Process for the preparation of C1-C4-alkyl nitrites
A technology of alkyl nitrite and esterification, which is applied in the preparation of nitrite, organic chemistry, etc., can solve the problems of loss of economy, removal of reaction heat can not be carried out uniformly and fully, etc., achieve short residence time, increase yield rate, increasing the effect of selectivity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
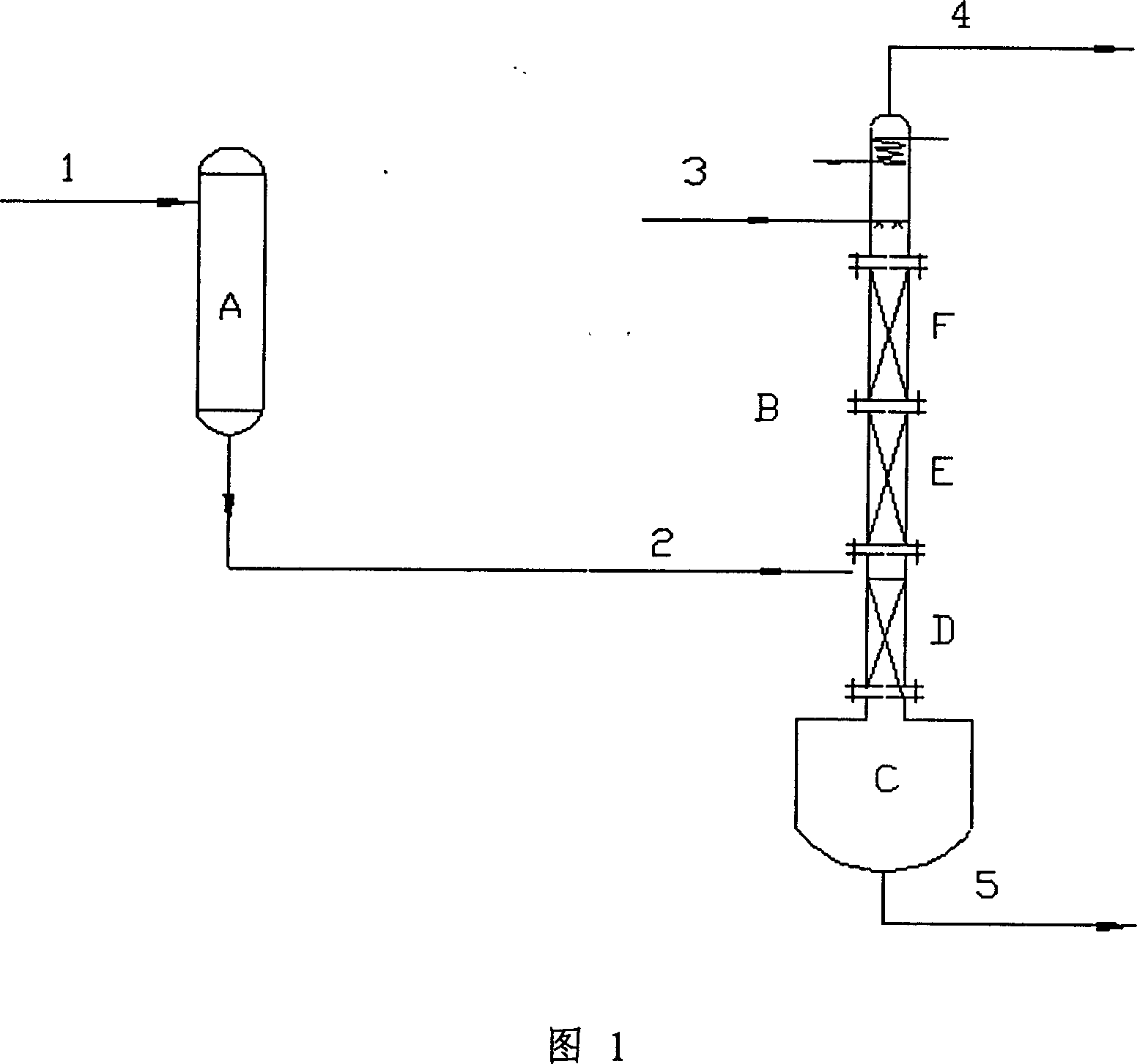
[0028] Mix gas (N 2 2.43L / min, NO 284mL / min, O 2 70mL / min) enters the oxidation reactor from the position in Figure 1, the reaction temperature of the oxidation reactor is controlled to be 100°C, and the methanol at the top of the esterification reactor enters the reaction system at 4.5mL / min. A certain liquid level is established in the tower kettle in advance, and the temperature is raised for heating. The pressure of the whole reaction system (oxidation reactor and esterification reactor) is 0.1Mpa. The temperature of the tower kettle is about 70°C, the temperature below the reaction section is a temperature distribution between 45-70°C, the temperature of the reaction section is controlled at a temperature distribution between 35-45°C, and the temperature of the refrigerant at the top of the tower is controlled at about -7°C.
[0029] After analysis, the nitric acid and methyl nitrite in the tower bottom material 5 are almost undetectable, and the nitric acid and water...
Embodiment 2-8
[0031] Experiments have been carried out according to the experimental device designed in accompanying drawing (1), wherein the alcohol used is methyl alcohol, and the results are shown in Table 1.
[0032] Table 1
[0033]
Embodiment 9
[0035] Mix gas (N 2 2.43L / min, NO 284mL / min, O 2 70mL / min) enters the oxidation reactor from the position in Figure 1, the reaction temperature of the oxidation reactor is controlled to be 100°C, and the ethanol at the top of the esterification reactor enters the reaction system at 4.5mL / min. A certain liquid level is established in the tower kettle in advance, and the temperature is raised for heating. The pressure of the whole reaction system (oxidation reactor and esterification reactor) is 0.1Mpa. The temperature of the tower kettle is about 100°C, the temperature below the reaction section is a temperature distribution between 50-90°C, the temperature of the reaction section is controlled at a temperature distribution between 35-45°C, and the temperature of the refrigerant at the top of the tower is controlled at about -7°C.
[0036] After analysis, the nitric acid and ethyl nitrite in the tower bottom material 5 are almost undetectable, and the nitric acid and water ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com