Method for synthesizing lithium borohydride
A lithium borohydride and synthesis method technology, which is applied in the synthesis of lithium borohydride and the preparation of lithium borohydride, can solve the problems of low product purity, complicated process, and harsh synthesis conditions, and achieve high purity, simple preparation process, and reaction short time effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
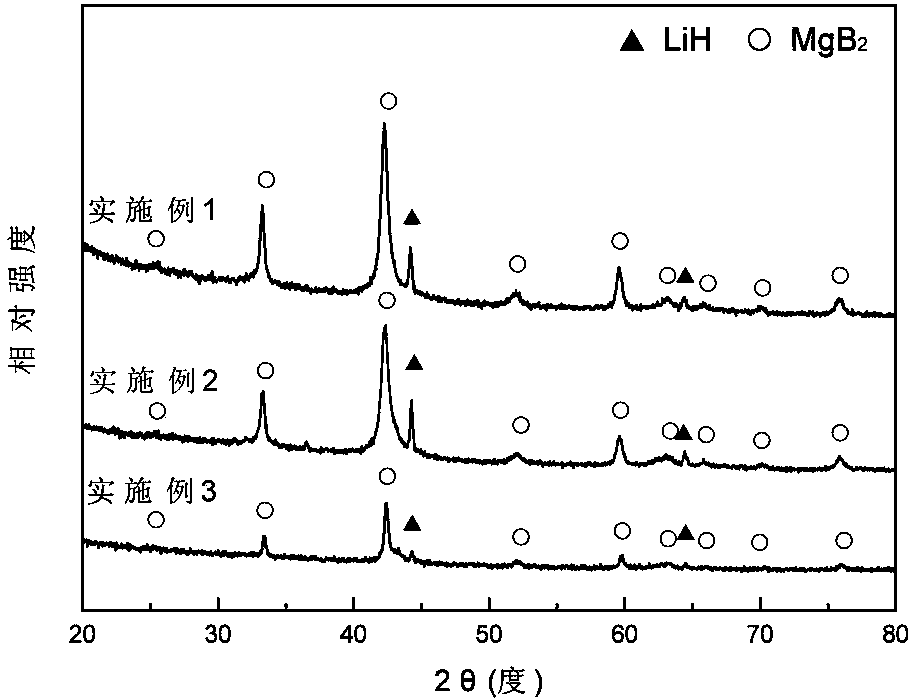
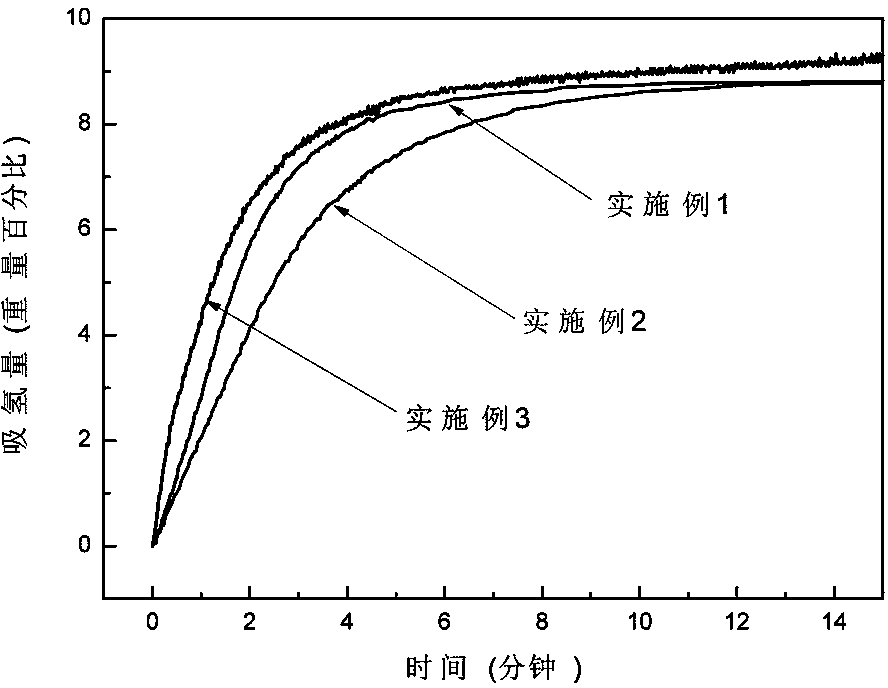
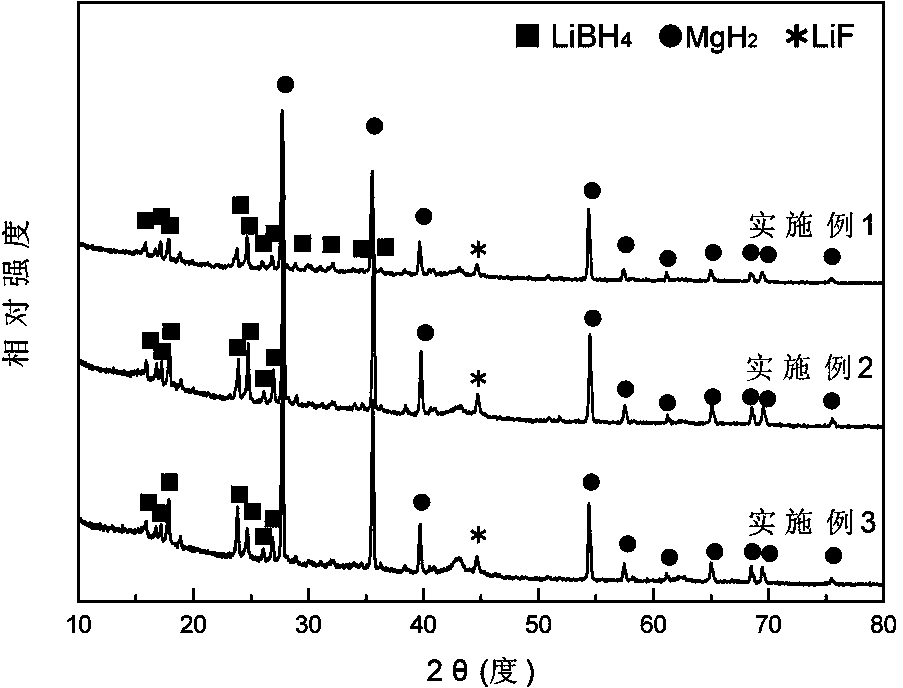
[0042] In a glove box filled with argon, weigh LiH and MgB at a molar ratio of 2:1 2 Two kinds of raw material powders, then add LiH and MgB 2 3 mol% NbF in the total mixture 5 The powder was poured into a stainless steel ball mill tank with a volume of 100 ml, and ball milled with a planetary ball mill for 2 h at a ball-to-material ratio of 40:1, a speed of 400 rpm, and an argon atmosphere, and mechanically mixed to obtain the product after ball milling , its XRD pattern is as figure 1 shown. Put the ball-milled product into the reactor, vacuumize it, place the reactor in a heating furnace to heat the reactor to 400°C, then quickly fill the reactor with hydrogen with a purity of 99.99% to 7 MPa, and carry out Hydrogen absorption reaction for 15 min. Its hydrogen absorption reaction curve is as follows figure 2 It can be seen from the figure that the hydrogen absorption is basically completed after the reaction is carried out for about 10 minutes. Afterwards, the reacto...
Embodiment 2
[0044] In a glove box filled with argon, weigh LiH and MgB at a molar ratio of 2:1 2 Two kinds of raw material powders, then add LiH and MgB 2 3 mol% TiF in the total mixture 3 The powder was poured into a stainless steel ball mill tank with a volume of 100 ml, and was milled with a planetary ball mill for 5 h at a ball-to-material ratio of 30:1, a rotational speed of 350 rpm, and an argon atmosphere, and mechanically mixed to obtain the milled powder. product, its XRD pattern is as figure 1 shown. Put the ball-milled mixture into the reactor, vacuumize it, place the reactor in a heating furnace to heat the reactor to 400°C, and then quickly fill the reactor with hydrogen gas with a purity of 99.99% to 6.5 MPa to carry out Hydrogen absorption reaction for 20 min. Its hydrogen absorption reaction curve is as follows figure 2 It can be seen from the figure that the hydrogen absorption is basically completed after the reaction is carried out for about 14 minutes. Afterward...
Embodiment 3
[0046] In a glove box filled with argon, weigh LiH and MgB at a molar ratio of 2:1 2 Two kinds of raw material powders, then add LiH and MgB 2 5 mol% NbF in the total mixture 5 The powder is poured into a stainless steel ball mill tank with a volume of 100 ml, and ball milled with a planetary ball mill for 5 h at a ball-to-material ratio of 40:1, a speed of 400 rpm, and an argon atmosphere, and mechanically mixed. The mixture after ball milling The XRD pattern such as figure 1 shown. Put the ball-milled mixture into the reactor, vacuumize it, place the reactor in a heating furnace to heat the reactor to 450°C, and then quickly fill the reactor with hydrogen gas with a purity of 99.99% to 7 MPa to carry out Hydrogen absorption reaction for 15 min. Its hydrogen absorption reaction curve is as follows figure 2 It can be seen from the figure that the hydrogen absorption is basically completed after the reaction is carried out for about 8 minutes. Afterwards, the reactor is ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com