Production method of lithium ferric metasilicate anode material
A positive electrode material, lithium iron silicate technology, applied in the field of electrochemistry, can solve the problem of difficult balance between material density and electrochemical performance, and achieve the effects of avoiding side reactions of impurity compounds, promoting the roasting process, and facilitating industrial production
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
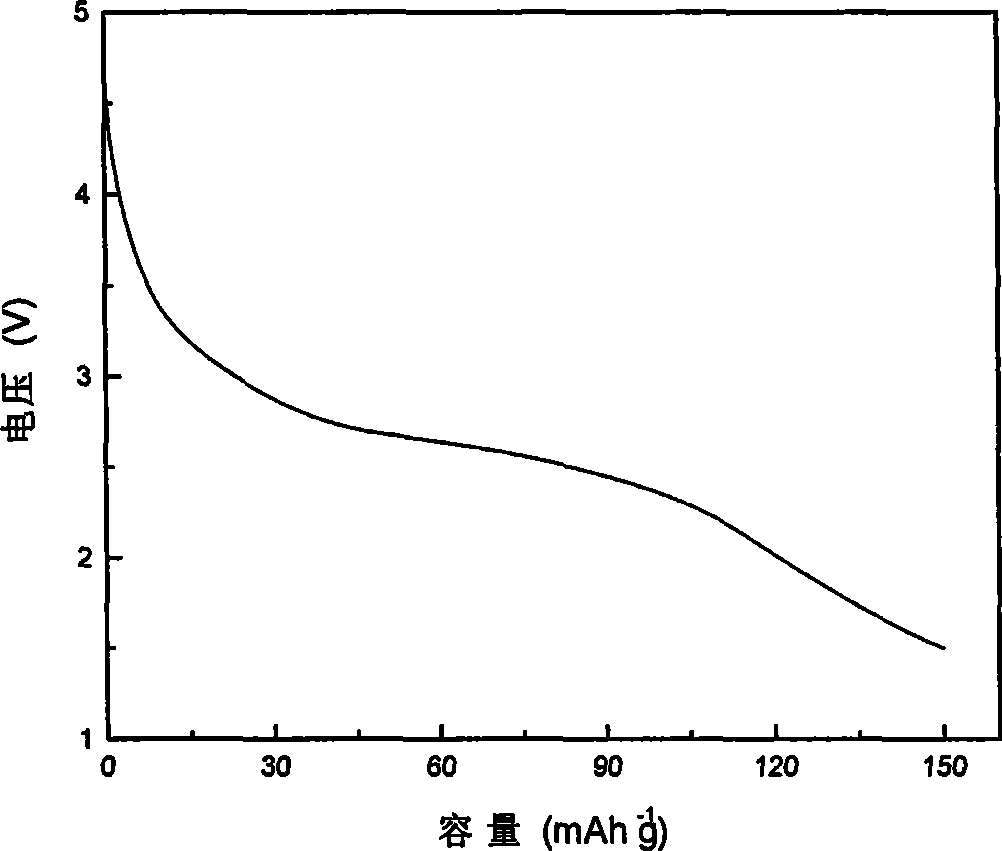
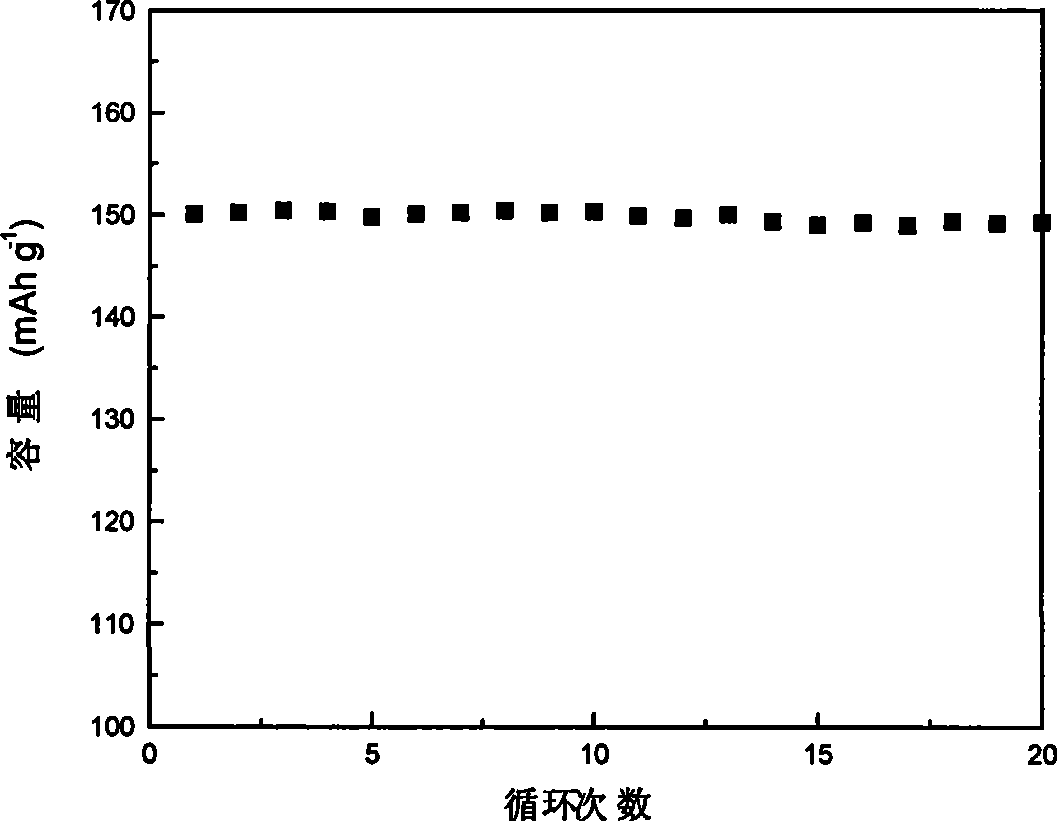
Examples
Embodiment 1
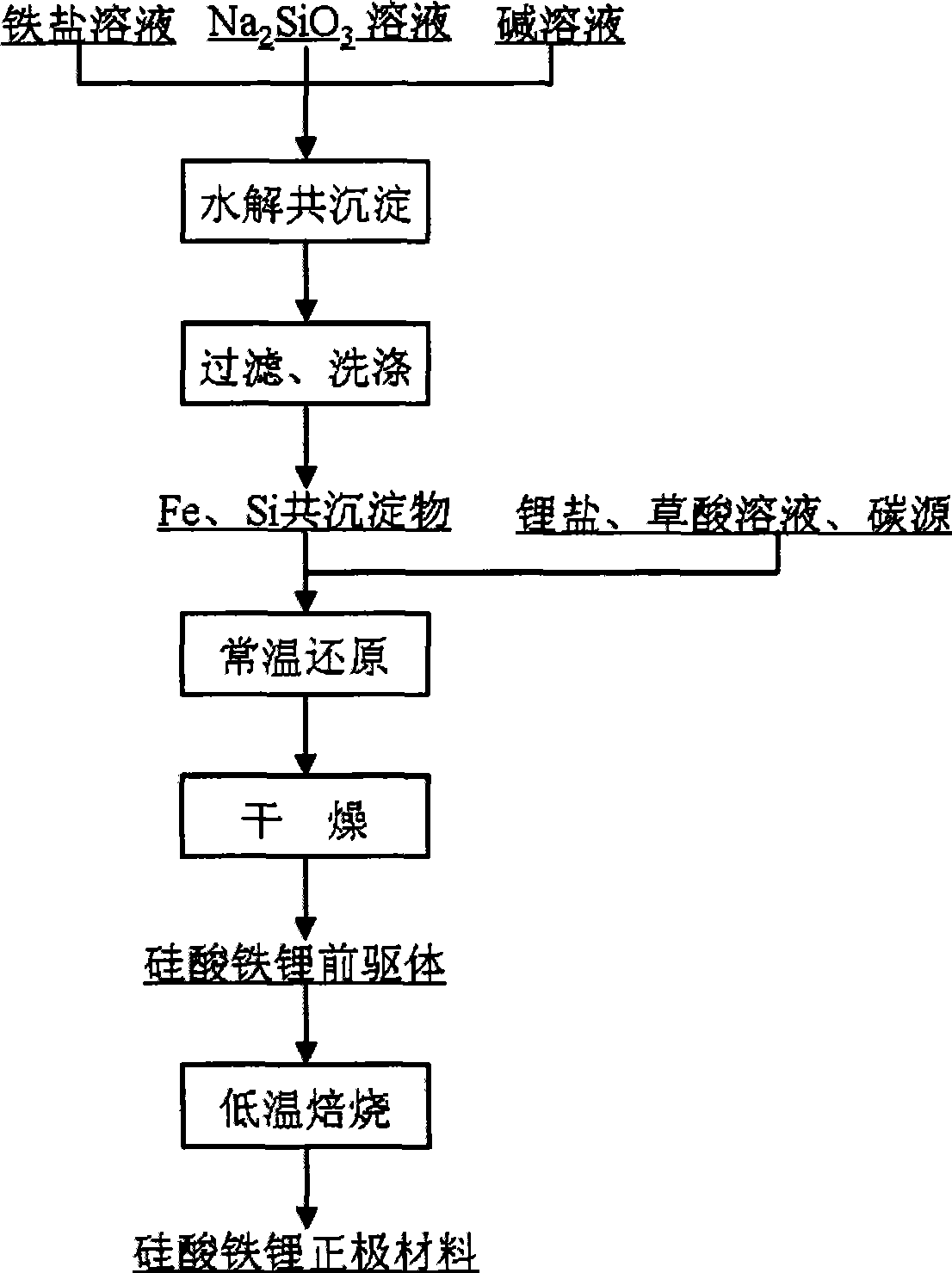
[0025] FeCl 3 Aqueous solution, NaOH solution and Na 2 SiO 3 The solution is added into the stirred reactor in parallel, and the pH value of the reaction system is controlled to be 4.5±0.2, so that ferric iron and SiO 3 2- Hydrolysis occurs, and after filtration and washing, a co-precipitate of iron and silicon, FeCl 3 with Na 2 SiO 3 The amount added is controlled by Fe / Si molar ratio of 1:1.
[0026] Mix iron and silicon co-precipitate with lithium oxalate solution, oxalic acid solution and sucrose to form a slurry, ball mill, and Fe 3+ reduced to Fe 2+ , the precursor material for the synthesis of lithium iron silicate is obtained after drying, wherein the amount of oxalic acid added is based on oxalic acid / Fe 3+ The molar ratio is 0.6, the Li / Si molar ratio in the raw material is controlled to be 2, and the amount of sucrose added is controlled according to the residual carbon content in the obtained lithium iron silicate cathode material being 5wt%±0.2.
[0027] ...
Embodiment 2
[0030] Fe(NO 3 ) 3 Aqueous solution, KOH solution and Na 2 SiO 3 The solution is added into the stirred reactor in parallel, and the pH value of the reaction system is controlled to be 3.2±0.2, so that ferric iron and SiO 3 2- Hydrolysis occurs, and after filtration and washing, a co-precipitate of iron and silicon is obtained, Fe(NO 3 ) 3 with Na 2 SiO 3 The amount added is controlled by Fe / Si molar ratio of 1:1.
[0031] Mix iron and silicon co-precipitate with lithium hydroxide solution, oxalic acid solution and carbon gel to form a slurry, ball mill, and Fe 3+ reduced to Fe 2+ , the precursor material for the synthesis of lithium iron silicate is obtained after drying, wherein the amount of oxalic acid added is based on oxalic acid / Fe 3+ The molar ratio is 0.5, the Li / Si molar ratio in the raw material is controlled at 1.96, and the amount of carbon gel added is controlled at 3wt%±0.2 in the obtained lithium iron silicate cathode material.
[0032] The above pre...
Embodiment 3
[0035] Fe 2 (SO 4 ) 3 Aqueous solution, ammonia solution and Na 2 SiO 3 The solution is added into the stirred reactor in parallel, and the pH value of the reaction system is controlled to be 5.0±0.2, so that ferric iron and SiO 3 2- Hydrolysis occurs, and after filtration and washing, a co-precipitate of iron and silicon, Fe 2 (SO 4 ) 3 with Na 2 SiO 3 The amount added is controlled by Fe / Si molar ratio of 1:1.
[0036] Mix iron and silicon co-precipitate with lithium carbonate, oxalic acid solution, carbon nanotubes, and phenolic resin to form a slurry, ball mill, and Fe 3+ reduced to Fe 2+ , the precursor material for the synthesis of lithium iron silicate is obtained after drying, wherein the amount of oxalic acid added is based on oxalic acid / Fe 3+ The molar ratio is 0.6, the Li / Si molar ratio in the raw material is 2 control, the addition amount of carbon nanotube and phenolic resin is 1% according to the content of carbon nanotube in the lithium iron silicat...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com