Method for preparing intermediate of Entecavir antiviral agent
An inert gas and compound technology, applied in the field of preparation of antiviral drug entecavir intermediates, can solve the problems of expensive raw material reagents, low yield of entecavir intermediates, and unsuitability for industrial production, and achieve low cost and simple reaction conditions , The effect of cheap raw materials
Inactive Publication Date: 2010-12-08
HANDE PHARMA
View PDF1 Cites 22 Cited by
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
The purpose of the present invention is to provide a preparation method of an entecavir intermediate, which solves the problems of low yield of entecavir intermediate in the prior art, high cost, expensive raw material reagents, and unsuitability for industrialized production.
Method used
the structure of the environmentally friendly knitted fabric provided by the present invention; figure 2 Flow chart of the yarn wrapping machine for environmentally friendly knitted fabrics and storage devices; image 3 Is the parameter map of the yarn covering machine
View moreImage
Smart Image Click on the blue labels to locate them in the text.
Smart ImageViewing Examples
Examples
Experimental program
Comparison scheme
Effect test
Embodiment 1
the structure of the environmentally friendly knitted fabric provided by the present invention; figure 2 Flow chart of the yarn wrapping machine for environmentally friendly knitted fabrics and storage devices; image 3 Is the parameter map of the yarn covering machine
Login to View More PUM
 Login to View More
Login to View More Abstract
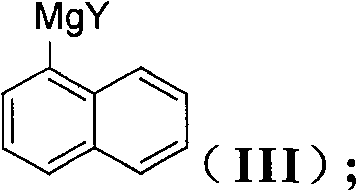
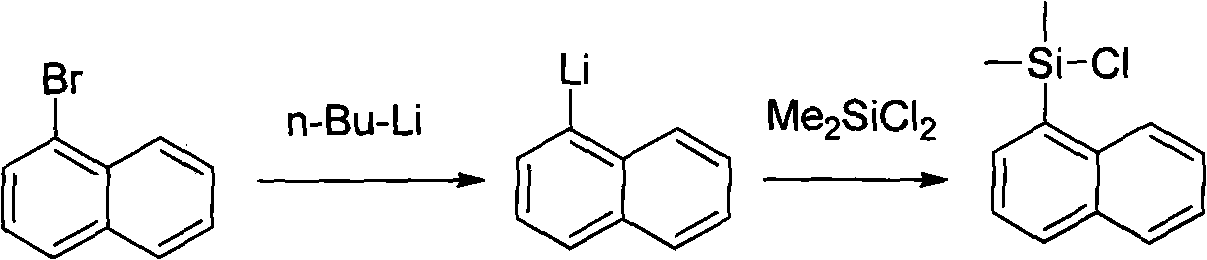
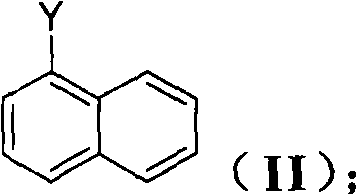
The invention discloses a method for preparing an intermediate (I) of an Entecavir antiviral agent, which comprises the following steps: (1) reacting a compound of a formula (II) with magnesium under a condition of a proper solvent to generate a compound of a formula (III); and performing the Grignard reaction of the compound of the formula (III) and a compound of a formula (IV). In the formulae, X and Y are identical or different and may be F, Cl and Br atoms. The method has the advantages of simple reaction conditions, cheap and readily available raw materials, simple and safe process, low cost and easy industrial production.
Description
technical field The invention belongs to the field of pharmaceutical technology, and in particular relates to a preparation method of an antiviral drug entecavir intermediate. Background technique Chronic hepatitis B is a chronic inflammatory necrotic disease of the liver caused by persistent hepatitis B virus (HBV) infection. Fatigue, general malaise, loss of appetite, discomfort or pain in the liver area, abdominal distension, insomnia, low-grade fever, etc. appear clinically. The disease is a common disease that seriously endangers human health. The prevention and treatment of chronic hepatitis B is a global public health problem, which has attracted the attention of countries all over the world. my country is a high incidence area of viral hepatitis, with an average annual incidence rate of 120-140 / 100,000. Among them, hepatitis B (HB) is the most prominent. The infection rate of hepatitis B virus (HBV) in my country is as high as 57.63%, that is, at least 600 milli...
Claims
the structure of the environmentally friendly knitted fabric provided by the present invention; figure 2 Flow chart of the yarn wrapping machine for environmentally friendly knitted fabrics and storage devices; image 3 Is the parameter map of the yarn covering machine
Login to View More Application Information
Patent Timeline
 Login to View More
Login to View More Patent Type & Authority Applications(China)
IPC IPC(8): C07F7/12
Inventor 方洋李晨曦殷飞
Owner HANDE PHARMA
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com