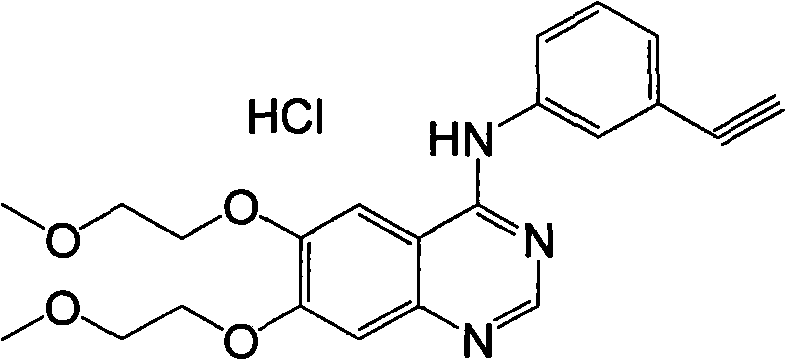
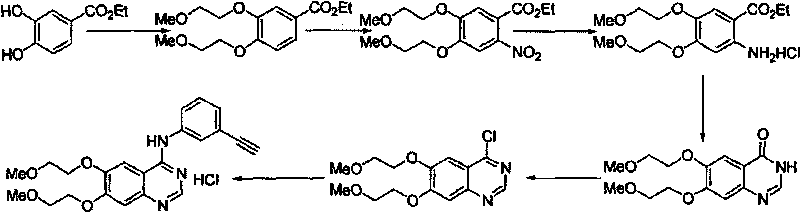
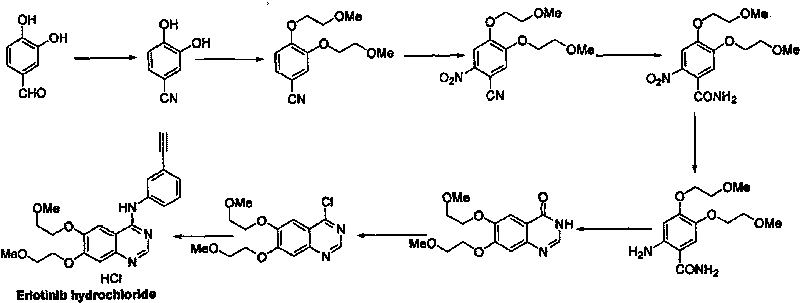
Preparation method of erlotinib hydrochloride
A technology of erlotinib hydrochloride and amino hydrochloride, applied in the field of N--6, can solve the problems of large reaction irritation and pollution, poor stability of substitution reagents, strong irritation, etc., and achieves simple and cheap raw materials, The effect of avoiding side effects and improving safety
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0076] Preparation of Intermediate I:
[0077] Add 10.0L of water, 0.64kg of chloral hydrate, and 9.1kg of anhydrous sodium sulfate into the reaction kettle, and raise the temperature to 30°C for use. Dissolve 0.53kg of 3,4-dimethoxyaniline and 0.78kg of hydroxylamine hydrochloride in 6L of 6.23% dilute hydrochloric acid (v / v), and stir to clarify. Control the temperature below 30°C, add the solution dropwise into the reaction kettle, raise the temperature to 70°C after dropping, and react for 4 hours. TLC monitoring (developer: petroleum ether / ethyl acetate = 2 / 1), filtered after the reaction was complete, washed the filter cake three times with water (0.5L×3), recrystallized with 2.0L ethanol, and filtered. The obtained solid was air-dried at 70° C. for 6 hours to obtain 0.43 kg of an off-white solid, which was the product, and the yield was 55.0%. The H-NMR spectrum identified the product as 2-oximino-N-(3,4-dimethoxyphenyl)acetamide.
[0078] Preparation of Intermediate...
Embodiment 2
[0097] Preparation of Intermediate I:
[0098] Add 5.0L of water, 0.86kg of chloral hydrate, and 5.0kg of anhydrous sodium sulfate into the reaction kettle, and raise the temperature to 40°C for use. Dissolve 0.53 kg of 3,4-dimethoxyaniline and 0.96 kg of hydroxylamine hydrochloride in 6 L of 10% dilute hydrochloric acid (v / v), and stir to clarify. Control the temperature below 40°C, add the solution dropwise to the reaction kettle, heat up to 100°C to reflux after dropping, and react for 3 hours. TLC monitoring (developer: petroleum ether / ethyl acetate = 2 / 1), filtered after the reaction was complete, washed the filter cake three times with water (0.5L×3), recrystallized with 2.5L ethanol, and filtered. The obtained solid was air-dried at 70° C. for 6 hours to obtain 0.40 kg of an off-white solid, which was the product, and the yield was 51.6%. The H-NMR spectrum identified the product as 2-oximino-N-(3,4-dimethoxyphenyl)acetamide.
[0099] Preparation of Intermediate II: ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com