Synthetic method of hydroxytyrosol
A synthetic method, the technology of hydroxytyrosol, which is applied in the field of chemical synthesis of hydroxytyrosol, can solve the problems of high cost and poor stability of raw materials, and achieve the effects of reduced reaction cost, mild reaction conditions, and good industrial application prospects
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
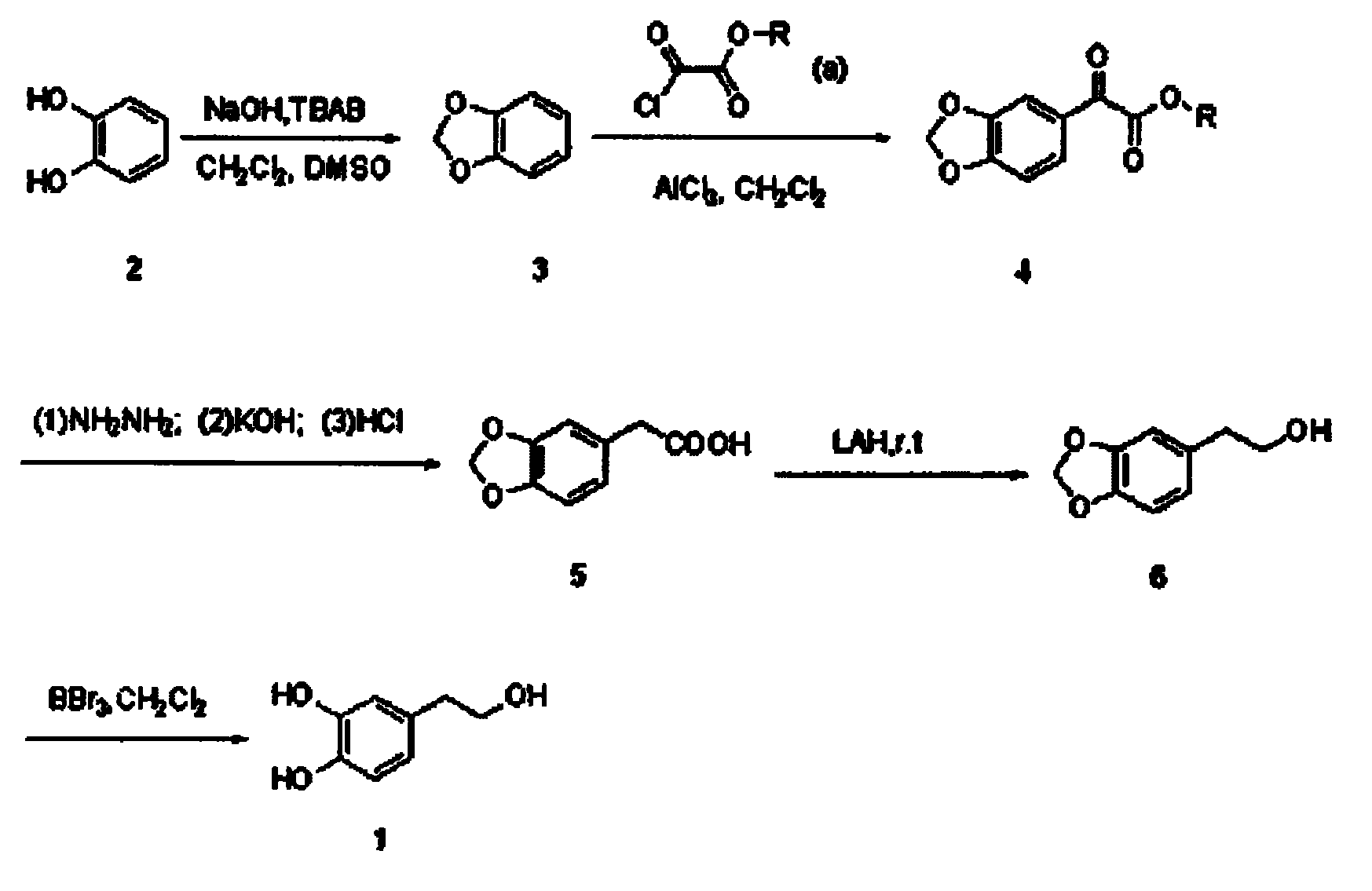
[0027] Embodiment one: according to figure 1 Synthesis of hydroxytyrosol by the synthetic route shown
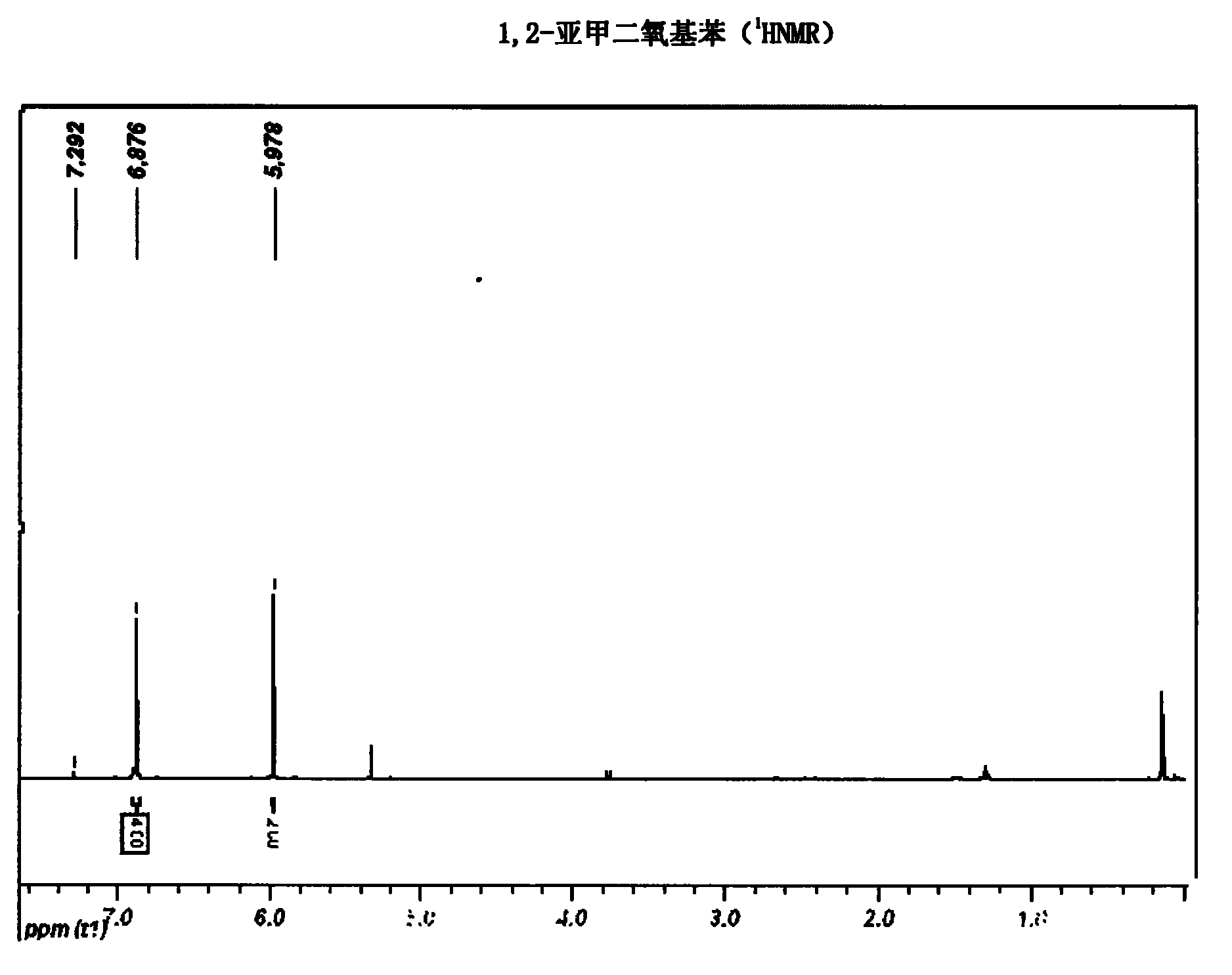
[0028] 1. Preparation of 1,2-methylenedioxybenzene (3)
[0029] Add DMSO (80ml), dichloromethane (20ml), and TBAB (1.9g, 6mmol) to a 250ml three-necked flask, and slowly heat up to 110°C. When reflux is seen, slowly add catechol ( 11g, 0.1mol) of DMSO (60mL) solution, add dropwise 50% sodium hydroxide aqueous solution (sodium hydroxide 8g, 0.2mol, water 8mL) at the same time, add dropwise for about 2h, continue to react for about 1h, and monitor the reaction by TLC Finish.
[0030] Add 100mL of water to the reaction flask, continue heating, and collect the fraction at 99-104°C. This distillate is an azeotrope of water and 1,2-methylenedioxybenzene, which is white and turbid. When the distillate is clear, it does not contain 1,2-methylenedioxybenzene. The distillate was treated to obtain a colorless transparent liquid (9.97 g, 81.7%).
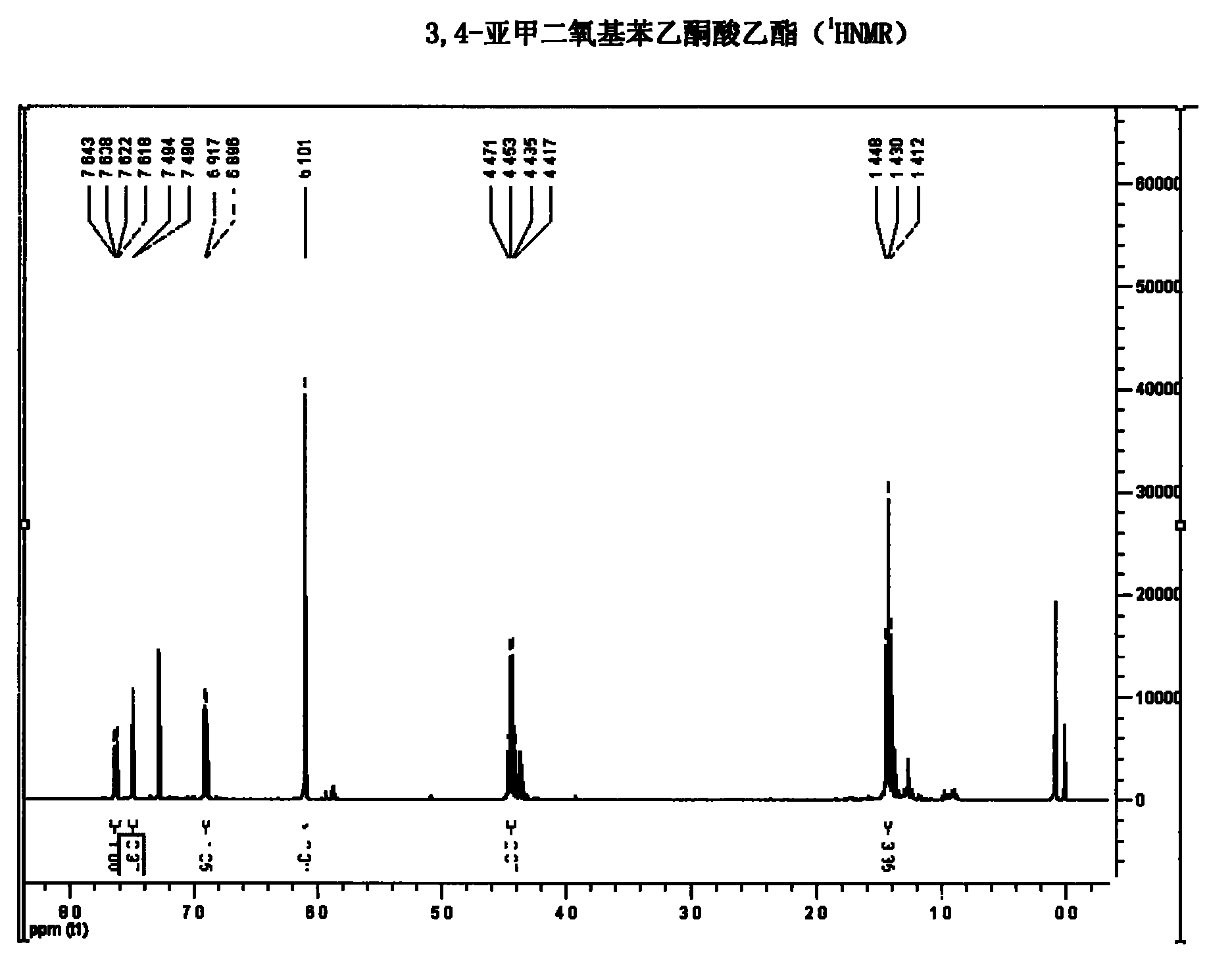
[0031] NMR analysis was carried ou...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com