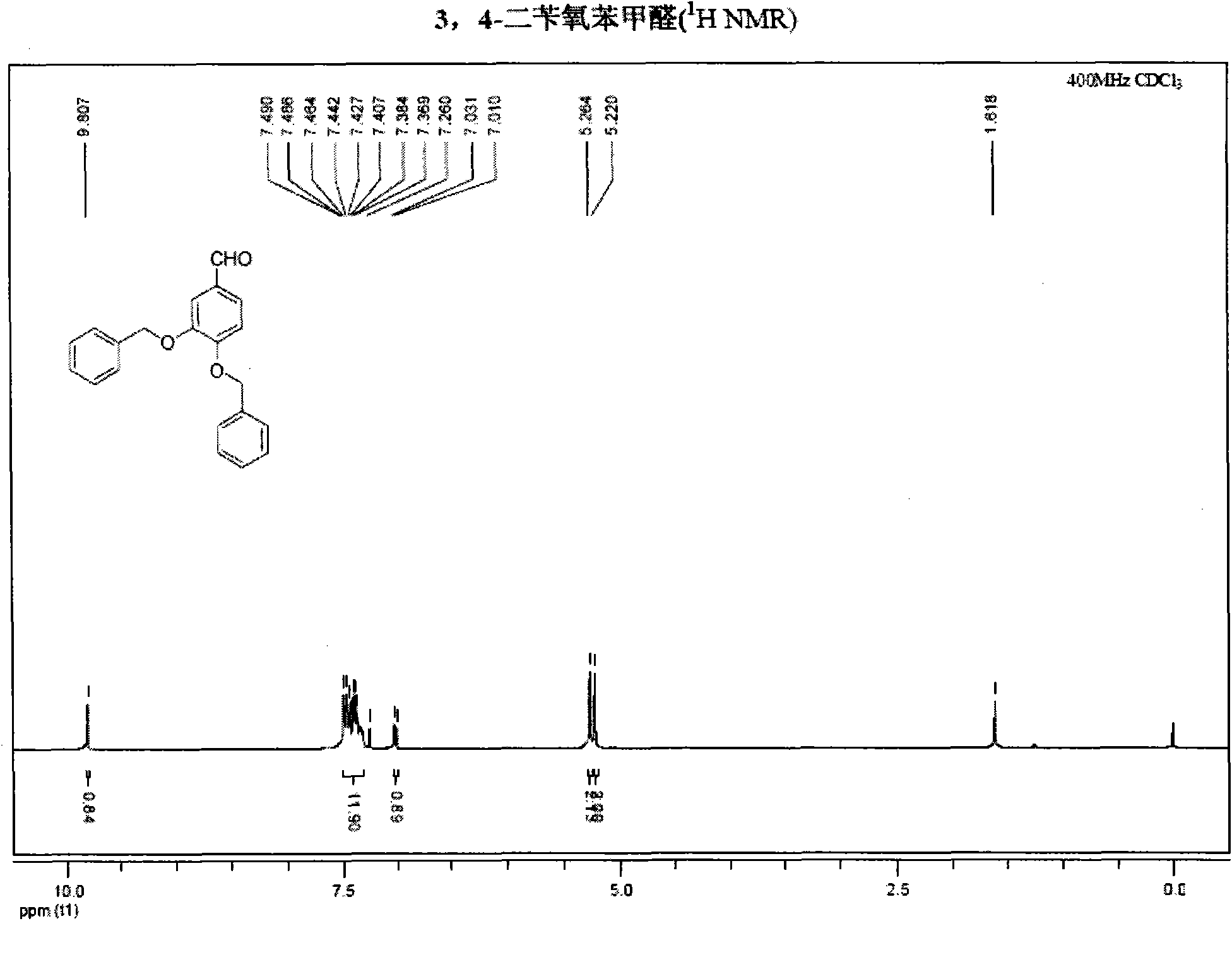
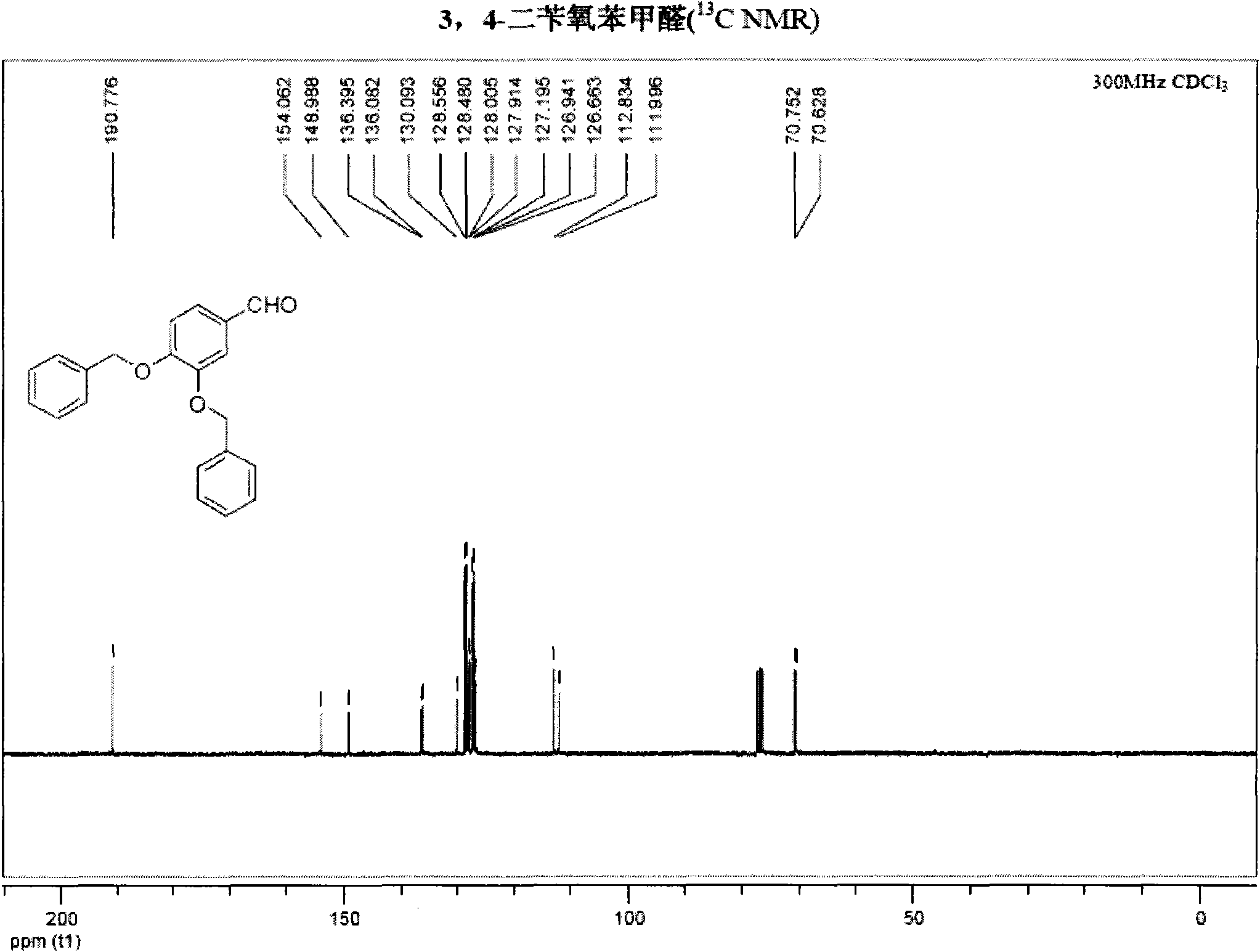
[0045] NMR analysis was carried out to obtain figure 2 , image 3 , the result is as follows:
[0046] 1 H NMR (400MHz, CDCl 3 )δ9.81(s, 1H), 7.49-7.37(m, 12H), 7.02(d, 1H, J=8.0Hz), 5.26(s, 2H), 5.22(s, 2H); 13 C NMR (75MHz, CDCl 3 )δ190.77, 154.06...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com